Summary
Some missense mutations and small deletions in the NOTCH3 gene, not involving cysteine residues, have been described in patients considered to be affected by paucisymptomatic CADASIL. However, the significance of such molecular variants is still unclear. We describe a 49-year-old woman with a CADASIL-like phenotype, carrying a novel cysteine-sparing mutation in exon 29 of the NOTCH3 gene, and discuss the possible pathogenetic role of this molecular variant. Even though atypical clinical and MRI findings make a diagnosis of CADASIL unlikely in this patient, our report nevertheless underlines the intriguing genotype-phenotype relationship in NOTCH3 mutations and the importance of functional investigation to ascertain the role of new NOTCH3 mutations in CADASIL pathogenesis.
Keywords: CADASIL, cysteine residue, NOTCH3 mutations, white matter lesions
Introduction
CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; OMIM#125310) is an autosomal dominant disease of the cerebral small vessels (Sourander and Wålinder, 1977). The clinical phenotype, which is highly variable even within single families, is characterized by recurrent ischemic episodes (TIA or stroke), typically lacunar, migraine with aura, mood disorders, cognitive deficits and epileptic seizures (Dichgans et al., 1998). Magnetic resonance imaging (MRI) is characterized by lacunar infarcts and diffuse and severe white matter changes that, usually preceding the onset of symptoms, progressively extend to the anterior pole of the temporal lobes, external and internal capsule, basal ganglia and thalamus (Chabriat et al., 1998). Skin biopsy, although positive in only 45% of CADASIL patients, can show the typical and highly disease-specific finding of ‘granular osmiophilic material’ (GOM) (Markus et al., 2002; Malandrini et al., 2007). CADASIL is due to a mutation of NOTCH3, a 34-exon gene located on chromosome 19q12 coding for a transmembrane receptor containing a large number of tandemly arranged epidermal growth factor (EGF)-like repeated domains (Tournier-Lasserve et al., 1993; Artavanis-Tsakonas et al., 1995). Several mutations, mostly missense, but also splice site mutations and small inframe deletions, have been identified in NOTCH3, clustering in exons 3 and 4 (Artavanis-Tsakonas et al., 1995; Dichgans et al., 2000). However, in recent years, mutations in almost all exons within the 34 EGF-like repeats (EGFRs) have been reported (Artavanis-Tsakonas et al., 1995; Dichgans et al., 2000; Dotti et al., 2005; Lesnik Obserstein et al., 2003; Ragno et al., 2006). It is currently not known how CADASIL mutations become pathogenic, although some mechanisms of Notch3 activation and abnormal signal transduction have been hypothesized. Since the reported mutations usually involve either the creation or destruction of a cysteine residue, some authors have hypothesized that unpaired cysteine residues might cause aberrant interaction between the Notch3 receptor and other proteins (Oberstein et al., 1999). Recently, some cysteine-sparing mutations have been described in patients with different MRI findings and a slower clinical course, leading to the suggestion that they might be responsible for milder CADASIL phenotypes rather than rare polymorphisms (Joutel et al.,1997; Mazzei et al., 2004; Kim et al., 2006; Santa et al., 2003; Uchino et al., 2002; Scheid et al., 2008).
We here describe the case of a 49-year-old woman with migraine, sudden falls without loss of consciousness, psychiatric disorders and widespread white matter lesions on MRI carrying a novel cysteine-sparing mutation in exon 29 of the NOTCH3 gene.
Materials and methods
Patient history and clinical features
In July 2007 a 49-year-old woman was admitted to our hospital due to recurrent attacks of right temporal migraine without aura, followed, after a few seconds, by weakness and sudden falls, sometimes associated with brief episodes of loss of consciousness. Morsus and urinary incontinence were never reported. However, the patient had no memory of these episodes. Migraine attacks, which were reported from early childhood, were characterized by diffuse headache, lasting for minutes to hours and associated with nausea, vomiting and photophobia. During the year preceding her admission, the patient reported a significant worsening of migraine, which occurred about twice a week and was associated with falls to the ground. She also reported progressive memory impairment. The patient’s past history included depression and panic disorder, increased prolactin levels with MRI detection of pituitary microadenoma, hypercholesterolemia not controlled by therapy, and smoking (up to 30–40 cigarettes per day). No other cerebrovascular risk factor was reported. Her 88-year-old mother (I-1), still alive, reported hypertension, recurrent thrombophlebitis and severe depression at the age of 30, during pregnancy, whereas her father, who died of prostate cancer at the age of 88, had had a positive history of heart disease. The proband (II-3) is the third of three siblings. The first, a 60-year-old male (II-1), was affected by hypertension, whereas the second, a 55-year-old male (II-2), had an unremarkable medical history, except for mood disorders. The patient’s maternal aunt reported an ischemic stroke whereas three paternal aunts reported intracerebral hemorrhages. There was no family history of migraine, seizures, or cognitive impairment. Neurological examination of the patient, performed during hospitalization, was normal except for the presence of postural instability consisting of body sway to the right on the Romberg test. Repeated measurements of arterial blood pressure over 24 hours were normal and no significant reduction in pressure was detected during the test. Likewise, 24-hour electrocardiography was normal, except for the presence of type I atrioventricular block. Structural cardiac defects were excluded by echocardiography. Interestingly, head-up tilt testing showed a rapid and full compensatory reflex adaptation to the upright position during the preparatory phase, followed by an abrupt drop in pressure with heart rate decrease consistent with a type 1 (mixed) vasovagal syncope. Biochemistry, including blood tests for coagulation and autoimmune disorders, was normal. Electroencephalogram (EEG) revealed only mild slow activity from bilateral frontotemporal derivations. Furthermore, 17-hour EEG polygraphic recording did not show epileptic activity. Neuropsychological tests were normal. The psychiatric assessment resulted in a diagnosis of panic attacks, personality disorder and cyclothymia. T2-weighted and fluid-attenuating inversion recovery (FLAIR) brain MRI (Philips Eclipse 1.5 T MRI scanner, Eindhoven, the Netherlands) revealed severe diffuse leukoencephalopathy with cortical-subcortical atrophy and multiple confluent signal abnormalities in the periventricular white matter, semioval centers and the central part of the pons bilaterally, without gadolinium enhancement (Fig. 1), findings consistent with severe cerebral small vessel disease. Cerebral angiography did not reveal vasculitic signs, such as multifocal segmental stenosis of the intracranial vessels, or display any vascular malformation or aneurysm. On the basis of the clinical history and neuroimaging data, a diagnosis of CADASIL was suspected and, after obtaining written consent, genetic screening of the NOTCH3 gene was performed. Skin biopsy did not reveal GOM deposits or vessel abnormalities. The patient began treatment with valproic acid 1 g/day for the psychiatric disorder, obtaining partial benefit. Given the persistence of migraine accompanied by falls, in April 2009 she was hospitalized again elsewhere. At that time, the neurological examination was normal and the one-year follow-up MRI did not show any significant change in comparison to previous examinations. Valproic acid was stopped on the patient’s request and substituted with lamotrigine 100 mg/day.
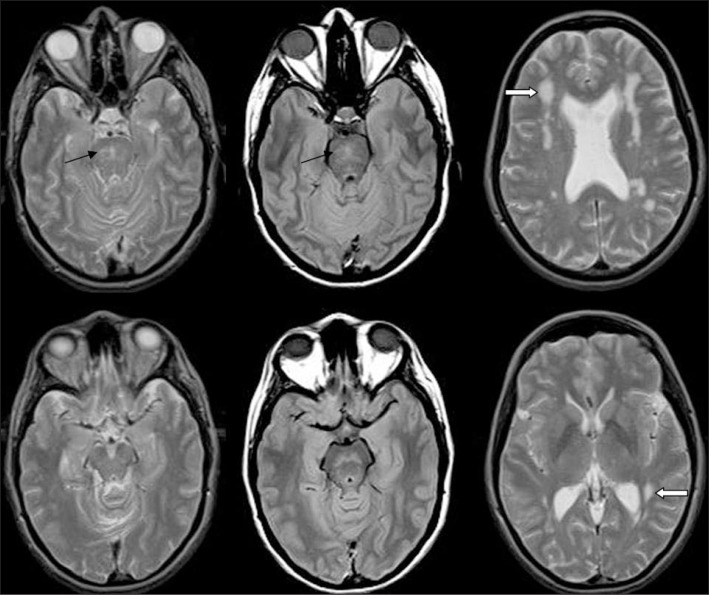
Figure 1.
Patient’s brain MRI
DP and T2-weighted MRI showing diffuse hyperintensities in the pons (black arrows) and the deep and periventricular white matter (white arrows). Note the absence of involvement of the anterior temporal pole and of the external capsule.
Genetic analysis
After obtaining written informed consent we purified DNA from blood of the proband and relatives using the Iso-Quick Nucleic Acid Extraction kit (ORCA Research, Bothell, WA, USA). Primers for PCR reactions were designed following the NOTCH3 genomic sequences accessible at the NCBI database (Acc N. NC 00019.9). Mutation analysis of all the exons of the NOTCH3 gene was performed by direct sequencing of the PCR products obtained with a Big Terminator Sequencing Kit (version 3.1; Applied Biosystems, Life Technology, Carlsbad, CA, USA) in an automated sequencer (ABI 3130 XL, Applied Biosystems). Genomic DNA from 200 unrelated healthy white Italian individuals was available as a control group. Multiplex ligation-dependent probe amplification (MLPA) was performed using the SALSA MLPA P072-A1 probe mix kit (MRC-Holland, Amsterdam, the Netherlands) according to the manufacturer’s instructions.
Results
Genetic analysis
The proband’s DNA was screened for mutations in all exons of the NOTCH3 gene. A single heterozygous base change was found in exon 29 in position c. 5284G>A, which leads to amino acid substitution p.V1762M. The V1762 amino acid residue is partly conserved through evolution in mammals while the substituting residue, methionine, is present in zebrafish (Fig. 2). The change although occurring within an evolutionarily conserved region, lies outside the recognized protein functional domains of EGF or Notch/Lin12. The same change was also detected in the mother (I-1) and in the younger of the two brothers (II-2) whereas none of the healthy subjects carried this molecular variant. No other known potentially pathogenic sequence changes were detected within the analyzed gene region. Intragenic deletion/duplication and/or rearrangements were excluded by MLPA analysis. Cerebral MRI was not performed in the patient’s mother, who refused to undergo the examination on the grounds of her old age, and in the younger of the two brothers who suffered from severe claustrophobia. The CT scan of the patient’s mother showed widespread leukoarariosis whereas that of the brother (II-2) was completely normal. The patient’s brother also underwent skin biopsy, which did not show vessel abnormalities.
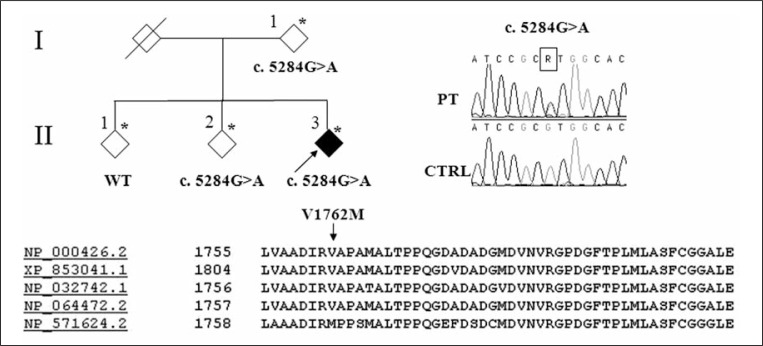
Figure 2.
Family pedigree
The pedigree of the family analyzed is shown in the upper left part of the figure. Asterisks indicate the subjects from whom DNA was obtained. The arrow indicates the proband. Rhombic symbols are used for privacy reasons. Electropherograms of the sequence encompassing the nucleotide change in the patient (PT) with respect to the control (CTRL) are shown on the right. At the bottom the alignment of the Nothc3 homologs from different species is shown: NP_000426 H. sapiens; XP_853041 C. lupus familiaris; NP_032742 M. musculus; NP_064472 R. norvegicus; NP_571624 D. rerio.
Discussion
The majority of disease-causing mutations in the NOTCH3 gene described to date occur within the 34 EGFRs of the Notch3 extracellular domain (ECD) subunit, encoded by exons 2–24, and lead to an odd number of cysteine residues. They have been shown to cause mutant Notch3 ECDs to abnormally accumulate at the cell surface of vascular smooth muscle cells over the disease course (Dichgans et al., 2000; Joutel et al., 2001). The exact mechanisms leading to Notch3 ECD accumulation remain unknown. It is believed that mutations causing CADASIL produce various dysfunctional Notch3 proteins through abnormal folding, dimerization, or tertiary structure or aberrant interactions with other proteins which could cause vascular dysfunction even before the appearance of pathological CADASIL hallmarks (GOM deposits) (Peters et al., 2005). Herein, we describe a patient carrying a missense variant in exon 29 of the NOTCH3 gene, consisting of a methionine/valine substitution. Methionine and valine are two residues with similar biophysical properties in terms of charge and polarity but different steric hindrance. The 5284G>A variant does not constitute a typical CADASIL mutation since it does not lie within an EGFR and does not lead to cysteine substitution.
NOTCH3 gene mutations not involving cysteine residues have already been described in a few patients. Carriers had atypical MRI findings (i.e. a less frequent involvement of the anterior temporal lobes), showed a later disease onset and presented a slower and a milder clinical course than CADASIL patients (Joutel et al.,1997; Mazzei et al., 2004; Kim et al., 2006; Santa et al., 2003; Uchino et al., 2002; Scheid et al., 2008; Quattrone et al., 2009) (Table 1).
Table 1.
Notch 3 mutations not involving cysteine residues reported in the literature
| Authors | Families and patients | Origin | NOTCH3 mutation | Exon | Phenotype/Onset | Age of onset | MRI | GOM |
|---|---|---|---|---|---|---|---|---|
| Uchino M et al., 2002 | 1A* | Japanese | R213K | 4 | Mild migraine, moderate dementia and gait disturbance | 63 | + | − |
| 2A* | Japanese | V237M | 5 | Mild dementia and gait disturbance | 71 | + | − | |
|
| ||||||||
| Santa Y et al., 2003 | 3A* | Japanese | R213K | 4 | Migraine with aura, emotional instability, character change and gait disturbance, disorientation, urinary incontinence, pseudobulbar palsy and dementia | 63 | + | + |
|
| ||||||||
| Mazzei et al., 2004 | 4A* | Italian | delta 88–91 | 3 | Emotional disorders, mild depression, gait difficulties, progressive cognitive decline, stroke-like episodes | 62 | + | + |
| 4B | Italian | delta 88–91 | 3 | Migraine with aura preceded by numbness of the right face and dysphasia | 45 | +/− | − | |
|
| ||||||||
| Kim et al., 2006 | 5A* | Korean | R75P | 3 | Dementia, ischemic episodes | 53 | + | NA |
| 5B | Asymptomatic | NA | − | NA | ||||
| 5C | Asymptomatic | NA | − | NA | ||||
| 6A | Korean | R75P | 3 | Ischemic episodes, dementia | 51 | NA | NA | |
| 6B | Emotional disturbance | 39 | + | NA | ||||
| 6C | Ischemic event, mood changes | 47 | + | NA | ||||
| 6D* | Ischemic episodes | NA | + | NA | ||||
| 6E | Asymptomatic | NA | + | NA | ||||
| 6F | Asymptomatic | NA | − | NA | ||||
| 7A* | Korean | R75P | 3 | Ischemic episodes, dementia | 55 | + | NA | |
| 7B | Asymptomatic | NA | − | NA | ||||
| 7C | Asymptomatic | NA | − | NA | ||||
| 7D | Asymptomatic | NA | − | NA | ||||
| 8A* | Korean | R75P | 3 | Ischemic events, dementia | 65 | + | NA | |
| 8B | Asymptomatic | NA | − | NA | ||||
| 8C | Asymptomatic | NA | − | NA | ||||
|
| ||||||||
| Scheid et al., 2008 | 9A* | German | A1020P | 19 | Sensorineural hearing loss, migraine, intermittent paresthesias and weakness of left extremities, reduced fine motor skills, cognitive slowing, impaired memory | 40 | + | + |
| 9B | Sensorineural hearing loss, nystagmus | 71 | + | NA | ||||
| 9C | Sensorineural hearing loss | NA | +/− | NA | ||||
| 10A* | German | A1020P | 19 | Depression, change of personality, loss of independence in daily living | 70 | + | NA | |
|
| ||||||||
| Our report | 11A* | Italian | c. 5284G>A | 29 | Migraine, psychiatric disorders (panic attacks, personality disorder and cyclothymia), leukoencephalopathy, vasovagal syncope | 49 | + | − |
| 11B | c. 5284G>A | Asymptomatic | 88 | NA | NA | |||
| 11C | c. 5284G>A | Asymptomatic | 60 | NA | NA | |||
| 11D | c. 5284G>A | Psychiatric disorders | 55 | NA | − | |||
Abbreviations and symbols: *=index case; GOM=granular osmiophilic material
The variant herein identified differs from previously described atypical mutations also because it does not lie within in any recognized functional domain of the Notch3 protein. Nevertheless the variant was not present in the set of controls and it occurred within a protein region that is highly evolutionarily conserved in mammals. These observations suggest that this variant is a rare polymorphism, rather than a cause of a milder CADASIL phenotype.
Fouillade et al. (2008) reported a patient with small vessel disease carrying a cysteine-sparing mutation in exon 25, not sharing any molecular, functional and histopathological features of CADASIL-associated NOTCH3 gene mutations. This mutation, unlike canonical NOTCH3 gene mutations, increased Notch3 signaling and the skin biopsy did not show GOM deposits. Given these findings the authors hypothesized that the mutation, through complex gene rearrangements, not revealed by classical molecular procedures, could, somehow, affect Notch3 signaling activity (Fouillade et al., 2008). In the same year, Scheid et al. reported a cysteine-sparing mutation within the EGFR in two German families that presented a CADASIL-like phenotype, except for the absence of GOM deposits on skin biopsy, suggesting the potential existence of more benign CADASIL variants (Scheid et al., 2008).
Taking into account the two above reports as well as the absence of the mutation in 200 healthy controls it can be suggested that the mutation herein described is responsible for a CADASIL-like phenotype. This may involve additional factors, both environmental and genetic, already suggested to influence stroke severity in CADASIL patients and variably accounting for the mild phenotype and the low familial penetrance (Singhal et al., 2004; Adib-Samii et al., 2010).
The interesting finding in our patient of a vasovagal syndrome on tilt testing, which could be purely coincidental since it has never previously been reported in CADASIL, could also be consistent with a major structural alteration of brain arteries leading to impaired ability of vessels to dilate when blood pressure falls, as shown in CADASIL experimental models (Lacombe et al., 2005). However, there are some clinical and molecular data that weaken the hypothesis of a pathogenetic role of the NOTCH3 5284G>A variant.
First, even though MRI studies in the asymptomatic family members are not available to complete the investigation, genotypic segregation within the family was not found to match phenotypic status (I-II and II-2 are healthy subjects despite being mutation carriers). Moreover, the absence of a history of stroke, both in the patient, and in the genetically affected mother and brother, the atypical MRI findings in the proband (lack of temporal lobe involvement and of confluent white matter lesions), and the absence of GOM on skin biospy in all the carriers are at odds with the typical CADASIL phenotype.
Finally, although a functional study is not available, MLPA analysis excluded possible intragenic rearrangements in the NOTCH3 gene, making an impact on Notch3 signaling activity unlikely.
In conclusion even though we are not able to demonstrate the causal role of c. 5284G>A, our report contributes to the ongoing debate on the role of cysteine-sparing mutations and underlines the importance of deeper investigation and functional studies in order to ascertain the role of atypical mutations in CADASIL pathogenesis. In addition, we advocate the utility of complete screening of Notch3 coding exons in suspected cases to better define CADASIL phenotype and genotype spectrum.
Acknowledgments
The patient gave her informed consent for publication of the data.
References
- Adib-Samii P, Brice G, Martin RJ, et al. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010;41:630–634. doi: 10.1161/STROKEAHA.109.568402. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Levy C, Taillia H, et al. Patterns of MRI lesions in CADASIL. Neurology. 1998;51:452–457. doi: 10.1212/wnl.51.2.452. [DOI] [PubMed] [Google Scholar]
- Dichgans M, Mayer M, Uttner I, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol. 1998;44:731–739. doi: 10.1002/ana.410440506. [DOI] [PubMed] [Google Scholar]
- Dichgans M, Ludwig H, Müller-Höcker J, et al. Small inframe deletions and missense mutations in CADASIL: 3D models predict misfolding of Notch3 EGF-like repeat domains. Eur J Hum Genet. 2000;8:280–285. doi: 10.1038/sj.ejhg.5200460. [DOI] [PubMed] [Google Scholar]
- Dotti MT, Federico A, Mazzei R, et al. The spectrum of Notch3 mutations in 28 Italian CADASIL families. J Neurol Neurosurg Psychiatry. 2005;76:736–738. doi: 10.1136/jnnp.2004.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillade C, Chabriat H, Riant F, et al. Activating NOTCH3 mutation in a patient with small-vessel-disease of the brain. Hum Mutat. 2008;29:452. doi: 10.1002/humu.9527. [DOI] [PubMed] [Google Scholar]
- Joutel A, Vahedi K, Corpechot C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]
- Joutel A, Favrole P, Labauge P, et al. Skin biopsy immunostaining with a Notch3 monoclonal antibody for CADASIL diagnosis. Lancet. 2001;358:2049–2051. doi: 10.1016/S0140-6736(01)07142-2. [DOI] [PubMed] [Google Scholar]
- Kim Y, Choi EJ, Choi CG, et al. Characteristics of CADASIL in Korea: a novel cysteine-sparing Notch3 mutation. Neurology. 2006;66:1511–1516. doi: 10.1212/01.wnl.0000216259.99811.50. [DOI] [PubMed] [Google Scholar]
- Lacombe P, Oligo C, Domenga V, et al. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke. 2005;36:1053–1058. doi: 10.1161/01.STR.0000163080.82766.eb. [DOI] [PubMed] [Google Scholar]
- Lesnik Oberstein SA, van Duinen SG, van den Boom R, et al. Evaluation of diagnostic NOTCH3 immunostaining in CADASIL. Acta Neuropathol. 2003;106:107–111. doi: 10.1007/s00401-003-0701-6. [DOI] [PubMed] [Google Scholar]
- Malandrini A, Gaudiano C, Gambelli S, et al. Diagnostic value of ultrastructural skin biopsy studies in CADASIL. Neurology. 2007;68:1430–1432. doi: 10.1212/01.wnl.0000264018.46335.c8. [DOI] [PubMed] [Google Scholar]
- Markus HS, Martin RJ, Simpson MA, et al. Diagnostic strategies in CADASIL. Neurology. 2002;59:1134–1138. doi: 10.1212/wnl.59.8.1134. [DOI] [PubMed] [Google Scholar]
- Mazzei R, Conforti FL, Lanza PL, et al. A novel Notch3 gene mutation not involving a cysteine residue in an Italian family with CADASIL. Neurology. 2004;63:561–564. doi: 10.1212/01.wnl.0000133399.37716.84. [DOI] [PubMed] [Google Scholar]
- Oberstein SA, Ferrari MD, Bakker E, et al. Diagnostic Notch3 sequence analysis in CADASIL: three new mutations in Dutch patients. Dutch CADASIL Research Group. Neurology. 1999;52:1913–1915. doi: 10.1212/wnl.52.9.1913. [DOI] [PubMed] [Google Scholar]
- Peters N, Opherk C, Bergmann T, et al. Spectrum of mutations in biopsy-proven CADASIL: implications for diagnostic strategies. Arch Neurol. 2005;62:1091–1094. doi: 10.1001/archneur.62.7.1091. [DOI] [PubMed] [Google Scholar]
- Quattrone A, Mazzei R. Cysteine-sparing notch3 mutations: cadasil or cadasil variants? Neurology. 2009;72:2135–2136. doi: 10.1212/01.wnl.0000349699.12456.06. [DOI] [PubMed] [Google Scholar]
- Ragno M, Fabrizi GM, Cacchiò G, et al. Two novel Italian CADASIL families from Central Italy with mutation CGCTGC at codon 1006 in the exon 19 Notch3 gene. Neurol Sci. 2006;27:252–256. doi: 10.1007/s10072-006-0679-7. [DOI] [PubMed] [Google Scholar]
- Santa Y, Uyama E, Chui DH, et al. Genetic, clinical and pathological studies of CADASIL in Japan: a partial contribution of Notch3 mutations and implications of smooth muscle cell degeneration for the pathogenesis. J Neurol Sci. 2003;212:79–84. doi: 10.1016/s0022-510x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Scheid R, Heinritz W, Leyhe T, et al. Cysteine-sparing notch3 mutations: cadasil or cadasil variants? Neurology. 2008;71:774–776. doi: 10.1212/01.wnl.0000324928.44694.f7. [DOI] [PubMed] [Google Scholar]
- Singhal S, Bevan S, Barrick T, et al. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain. 2004;127:2031–2038. doi: 10.1093/brain/awh223. [DOI] [PubMed] [Google Scholar]
- Sourander P, Wålinder J. Hereditary multi-infarct dementia. Morphological and clinical studies of a new disease. Acta Neuropathol. 1977;39:247–254. doi: 10.1007/BF00691704. [DOI] [PubMed] [Google Scholar]
- Tournier-Lasserve E, Joutel A, Melki J, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy maps to chromosome 19q12. Nat Genet. 1993;3:256–259. doi: 10.1038/ng0393-256. [DOI] [PubMed] [Google Scholar]
- Uchino M, Hirano T, Uyama E, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and CADASIL-like disorders in Japan. Ann N Y Acad Sci. 2002;977:273–278. doi: 10.1111/j.1749-6632.2002.tb04826.x. [DOI] [PubMed] [Google Scholar]