Abstract
Enhancing chondrogenic and osteogenic differentiation is of paramount importance in providing effective regenerative therapies and improving the rate of fracture healing. This study investigated the potential of non-thermal atmospheric dielectric barrier discharge plasma (NT-plasma) to enhance chondrocyte and osteoblast proliferation and differentiation. Although the exact mechanism by which NT-plasma interacts with cells is undefined, it is known that during treatment the atmosphere is ionized generating extracellular reactive oxygen and nitrogen species (ROS and RNS) and an electric field. Appropriate NT-plasma conditions were determined using lactate-dehydrogenase release, flow cytometric live/dead assay, flow cytometric cell cycle analysis, and Western blots to evaluate DNA damage and mitochondrial integrity. We observed that specific NT-plasma conditions were required to prevent cell death, and that loss of pre-osteoblastic cell viability was dependent on intracellular ROS and RNS production. To further investigate the involvement of intracellular ROS, fluorescent intracellular dyes Mitosox (superoxide) and dihydrorhodamine (peroxide) were used to assess onset and duration after NT-plasma treatment. Both intracellular superoxide and peroxide were found to increase immediately post NT-plasma treatment. These increases were sustained for one hour but returned to control levels by 24 hr. Using the same treatment conditions, osteogenic differentiation by NT-plasma was assessed and compared to peroxide or osteogenic media containing β-glycerolphosphate. Although both NT-plasma and peroxide induced differentiation-specific gene expression, neither was as effective as the osteogenic media. However, treatment of cells with NT-plasma after 24 hr in osteogenic or chondrogenic media significantly enhanced differentiation as compared to differentiation media alone. The results of this study show that NT-plasma can selectively initiate and amplify ROS signaling to enhance differentiation, and suggest this technology could be used to enhance bone fusion and improve healing after skeletal injury.
Introduction
The goal of this investigation was to examine the effect of non-thermal (NT)-plasma on skeletal cell differentiation. Medical use of plasma technology is most commonly associated with thermal plasmas, such as the plasma knife used in surgery to cut and simultaneously cauterize vessels as a result of the high temperature generated by plasma [1]. Advancements in atmospheric pressure plasma systems led to the development of a novel NT dielectric barrier discharge plasma with a discharge sufficiently uniform and cold to safely apply to living cells and tissues [2]–[6]. The NT-plasma discharge is generated by applying a high voltage, time-varying waveform between a dielectric covered electrode and the biological target [7], [8]. To prevent high temperature build-up and transition to arc, high voltage current is alternated between the two electrodes, one of which is covered with a dielectric. Within the NT-plasma discharge, the molecules present in air (O2, N2, H2O, CO2, etc) are ionized resulting in the direct formation of numerous reactive oxygen species (ROS) and reactive nitrogen species (RNS) [8]–[10].
Most biomedical studies on the effect of NT-plasma have focused on the bacteriostatic and bactericidal properties of this new technology [11]–[13]. Recently, it was reported that NT-plasma exposure promoted endothelial cell proliferation, which was abrogated by fibroblast growth factor (FGF)-2 neutralizing antibody [5]. Proliferation and FGF-2 release were blocked by N-acetyl-cysteine (NAC), which prevented changes in intracellular redox. Mechanistically, these studies directly link NT-plasma effects to ROS or RNS generation.
ROS and RNS are known to directly activate multiple proteins involved in signaling pathways that regulate cell function. ROS-responsive MAP kinases are known to control a wide range of cellular processes including: cellular differentiation, cell cycle control, cytokine and growth factor signaling, survival, hypertrophy and/or apoptosis [14]–[17]. For example, the Map5kinase Apoptosis signal-regulating kinase 1 (ASK1), is particularly sensitive to ROS as its activity is tightly regulated by four ROS sensitive proteins thioredoxin, glutaredoxin, Akt and 14-3-3 [17]–[21]. ROS activated ASK1 phosphorylates and activates both p38 and jnk kinases, which play key roles in cellular differentiation [22], [23] as well as the regulation of apoptosis [24]. Activation of ASK1, p38 and/or jnk promotes the differentiation of several cell lineages including chondrocytes [25]–[27], osteoblasts, neuronal [28], myoblasts [29] and keratinocytes [14], [23], [27].
In the case of mesenchymal cell differentiation into osteoblast lineages there is precedence for ROS stimulation to both direct and enhance this process [30]–[32]. Similarly, enhanced chondrogenesis has also been associated with ROS stimulation [27] and the occurrence of oxidative spikes as a driving force for transitional stages throughout developmental and regenerative processes is a commonly observed phenomena. The possibility of using NT-plasma technology to artificially trigger these transitions and direct these processes is intriguing. Herein, we show that specific exposure conditions to NT-plasma promote intracellular ROS production and skeletal cell differentiation.
Materials and Methods
Cell Culture
The pre-osteocytic cell line MLO-A5 was obtained from Dr. Linda Bonewald and cultured using the method described previously [33]. To induce maturation for qRT-PCR experiments MLO-A5, cells were placed in osteogenic differentiation media (Dulbecco’s Modified Eagle’s Medium (DMEM) (Cellgro, Fisher Scientific, USA) with 10% Fetal Bovine Serum (FBS) (Invitrogen, Life Science Technologies, USA), and supplemented with 100 units/ml penicillin, 100 µg/ml streptomycin (Cellgro, Fisher Scientific, USA), 0.5 mM beta glycero-phosphate and 100 µg/ml ascorbic acid (Sigma-Aldrich, St. Louis, MO) and incubated in 5% CO2 at 37°C with media changes every 2 days. N1511 mouse chondrocytes were obtained from Dr. Motomi Enomoto-Iwamoto. The cells were maintained in culture using the method described previously [34], [35]. Briefly, the cells were plated at a concentration of 50,000 cells/ml in/−MEM containing 10% FBS, 0.2% L-glutamine, and penicillin/streptomycin. To induce maturation, after 24 h, the adherent cells were treated with 200 ng/ml recombinant BMP2 (Alpha Diagnostic Intl., San Antonio, TX). Assessment of the maturation was performed at day 5 by measuring alkaline phosphatase activity and the expression of chondrogenic markers.
Power Measurement for Calibration of Plasma Device
Tektronix DPO4104B (1 GHz bandwidth, 5 GS/sec) digital oscilloscope with Tektronix P6015A high voltage divider (1000∶1) and Ion Physics Corp CM-10-L current probe (1 V/1 A, 5 kA max) was used to record voltage and current waves at maximum sampling rate. Instant values for both were multiplied and resulting instant power was then integrated over the entire cycle. Ten measurements were taken for statistics analysis.
Non-Thermal Plasma Treatment
NT-plasma was generated by applying alternating polarity pulsed voltage of 20 kV magnitude (peak to peak), a 10 µs pulse width, at a frequency between 50 to 3500 Hertz (Hz), with a rise time of 5 V/ns between the quartz-insulated high voltage electrode and the sample undergoing treatment 2 mm from the electrode. General electrical schematics and description of the power supply (Plasma Power, LLC, Philadelphia USA) were as reported by the authors [4]. A 32 mm (6-well plate) electrode was used, which was composed of an inner core of copper surrounded by an outer insulating shell of acrylic, and a 0.5 mm quartz disc plasma-generating surface covered with a 0.01inch sheet of polyether ether ketone. Just before treatment, culture media was removed by pipet from the dish and replaced immediately following treatment.
Cell Viability and Cell Cycle Analysis
Cell viability was measured by live/dead flow cytometry in cells stained with propidium iodide (PI; Sigma), an indicator of cell death. PI was added immediately before analysis on the MACSQuant VYB flow cytometer (Miltenyi Biotec, Auburn, CA). The number of PI stained cells was counted and the data was analyzed with MACSQuant software (Miltenyi Biotec, Auburn, CA).
Lactate dehydrogenase (LDH) (Cayman Chemical, Ann Arbor, MI) assay was performed at 24 hr as per manufacturer’s instructions, and the % cytotoxicity (LDH release) was calculated from the standard curve and TritonX-100 positive control. Intensity measurements were performed using a Tecan Infinite 1000 plate reader (Tecan, Research Triangle, NC). H2O2 (100 µM) (Life Technologies, Grand Island, NY) or 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL; 6 mM) (Sigma, St. Louis, MO) was added to the medium for one hour before NT-plasma treatment. The media was replaced after 3 hr without H2O2 or TEMPOL.
To determine cell cycle kinetics, 24 hr after NT-plasma treatment cells were prepared for flow cytometric cell cycle analysis on the MACSQuant VYB (Miltenyi Biotec, Auburn, CA), following the Miltenyi cell cycle analysis protocol. Briefly, cells were collected in sample buffer (Ca+2 and Mg+2 free PBS, with 1 g/L glucose), washed and placed in 70% ethanol overnight at 4°C. After re-suspension in sample buffer, the propidium iodide (PI (1 ug/ml); Sigma, St. Louis, MO) was added and the stained cells were analyzed with MACSQuant software (Miltenyi Biotec, Auburn, CA). This procedure was repeated in quadruplicate on 3 separate days.
Western Blot Analysis for DNA and Mitochondrial Damage
Protein from NT-plasma treated cells was collected 24 hr after treatment. Cells were isolated with MPER®, 5 M NaCl, complete protease inhibitor cocktail, DTT, 100 µM NaF, and 100 µM Na3VO4 and quantified using the Bradford assay (Pierce Biotechnology Thermol Scientific, Rockford IL). Protein (40 µg) was then loaded onto 10% Tris polyacrylamide gels. Samples were transferred to PVDF membranes and subsequently blocked with ECL Blocking agent (RPN 2125, GE Health Life Sciences, Cleveland, OH) (2.0% TBS-T +2.0 g dried milk). Blots were treated overnight with antibodies to either H2Ax (Millipore, Billerica, MA) or Cytochrome C (BD Biosciences, Franklin Lakes, NJ) at a 1∶500 dilution in 2% blocking reagent. The blots were washed three times in TBST and species specific HRP conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX) were applied for 1 hr at room temperature. After three washes in TBST, the membrane was detected with Western Bright Quantum kit (Advansta, Menlo Park, CA) for 5 min at 25°C. Photographs of the membranes were taken in a GE Image quant LAS 4000 dark box with a GE Luminescent Image Analyzer LAS-4000 (GE Health Life Sciences, Cleveland, OH) and digital images were collected using the Image quant TL 1D gel analysis software (GE Health Life Sciences, Cleveland,OH).
ROS Measurements
Mitosox and dihydrorhodamine (DHR) dyes (Invitrogen, Carlsbad, CA) were used to detect the presence of intracellular superoxide anion and hydrogen peroxide, respectively. NT-plasma or sham treatments were performed on MLO-A5 cells plated overnight in 6 well plates and fluorescent intensity was measured using a Tecan Infinite 1000 plate reader (Tecan, Research Triangle, NC). Inhibitors (NAC, 200 µM and TEMPOL, 6 mM (Sigma, St. Louis, MO) were added to the medium for one hour before NT-plasma treatment and replaced 3 hr after with regular media.
Real Time-PCR for Differentiation Markers and Comparative Analyses
Confluent cells in 6 well dishes were either treated with differentiation media (β-GP), H2O2 (100 µM) or treated with 1000 Hz NT-plasma for 10 sec and harvested after 24 hr. Alternatively, confluent cells from 6 well dishes were placed in differentiation media (MLO-A5 with β-GP or N1511 with BMP) 24 hr before NT-plasma or sham-plasma control treatment and harvested after 24 hr or 56 hr. All harvested cells were washed with DEPC water before total RNA was isolated using the QiagenRNeasy® Mini kit (Qiagen, Valencia, CA). RNA yield was determined spectrophotometrically and integrity confirmed by gel electrophoresis. RNA was reverse-transcribed and then amplified using the Superscript™ One-Step RT-PCR with Platinum Taq® (Invitrogen, Carlsbad, CA) kit. PCR products were analyzed by 1.0% agarose gel electrophoresis. Template cDNA and gene specific primers were added to Fast SYBR Green master mix (Applied Biosystems, Carlsbad, CA) and mRNA expression was quantified using the MyIQ Real-Time PCR System (BioRad, Hercules, CA). The expression level of the housekeeping gene, β-actin was used to normalize the data presented. Melting curves were analyzed to verify the specificity of the RT-PCR reaction and the absence of primer dimer formation. Each sample is analyzed in duplicate and included a template-free control. All the primers (Table 1) used were synthesized by Integrated DNA Technologies, Inc. (Coralville, IN).
Table 1. PCR primer information.
| Primer Name | Strand | Primer Sequence |
| actin | FWD | gct aca gct cac cac cac a |
| actin | REV | 251658240tct cca ggg agg aag agg a |
| Osteocalcin | FWD | gac aaa gcc ttc atg tcc aag c |
| Osteocalcin | REV | aaa gcc gag ctg cca gag ttt g |
| BMP2 | FWD | aag aag ccg tgg agg aac tt |
| BMP2 | REV | ttc ccg gaa gat ctg gag tt |
| BSP | FWD | agg gag gca gtg act ctt ca |
| BSP | REV | aca ccc gag agt gtg gaa ag |
| Runx2 | FWD | gcc ggg aat gat gag aac ta |
| Runx2 | REV | gga ccg tcc act gtc act tt |
| Fgf2 | FWD | agc ggc tct act gca aga ac |
| Fgf2 | REV | gcc gtc cat ctt cct tca ta |
| ALK PHOS | FWD | tat gtc tgg aac cgc act ga |
| ALK PHOS | REV | cca gca aga aga agc ctt tg |
| Col X | FWD | cgt gtc tgc ttt tac tgt ca |
| Col X | REV | acc tgg tca ttt tct gtg ag |
| MMP-13 | FWD | atc ctg gcc acc ttc ttc tt |
| MMP-13 | REV | ttt ctc gga gcc tgt caa ct |
| OSTERIX | FWD | act ggc tag gtg gtg gtc ag |
| OSTERIX | REV | ggt agg gag ctg ggt taa gg |
Statistical Analysis
Statistical analysis between groups was performed using a one-way ANOVA for normality and student’s t-test for continuous variables. A level of significance (α), or a p-value of less than 0.05, with a 95% confidence interval was determined. Representative data are presented as the mean ± SD of individual samples from 2 to 16 independent analyses, unless otherwise stated. In this work “n” indicates number of separate experimental trials, with repeats varying from 3 to 8, or more. For normally distributed data, differences between groups were determined by independent t-tests. For non-parametric data, Mann Whitney U tests were used to evaluate differences.
Results
Determining the Appropriate NT-Plasma Dose
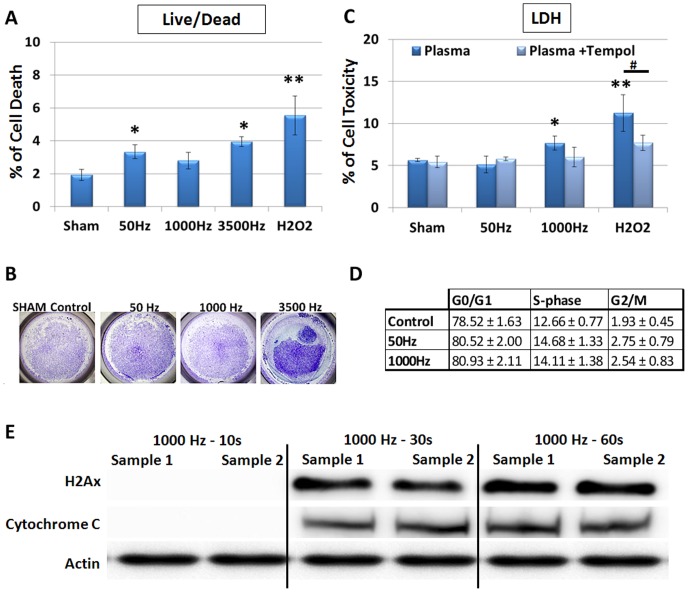
To investigate the appropriate NT-plasma dose, MLO-A5 osteoblastic cells in 6 well plates were treated with NT-plasma for 10 sec at a voltage of 20 kV and pulse width of 10 µs), varying only the frequency at either 50, 1000, or 3500 Hertz (Hz). Controls included the NT-plasma electrode without power as a sham treatment, and H2O2 (100 µM) as a positive control for ROS. Cell death was determined 24 hr after NT-plasma treatment using a fluorescent flow cytometric live/dead assay. Less than 4% of the cells underwent cell death in response to NT-plasma treatment, although the 50 Hz (p<0.05) and 3500 Hz (p<0.05) treatments were significantly above control (Fig. 1A). H2O2 induced a slightly higher 5.6% increase in cell death (p<0.01). Results for the 3500 Hz treatment, were an under estimate of cell damage, as large areas of the culture plate were devoid of cells immediately after treatment (Fig. 1B). Due to cell detachment this setting was not used in subsequent assays.
Figure 1. Direct effects of NT-plasma on cytotoxicty and cell proliferation.
Osteoblast-like, MLO-A5 cells were treated with NT-plasma at frequencies of 50, 1000 and 3500 Hz for 10 seconds. (A) Cell death measured by nuclear PI incorporation at 24 hr showed increased cell death in response to frequency 50 Hz, 3500 Hz (p<0.01) and H2O2 (p<0.001) (n = 3). There was no significant cell death in response to frequency 1000 Hz. (B) Cell detachment was observed at 3500 Hz as shown by toluidine staining of cells after NT-plasma treatment. (C) Cell viability after NT-plasma was assessed by a lactate dehydrogenase (LDH) release assay. 1000 Hz and H2O2 both showed an increase in LDH release (p<0.01 and p<0.001). LDH release was reduced in the presence of TEMPOL for both H2O2 (p<0.01) and NT-plasma treatments (ns, n = 3). (D) No difference in cell cycle profile was observed between NT-plasma at 50 Hz or 1000 Hz as compared to sham control. (E) Western blots show no H2Ax or cytochrome c release in cells treated at 1000 Hz for 10 s as compared to the 30 and 60 sec NT-plasma treatments. Statistical significance was determined by the Mann-Whitney U test for non-parametric data; * or # (p<0.01) ** or ## (p<0.001.
To further assess the 50 and 1000 Hz dose rates, MLO-A5 cell cytotoxicity was measured using lactate dehydrogenase (LDH) (Fig. 1C). At 24 h LDH release in response to 50 Hz was 5.1% (not significant; ns) and 1000 Hz was 7.7% (p<0.01) above NT-plasma sham control. Both treatments were less than H2O2, which induced an 11.2% increase in cell death (p<0.01). To prevent changes in intracellular oxidant levels, TEMPOL (6 mM) a membrane permeable scavenger of both ROS and RNS, was added 1 hr before NT-plasma or H2O2 treatment [36], [37]. TEMPOL reduced the release of LDH in response to 1000 Hz NT-plasma (ns) and significantly decreased LDH release in response to H2O2 (p<0.01), indicating ROS/RNS are being generated in response to NT-plasma and cause cell damage.
To determine whether NT-plasma treatment affected cell proliferation (increased S phase) or caused DNA damage (blockage in G2), flow cytometric analysis of cell cycle phase was determined 24 hours after treatment of MLO-A5 cells with 50 and 1000 Hz NT-plasma or H2O2 (Fig. 1D). No significant differences were observed in the G1, S or G2/M phase after any treatment, indicating no increase in cell proliferation or blockage of the cell cycle due to DNA damage.
Additionally, Western blot analysis was performed to assess damage to DNA or mitochondrial membranes using antibodies to the histone2A variant (H2AX) and cytoplasmic cytochrome c, respectively (Fig. 1E). The 1000 Hz NT-plasma treatment dose was achieved by varying the time to include 10 sec (4.08 Joules (J)/cm2), 30 sec (12.24 J/cm2) and 60 sec (24.48 J/cm2) dose. The 10 sec dose did not induce DNA or mitochondrial damage within 1 hr after treatment, but damage after both the 30 and 60 sec treatments was observed.
Taken together the results show that NT-plasma treatment at 1000 Hz for 10 sec did not significantly reduce cell viability, alter the cell cycle or cause DNA or mitochondrial damage in MLO-A5 cells. Based on these findings the 1000 Hz, 10 sec NT-plasma treatment was chosen as the NT-plasma optimal treatment.
Production of Intracellular ROS in Response to NT-Plasma
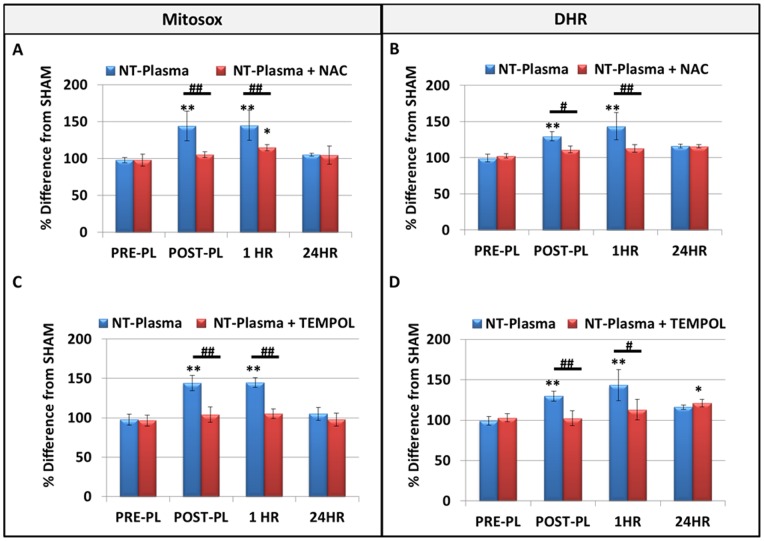
To evaluate intracellular reactive species levels in response to NT-plasma at 1000 Hz, 10 sec, mitochondrial superoxide anion (O2 −.) and cytosolic/mitochondrial H2O2 levels were measured using fluorescent indicators MitoSox and dihydrorhodamine (DHR), respectively. Measurements were done immediately post-treatment (POST-PL), at 1 hr and 24 hr after NT-plasma and results were compared to sham controls (pre-plasma, PRE-PL). Immediately post-treatment and at 1 hr, NT-plasma treated cells showed significantly increased intracellular O2 −. (Fig. 2A) and H2O2 generation (p<0.001) (Fig. 2B) as compared to PRE-PL. Levels of both oxidants returned to PRE-PL levels by 24 hr. The immediate increase in H2O2 may be due to ROS produced by NT-plasma but the extended detection indicates ROS are being actively produced in response to NT-plasma.
Figure 2. Direct effects of NT-plasma on intracellular ROS production.
(A and B) Intracellular O2 −. and H2O2 levels were measured using fluorescent indicators MitoSox (n = 3) and dihydrorhodamine (DHR), (n = 3) respectively. Immediately post-treatment (POST-PL) and at 1 hr, 1000 Hz NT-plasma treated cells generated significantly increased amounts of O2 −. and H2O2 (p<0.001) as compared to sham control or pre-treatment levels (PRE-PL). Amounts of both ROS were decreased by 24 hr. NAC and TEMPOL quenched the ROS increase POST-PL and at 1 hr (p<0.01–0.001). However, H2O2 levels in the TEMPOL (p<0.01) inhibitor group were significantly increased above control at 24 hr, presumably due to removal of baseline NO. The results are expressed as the mean ± standard deviation. Statistical significance was determined by the Mann-Whitney U test for non-parametric data; * or # (p<0.01) ** or ## (p<0.001.
To confirm the production of ROS in response to NT-plasma treatment, MLO-A5 cells were pretreated for 1 hr with either NAC (200 µM), to maintain intracellular redox balance and interfere with ROS production [38] or TEMPOL (6 mM), a scavenger of ROS as well as RNS. After pre-incubation with the inhibitor, the cells were subjected to NT-plasma treatment at 1000 Hz for 10 sec. NAC significantly inhibited the POST-PL and 1 hr NT-Plasma increase in both O2 −. (Fig. 2A) and H2O2 (Fig. 2B). TEMPOL also significantly inhibited the POST-PL and 1 hr increase in O2 −. (Fig. 2C), and it inhibited the POST-PL increase in H2O2 (Fig. 2D). To lesser extent TEMPOL inhibited the 1 hr increase in H2O2 in response to NT-Plasma, and at 24 hr H2O2 production was increased (p<0.01). One possible explanation for the decreased effectiveness of TEMPOL after 1 hr and 24 hr after NT-plasma/TEMPOL treatment is that removal of NO. prevents its reaction with O2 −. and the production of peroxynitrite (ONOO-). Thus, by scavenging NO. TEMPOL allows H2O2 to be generated from O2 −., rather than ONOO-, indicating some NO. is being generated although at significantly lower amounts compared to H2O2.
NT-plasma Differentiation of Osteoblasts and Chondrocytes in vitro
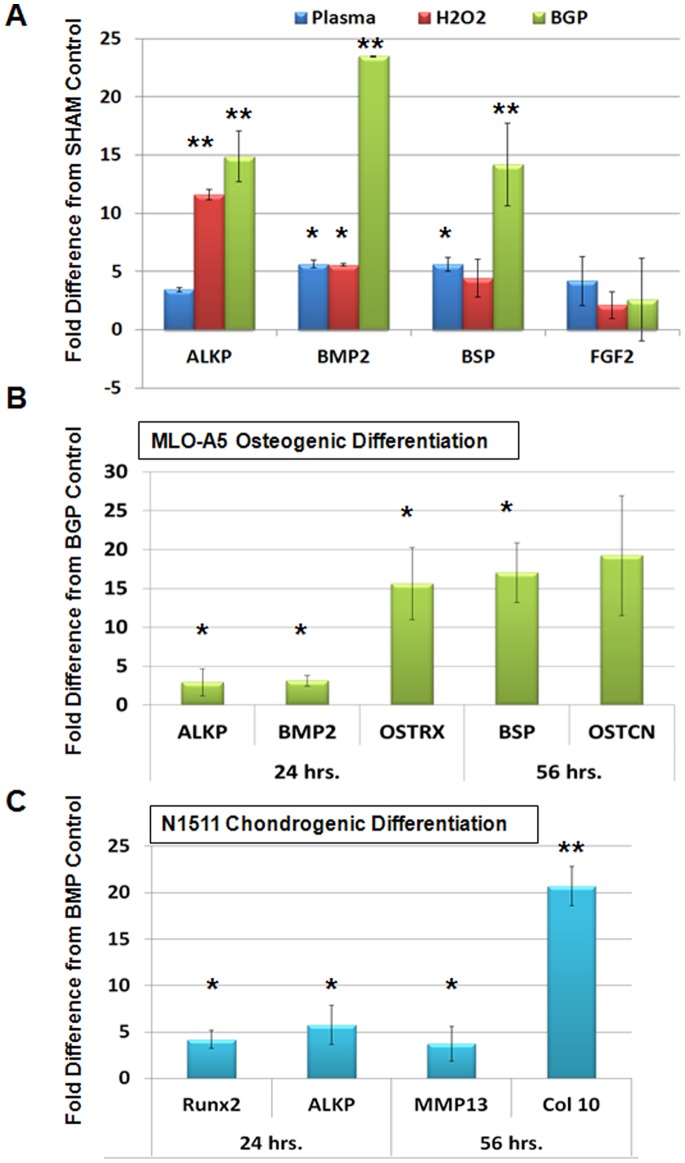
To determine if NT-plasma treatment influenced the differentiation of MLO-A5 cells, alkaline phosphatase (ALKP), bone morphogenetic protein 2 (BMP2), bone sialoprotein (BSP) and fibroblast growth factor-2 (FGF-2) expression was evaluated by qPCR (Fig. 3). We compared differentiation media containing β-glycerophosphate (βGP) to either NT-plasma or H2O2 treatment; used to evaluate the effects of redox. Both NT-plasma and H2O2 increased the expression of osteogenic genes above controls, which were undifferentiated cells sham treated with NT-plasma (Fig. 3A). ALKP expression was upregulated 3 (ns) and 11 (p<0.01) fold, respectively, and BMP2 and BSP were increased 5 fold by 24 hr (p<0.05). However, compared to the 14–23 fold increases induced by βGP alone (p<0.01), these changes were relatively small. FGF-2 expression, which is increased in endothelial cells in response to NT-plasma [5], was not affected in MLO-A5 cells by NT-plasma, H2O2 or βGP. These results indicate NT-plasma does not significantly promote osteoblast differentiation when compared to βGP, although it induces some differentiation specific protein expression.
Figure 3. NT-plasma induced osteogenic differentiation using qPCR markers.
(A) Fold increases in the expression of alkaline phosphatase (ALKP), bone morphogenetic protein 2 (BMP2), bone sialoprotein (BSP) and fibroblast growth factor-2 (FGF-2) in response to NT-plasma treatment, H2O2 or β-glycerol phosphate (βGP) normalized to sham treated control cells after 24 hr. ALKP was upregulated 3- (NS) and 11-fold (p<0.01), respectively, and BMP2 and BSP were increased 5 fold (p<0.05). βGP increased expression 14–23 fold (p<0.01). FGF-2 expression was not affected by NT-plasma, H2O2 or βGP. (B) After a 24 hr incubation in βGP NT-plasma was applied. 24 hr later there was a 2–7 fold increase in the induction of BMP2, ALKP (p<0.05) and Osterix was increased 15 fold (p<0.01) compared to βGP treatment alone. At 56 hr, BSP and osteocalcin (OSTCN) were increased 17–24 fold above βGP-treated control (p<0.05). The results are expressed as the mean ± standard deviation (n = 2). Statistical significance was determined by the Wilcoxin Mann-Whitney test for non-parametric data; * (p<0.05), ** (p<0.01).
As NT-plasma treatment evoked only a weak induction of osteogenic gene expression, a potential synergism between βGP and NT-plasma was investigated. NT-plasma treatment applied 24 hr after incubation in βGP, induced a 2–7 fold increase in the expression levels of osteogenic markers BMP2 and ALKP above βGP alone (p<0.05) (Fig. 3B). The expression of osterix (ostrx), a key transcription factor for osteogenesis, was increased 15 fold (p<0.01). At 56 hr after NT-plasma treatment, the late osteoblast markers, BSP and osteocalcin (OSTCN) were increased 17–24 fold above βGP-treated control (p<0.05).
To determine if the differentiation effect of NT-plasma was osteoblast specific, the N1511 chondrocyte cell line was subjected to the same NT-plasma treatment in the presence of BMP2 (200 ng/ml), a known inducer of chondrocyte differentiation [34]. 24 hr after treatment, chondrocyte differentiation markers Runx2, ALKP were increased 3–6-fold above BMP-treated controls (p<0.05) (Fig. 3C). By 56 hr, collagen type X (Col X) and another late marker, matrix metalloprotease 13 (MMP13) were both increased 20 (p<0.01) and 4-fold (p<0.05), respectively above BMP-treated control.
We conclude from these studies that NT-plasma alone was not sufficient to initiate significant changes in cell differentiation. However, once differentiation had been initiated, NT-plasma enhanced osteogenesis and chondrogenesis at both early and late time points.
Discussion
This study investigated the potential of NT-plasma to enhance both chondrocyte and osteoblast differentiation. Initially, we determined the specific NT-plasma condition required to maintain cell viability and showed both an immediate and long-term increase in intracellular ROS and RNS in response to NT-plasma. Furthermore, we demonstrate that NT-plasma enhanced the expression of differentiation specific genes by MLO-A5 in media without differentiation factors and in differentiation media, In media alone, the levels of expression were equal to those induced by H2O2, but not as effective as differentiation medium. However, when NT-plasma was applied 24 hr after the cells were placed in differentiation media a synergistic enhancement of chondrogenic and osteogenic differentiation was observed.
The NT-plasma generated ROS and RNS at the cell/environment interface initiates an immediate intracellular oxidative response (Fig. 2). It is interesting to speculate that this reaction may mimic naturally occurring ROS-associated cell signaling known to be associated with stem cell differentiation, cell fate decisions and regeneration. Within the stem cell pool, quiescence and pluripotency is maintained by the repression of ROS generation [39], [40]. As such, mouse and human embryonic stem cells have immature mitochondria, reduced expression of OXPHOS enzymes, low metabolic activity, low oxygen consumption, decreased levels of ATP production [41], express modest levels of antioxidant enzymes [40] and have a high glycolytic flux [41]. In vivo, embryonic stem cells are maintained in hypoxic conditions (2 to 3%) before vascularization [42] and in vitro, low oxygen culture conditions promote maintenance of pluripotency [5]. Rapid changes in ROS production and oxidative status associated with vascularization, growth factor binding, or increased mitochondrial biogenesis and activity, all promote cellular differentiation and determine cell fate [30].
Our findings highlight the capability of NT-plasma to promote osteo and chondrogenesis. The mechanism(s) by which both proliferation and differentiation occur is dependent on both immediate and long-term signaling molecules. In the short term, O2 −. and H2O2 are produced during the 10 sec NT-plasma exposure, however their sustained increases at 1 and 24 hr after treatment is due to intracellular ROS production. The most likely sources of the increased intracellular ROS are NADPH oxidase and mitochondrial production of O2 −., H2O2 and NO. [42], [43]. All three are signaling factors known to participate in cellular proliferation and differentiation by directly activating ROS or RNS-responsive proteins or indirectly by altering the redox status of the cell [44]–[57]. Specifically, MAP kinases are known to respond rapidly to ROS stimulation and these kinases control a wide range of cellular processes including: cellular differentiation, cell cycle control, cytokine and growth factor signaling, survival, hypertrophy and/or apoptosis [14]–[17].
ROS also promote downstream cellular responses by affecting the activity and expression of specific transcription factors [24], [58], [59]. In osteoblast progenitors, continuous production of low levels of H2O2 stimulates proliferation and augments their potential to differentiate into mature osteoblasts through up-regulation of Runx2 and osterix. [22]. In turn, these transcription factors promote ALKP and osteocalcin expression in differentiating osteoblasts. Similarly, in the current study, NT-plasma induced ROS production increased the expression of Runx2, osterix, ALKP and osteocalcin in pre-osteoblastic MLO-A5 cells.
Conflicting findings have been reported concerning the role of ROS in cell differentiation, but these discrepancies may be due to differing methodologies used for the generation of ROS and the delicate balance required for the appropriate response [57]. In a recent study comparing glucose oxidase continuous production of low levels of H2O2 to a single bolus of chemical H2O2, only glucose oxidase generated H2O2 promoted osteoblast differentiation [22]. This finding is not unexpected as the glucose oxidase system mimics cellular levels of H2O2 production. In the same way, a 10 sec exposure to NT-plasma initiates low-level intracellular production of ROS. This emphasizes that both timing and ROS concentration are critical in directing cell function. In the same way, chondrocyte differentiation in response to NT-plasma may be directly linked to ROS induced expression of SOX-9 and Runx2, which control collagen and aggrecan expression [60]. In a related study, laser irradiation induced intracellular ROS production and enhanced SOX-9 expression leading to chondrocyte differentiation and expression of collagen and aggrecan [61]. The roles of ROS in progenitor cell proliferation and differentiation described above may also be directly applicable to more complex tissue systems such as regenerating limbs or limb development. While very little has been reported on the direct contribution of ROS in the developmental process, it is well established that a majority of the required signaling molecules (BMPs, WNTs and FGFs) either directly signal through ROS or are indirectly controlled by ROS [62]–[64].
Current studies investigating NT-plasma report multiple effects can be induced in living cells and tissues [5]: these include increased cell proliferation [3], enhanced cell transfection [65]–[67], selective killing of cancer cells [68] and improved wound healing [69], [70]. The multiple effects can be attributed to the in vitro conditions selected during cellular treatment with NT-plasma. Results of the current study highlight the use of NT-plasma as a tool to initiate a non-lethal oxidative cellular burst that promotes osteoblast differentiation. Furthermore, these findings suggest that ROS and RNS produced in response to NT-plasma influence signaling pathways that are responsible for cellular proliferation and differentiation. Thus, it would not be unreasonable to assume that NT-plasma could be used to promote musculoskeletal cell differentiation and tissue regeneration.
Acknowledgments
Special thanks go to Dr. Irving Shapiro for his valuable discussions and his edits to the manuscript. Special thanks to Carol Diallo and the Drexel Plasma Institute.
Funding Statement
This work was supported by NIH Grants 1 R01 EB 013011 - 01 (Freeman and Fridman) and 5R03 DE020840-03 (Freeman). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Glover JL, Bendick PJ, Link WJ, Plunkett RJ (1982) The plasma scalpel: a new thermal knife. Lasers Surg Med 2: 101–106. [DOI] [PubMed] [Google Scholar]
- 2.Arjunan KP, Friedman G, Fridman A, Clyne AM (2011) Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J R Soc Interface. [DOI] [PMC free article] [PubMed]
- 3.Dobrynin D, Fridman G, Friedman G, Fridman A (2009) Physical and biological mechanisms of direct plasma interaction with living tissue. New Journal of Physics: 115020.
- 4. Fridman G, Peddinghaus M, Ayan H, Fridman A, Balasubramanian M, et al. (2006) Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chemistry and Plasma Processing 26: 425–442. [Google Scholar]
- 5. Kalghatgi S, Friedman G, Fridman A, Morss Clyne A (2010) Endothelial Cell Proliferation is Enhanced by Low Dose Non-Thermal Plasma Through Fibroblast Growth Factor-2 Release. Annals of Biomedical Engineering 38: 748–757. [DOI] [PubMed] [Google Scholar]
- 6. Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, et al. (2011) Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 6: e16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vargo JJ (2004) Clinical applications of the argon plasma coagulator. Gastrointest Endosc 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 8. Fridman A, Chirokov A, Gutsol A (2005) Non-thermal atmospheric pressure discharges. Journal of Physics D: Applied Physics 38: R1–R24. [Google Scholar]
- 9. Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, et al. (2008) Applied Plasma Medicine. Plasma Processes and Polymers 5: 503–533. [Google Scholar]
- 10.Fridman A, Kennedy LA (2004) Plasma Physics and Engineering. Routledge, USA 853.
- 11. Weng C-C, Wu Y-T, Liao J-D, Kao C-Y, Chao C-C, et al. (2009) Inactivation of bacteria by a mixed argon and oxygen micro-plasma as a function of exposure time. International Journal of Radiation Biology 85: 362–368. [DOI] [PubMed] [Google Scholar]
- 12. Fridman G, Brooks AD, Balasubramanian M, Fridman A, Gutsol A (2007) Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Processes and Polymers 4: 370–375. [Google Scholar]
- 13. Laroussi M (2005) Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Processes and Polymers 2: 391–400. [Google Scholar]
- 14. Sayama K, Hanakawa Y, Shirakata Y, Yamasaki K, Sawada Y, et al. (2001) Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. Journal of Biological Chemistry 276: 999–1004. [DOI] [PubMed] [Google Scholar]
- 15. Liu H, Nishitoh H, Ichijo H, Kyriakis JM (2000) Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol 20: 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeda K, Noguchi T, Naguro I, Ichijo H (2008) Apoptosis signal-regulating kinase 1 in stress and immune response. Annu Rev Pharmacol Toxicol 48: 199–225. [DOI] [PubMed] [Google Scholar]
- 17. Kashiwase K, Higuchi Y, Hirotani S, Yamaguchi O, Hikoso S, et al. (2005) CaMKII activates ASK1 and NF-kappaB to induce cardiomyocyte hypertrophy. Biochemical & Biophysical Research Communications 327: 136–142. [DOI] [PubMed] [Google Scholar]
- 18. Kim AH, Khursigara G, Sun X, Franke TF, Chao MV (2001) Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 21: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, et al. (2001) Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J 20: 6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsieh CC, Papaconstantinou J (2006) Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J 20: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang L, Chen J, Fu H (1999) Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proceedings of the National Academy of Sciences of the United States of America 96: 8511–8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe Y, Yu JY, Son YO, Park SM, Kim JG, et al.. (2011) Continuously generated H(2) O(2) stimulates the proliferation and osteoblastic differentiation of human periodontal ligament fibroblasts. J Cell Biochem Dec 15. doi: 10.1002/jcb.24017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 23. Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, et al. (2011) Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. Journal of Biological Chemistry 286: 4809–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuzawa A, Ichijo H (2008) Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochimica et Biophysica Acta 1780: 1325–1336. [DOI] [PubMed] [Google Scholar]
- 25. Stanton LA, Sabari S, Sampaio AV, Underhill TM, Beier F (2004) p38 MAP kinase signalling is required for hypertrophic chondrocyte differentiation. Biochemical Journal 378: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Zhao Z, Liu J, Huang N, Long D, et al. (2010) MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif 43: 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stanton LA, Underhill TM, Beier F (2003) MAP kinases in chondrocyte differentiation. Dev Biol 263: 165–175. [DOI] [PubMed] [Google Scholar]
- 28. Faigle R, Brederlau A, Elmi M, Arvidsson Y, Hamazaki TS, et al. (2004) ASK1 inhibits astroglial development via p38 mitogen-activated protein kinase and promotes neuronal differentiation in adult hippocampus-derived progenitor cells. Mol Cell Biol 24: 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran P, Ho SM, Kim BG, Vuong TA, Leem YE, et al.. (2012) TGF-beta-activated kinase 1 (TAK1) and apoptosis signal-regulating kinase 1 (ASK1) interact with the promyogenic receptor Cdo to promote myogenic differentiation via activation of p38MAPK pathway. Journal of Biological Chemistry Feb 18. doi: 10.1074/jbc.M112.351601 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 30. Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, et al. (2008) TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 205: 2397–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen C-T, Shih Y-RV, Kuo TK, Lee OK, Wei Y-H (2008) Coordinated Changes of Mitochondrial Biogenesis and Antioxidant Enzymes During Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 26: 960–968. [DOI] [PubMed] [Google Scholar]
- 32. Ji A-R, Ku S-Y, Cho MS, Kim YY, Kim YJ, et al. (2010) Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp Mol Med 42: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosser J, Bonewald LF (2012) Studying Osteocyte Function Using the Cell Lines MLO-Y4 and MLO-A5. Methods Mol Biol 816: 67–81. [DOI] [PubMed] [Google Scholar]
- 34. Kamiya N, Jikko A, Kimata K, Damsky C, Shimizu K, et al. (2002) Establishment of a novel chondrocytic cell line N1511 derived from p53-null mice. J Bone Miner Res 17: 1832–1842. [DOI] [PubMed] [Google Scholar]
- 35. Terkhorn SP, Bohensky J, Shapiro IM, Koyama E, Srinivas V (2007) Expression of HIF prolylhydroxylase isozymes in growth plate chondrocytes: Relationship between maturation andapoptotic sensitivity. J Cell Physiol 210: 257–265. [DOI] [PubMed] [Google Scholar]
- 36. Castagna R, Davis PA, Vasu VT, Soucek K, Cross CE, et al. (2009) Nitroxide radical TEMPO reduces ozone-induced chemokine IL-8 production in lung epithelial cells. Toxicology in Vitro 23: 365–370. [DOI] [PubMed] [Google Scholar]
- 37. Suy S, Mitchell JB, Ehleiter D, Haimovitz-Friedman A, Kasid U (1998) Nitroxides tempol and tempo induce divergent signal transduction pathways in MDA-MB 231 breast cancer cells. Journal of Biological Chemistry 273: 17871–17878. [DOI] [PubMed] [Google Scholar]
- 38. Parasassi T, Brunelli R, Costa G, De Spirito M, Krasnowska EK, et al. (2010) Thiol Redox Transitions in Cell Signaling: a Lesson From N-Acetylcysteine. The Scientific World Journal 10: 1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. zur Nieden NI, Cormier JT, Rancourt DE, Kallos MS (2007) Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J Biotechnol 129: 421–432. [DOI] [PubMed] [Google Scholar]
- 40. Akterin S, Cowburn RF, Miranda-Vizuete A, Jimenez A, Bogdanovic N, et al. (2006) Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ 13: 1454–1465. [DOI] [PubMed] [Google Scholar]
- 41. Boota A, Zar H, Kim YM, Johnson B, Pitt B, et al. (1996) IL-1 beta stimulates superoxide and delayed peroxynitrite production by pulmonary vascular smooth muscle cells. American Journal of Physiology 271: L932–938. [DOI] [PubMed] [Google Scholar]
- 42. Geiszt M, Leto TL (2004) The Nox family of NAD(P)H oxidases: host defense and beyond. Journal of Biological Chemistry 279: 51715–51718. [DOI] [PubMed] [Google Scholar]
- 43. Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochemical Journal 417: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hancock JT (1997) Superoxide, hydrogen peroxide and nitric oxide as signalling molecules: their production and role in disease. British Journal of Biomedical Science 54: 38–46. [PubMed] [Google Scholar]
- 46. Stone JR, Yang S (2006) Hydrogen peroxide: a signaling messenger. Antioxidants & Redox Signaling 8: 243–270. [DOI] [PubMed] [Google Scholar]
- 47. Winterbourn CC (2008) Reconciling the chemistry and biology of reactive oxygen species. Nature Chemical Biology 4: 278–286. [DOI] [PubMed] [Google Scholar]
- 48. Mungrue IN, Bredt DS, Stewart DJ, Husain M (2003) From molecules to mammals: what’s NOS got to do with it? Acta Physiologica Scandinavica 179: 123–135. [DOI] [PubMed] [Google Scholar]
- 49. Gow AJ, Ischiropoulos H (2001) Nitric Oxide Chemistry and Cellular Signaling. Journal of Cellular Physiology 187: 277–282. [DOI] [PubMed] [Google Scholar]
- 50. Burdon RH (1995) Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biology & Medicine 18: 775–794. [DOI] [PubMed] [Google Scholar]
- 51. Linnane AW, Kios M, Vitetta L (2007) Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology 8: 445–467. [DOI] [PubMed] [Google Scholar]
- 52. Nicco C, Laurent A, Chereau C, Weill B, Batteux F (2005) Differential modulation of normal and tumor cell proliferation by reactive oxygen species. Biomedicine & Pharmacotherapy 59: 169–174. [DOI] [PubMed] [Google Scholar]
- 53. Pervaiz S, Clement M-V (2007) Superoxide anion: oncogenic reactive oxygen species? International Journal of Biochemistry & Cell Biology 39: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 54. Rigoulet M, Yoboue ED, Devin A (2011) Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxidants & Redox Signaling 14: 459–468. [DOI] [PubMed] [Google Scholar]
- 55. Lin IC, Smartt JM, Nah H-D, Ischiropoulos H, Kirschner RE (2008) Nitric Oxide Stimulates Proliferation and Differentiation of Fetal Calvarial Osteoblasts and Dural Cells. Plast Reconstr Surg 121: 1554–1566. [DOI] [PubMed] [Google Scholar]
- 56. Teixeira CC, Ischiropoulos H, Leboy PS, Adams SL, Shapiro IM (2005) Nitric oxide–nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone 37: 37–45. [DOI] [PubMed] [Google Scholar]
- 57. Steinbeck MJ, Kim JK, Trudeau MJ, Hauschka PV, Karnovsky MJ (1998) Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells. Journal of Cellular Physiology 176: 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hou Y, Xue P, Bai Y, Liu D, Woods CG, et al. (2012) Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein beta during adipogenesis. Free Radical Biology & Medicine 52: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Motohashi H, Kimura M, Fujita R, Inoue A, Pan X, et al. (2010) NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood 115: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL, et al.. (2011) Interactions between extracellular signal-regulated kinase 1/2 and P38 map kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J Bone Miner Res Nov 9. doi: 10.1002/jbmr.561. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 61. Kushibiki T, Tajiri T, Ninomiya Y, Awazu K (2010) Chondrogenic mRNA expression in prechondrogenic cells after blue laser irradiation. J Photochem Photobiol B 98: 211–215. [DOI] [PubMed] [Google Scholar]
- 62. Juarez JC, Manuia M, Burnett ME, Betancourt O, Boivin B, et al. (2008) Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proceedings of the National Academy of Sciences of the United States of America 105: 7147–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mandal CC, Ganapathy S, Gorin Y, Mahadev K, Block K, et al. (2011) Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochemical Journal 433: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, et al. (2007) Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci 1117: 40–50. [DOI] [PubMed] [Google Scholar]
- 65. Ahmad R, Rasheed Z, Ahsan H (2009) Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacology & Immunotoxicology 31: 388–396. [DOI] [PubMed] [Google Scholar]
- 66. Coulombe S, Léveillé V, Yonson S, Leask RL (2006) Miniature atmospheric pressure glow discharge torch (APGD-t) for local biomedical applications. Pure and Applied Chemistry 78: 1147–1156. [Google Scholar]
- 67. Leduc M, Guay D, Leask RL, Coulombe S (2009) Cell permeabilization using a non-thermal plasma Focus on Plasma Medicine. New Journal of Physics 11: 115021. [Google Scholar]
- 68. Vandamme M, Robert E, Pesnel S, Barbosa E, Dozias S, et al. (2010) Antitumor Effect of Plasma Treatment on U87 Glioma Xenografts: Preliminary Results. Plasma Processes and Polymers 7: 264–273. [Google Scholar]
- 69. Shekhter AB, Serezhenkov VA, Rudenko TG, Pekshev AV (2005) Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide: Biology and Chemistry 12: 210–219. [DOI] [PubMed] [Google Scholar]
- 70. Wu AS, Kalghatgi S, Dobrynin D, Sensenig R, Cerchar E, et al. (2013) Porcine intact and wounded skin responses to atmospheric nonthermal plasma. J Surg Res 179: e1–e12. [DOI] [PubMed] [Google Scholar]