Abstract
Alpine steppe is considered to be the largest grassland type on the Tibetan Plateau. This grassland contributes to the global carbon cycle and is sensitive to climate changes. The allocation of biomass in an ecosystem affects plant growth and the overall functioning of the ecosystem. However, the mechanism by which plant biomass is allocated on the alpine steppe remains unclear. In this study, biomass allocation and its relationship to environmental factors on the alpine grassland were studied by a meta-analysis of 32 field sites across the alpine steppe of the northern Tibetan Plateau. We found that there is less above-ground biomass (MA) and below-ground biomass (MB) in the alpine steppe than there is in alpine meadows and temperate grasslands. By contrast, the root-to-shoot ratio (R:S) in the alpine steppe is higher than it is in alpine meadows and temperate grasslands. Although temperature maintained the biomass in the alpine steppe, precipitation was found to considerably influence MA, MB, and R:S, as shown by ordination space partitioning. After standardized major axis (SMA) analysis, we found that allocation of biomass on the alpine steppe is supported by the allometric biomass partitioning hypothesis rather than the isometric allocation hypothesis. Based on these results, we believe that MA and MB will decrease as a result of the increased aridity expected to occur in the future, which will reduce the landscape’s capacity for carbon storage.
Introduction
Biomass allocation was an important character for the process of characterization of plant physiological ecology [1], moreover, it also was the result of the plant long-term adapted to different environmental conditions [2].The Biomass allocation also reflect show photosynthates are allocated between above-ground and below-ground biomass [3]. Biomass allocation above-ground and below-ground affects plant growth as well as the overall function of the ecosystem and biogeochemical cycles [4], [5]. Therefore, the mechanism by which plants respond to variations in the availability of resources in their environment is a major question in plant ecology [6]. Two important hypotheses regarding biomass allocation of plants have been proposed: (i) optimal partitioning and (ii) isometric allocation [2], [7], [8]. The optimal partitioning hypothesis suggests that plants respond to variations in the environment by partitioning biomass among various plant organs to maximize the plants’ growth rate [9], [10]. For example, plants in arid regions are rooted deeper than those in humid environments [11], [12]. On the contrary, the isometric allocation hypothesis predicts the net primary productivity of the roots vs the net primary productivity of the shoots (BNPP:ANPP) isometrically without considering the differences in plant species or community types [13]–[15]. Thus far, biomass allocation has been widely examined: investigations have focused on individual organisms as well as whole ecosystems. However, no conclusion about biomass allocation has yet been presented.
Optimal partitioning theory might explain the effect of environmental factors on the allocation of plants’ photosynthetic products, but this theory does not consider the size of the individual plants [8], [16]. The allometric biomass partitioning theory, on the other hand, may resolve biomass allocation patterns in terms of plant size by using standardized major axis (SMA) regression [8], [17]. However, this theory does not provide quantitative descriptions about how environmental factors affect biomass allocation. It also cannot explain the mechanism behind how photosynthates are allocated to different organs [18]. Furthermore, it is still hotly debated whether a uniform biomass allocation pattern is applicable to different ecosystems [19].
The alpine steppe is the largest grassland type in the Tibetan Plateau, which contributes significantly to the global carbon cycle [1]. In the alpine grassland ecosystem, few soil nutrients, aridity, and low temperatures limit plant growth [20], [21]. According to the optimal partitioning hypothesis, environmental factors likely affect how plant biomass is allocated. At the individual plant level, fewer soil nutrients (particularly nitrogen and water) results in an increase in root biomass. On the contrary, root biomass decreases and shoot biomass increases as soil nutrients increase. This partition model is appropriate for different types of vegetation and life forms of plants [22]–[26]. Studies have shown that plants allocate more biomass to their roots when water and nutrients in grassland ecosystems are limited [27], [28]. Moreover, studies have also suggested that plants allocate photosynthates to root in low-temperature environments, which may increase the rate of nutrient absorption and help the plants adapt to environmental conditions [29]–[31]. However, Yang et al. (2009a) reported that on the Tibetan alpine grasslands, the relationship between roots and shoots supports the isometric allocation hypothesis [32]. They also found that this isometric relationship is independent of soil nitrogen and moisture [32]. These results indicate that the mechanism of biomass allocation in the alpine steppe is still misunderstood and unverified in alpine and arid environments. Therefore, this subject requires further investigation. In the present study, we investigated (i) the mechanism behind allocating root and shoot biomass in the Tibetan alpine grassland and (ii) the main factors that affect biomass allocation in the alpine steppe of northern Tibet.
Materials and Methods
Collecting Biomass and Soil Samples
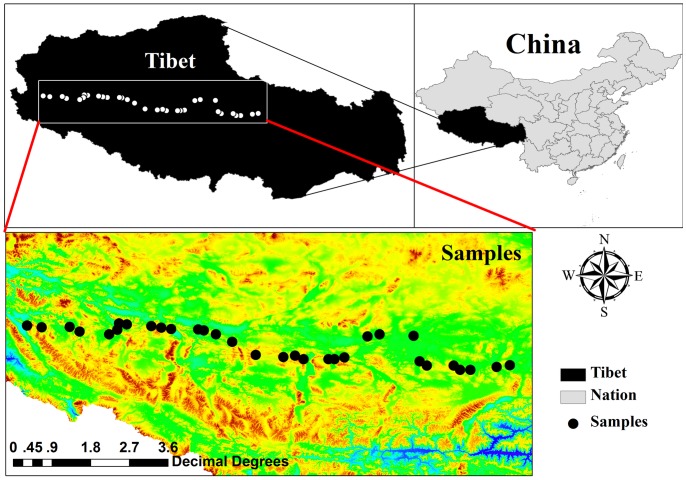
In August 2012, 32 sites were selected on Stipa purpurea alpine steppe from Nagqu County to Gar County in northern Tibet. Sampling sites were established at intervals of 30 km (Fig. 1, Table 1). In each site, no specific permits were required for collecting samples and the field studies did not involve endangered or protected species. We selected flat sites with well-protected vegetation. We harvested the aboveground biomass (MA) and the belowground biomass (MB) from three blocks of 0.5 m×0.5 m in each site. We collected MB from soil depths of 0 cm to 15 cm, where most of belowground biomass is located [33], [34].The root samples obtained from the blocks were immediately placed in a cloth bag and then soaked in water to remove the residual soil using a 0.5 mm sieve. Biomass was oven-dried at 65°C until a constant weight was reached, and then it was weighed to the nearest 0.01 g.
Figure 1. Spatial distribution of the sampling sites in S. Purpurea alpine steppe in northern Tibet.
Table 1. Site description of S. purpurea alpine steppe.
| Site | County | Dominant species | Mean annualprecipitation (MAP,mm) | Mean annual temperature(MAT,°C) |
| S1 | Nakchu | S. purpurea Kobre siahumilis | 428.1 | –1.5 |
| S2–S7 | Baingoin | S. purpurea Carex moorcroftii | 321.7 | –0.8 |
| S8 | Xainza | S. purpurea C.moorcroftii | 304.5 | –0.4 |
| S9–S17 | Nyima | S. purpurea C.m oorcroftii | 200 | –0.4 |
| S18–S24 | Gêrzê | S. purpurea | 170.1 | 0.10 |
| S25–S30 | Gêgyai | S. purpurea | 120 | 0.45 |
| S31–S32 | Gar | S. purpurea | 72.1 | 0.7 |
Soil samples were collected from two different depths (0–15 cm and 15–30 cm), air-dried, and sieved (2 mm mesh). The fine roots were extracted by hand picking for physical and chemical analyses. The total nitrogen content (TN; TN1∶0–15 cm, TN2∶15–30 cm) of the soil was determined using the micro-Kjeldahl digestion method. The available nitrogen content (AN; AN1∶0–15 cm, AN2∶15–30 cm) of the soil was determined using the alkaline hydrolysis diffusion method. All of the element concentrations were expressed as mg⋅g−1 on a dry weight basis.
Data Analysis
MA in grasslands can be considered as annual aboveground net primary productivity (ANPP). Blow-ground net primary productivity (BNPP) was calculated using Gill’s method:
| (1) |
where (live MB/MB) = 0.6 and turnover = 0.0009(g⋅m−2)×MA +0.25 [35], [36]. In the present study, the value for (live MB/MB) was 0.79, which was measured by Zhou (2001) in the Qinghai region [37]. The relationship between log ANPP and log BNPP was constructed using Model II regression [14], [15]. The slope (α) and y-intercept (log b) of the allocation function were determined by standardized major axis (SMA) tests [38]. The heterogeneity between slopes was determined by performing a permutation test and was rejected if P>0.05 [15]. We analyzed the correlations between environmental factors and the measured MA, MB, and root-to-shoot ratios (R:S) using the Pearson correlation. We also examined relationships between MA, MB, R:S, and environmental factors using regression and ordination space partitioning to find the main environmental factors that affected MA, MB, and R:S. Analyses were performed using SPSS software version 16.0 (IBM; Armonk, NY).
Results
Variations in the Chemical Properties of the Soil as well as MA, MB, and R:S
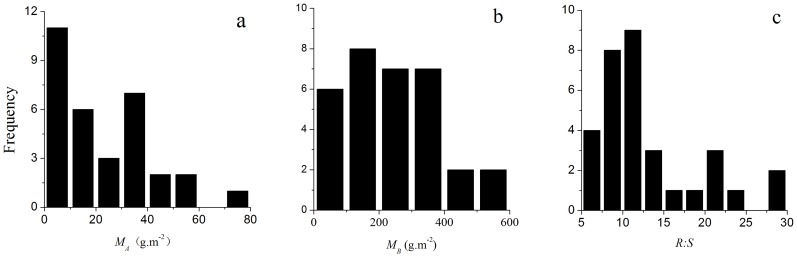
Small variations in the chemical properties of the soil along the sampled transect were found. There also was not significance in available nitrogen and total nitrogen between the two soil layers (Table 2). We found large variations in MA, MB, and R:S along the sampled transects (Fig. 2). MA ranged from 2.32 g⋅m−2 to 73.6 g⋅m− 2, while MB ranged from 22.40 g⋅m−2 to 587.32 g⋅m−2. R:S ranged from 6.19 to 29.15 (Table 3). The median values of MA, MB, and R:S in S. purpurea alpine steppe were 17.16 g⋅m−2, 233 g⋅m−2, and 11.83, respectively (Table 3).
Table 2. Chemical properties of soils in S. purpurea alpine steppe.
| Min | Max | Mean | Std. Error | Std. Deviation | |
| AN1 mg·g −1 | 0.013 | 0.110 | 0.057a | 0.004 | 0.025 |
| AN2 mg·g −1 | 0.008 | 0.095 | 0.051a | 0.004 | 0.023 |
| TN1 mg·g −1 | 0.386 | 1.630 | 1.008a | 0.061 | 0.342 |
| TN2 mg·g −1 | 0.292 | 1.921 | 0.980a | 0.064 | 0.362 |
Figure 2. Frequency distributions of (a) above-ground biomass (MA), (b) below-ground biomass (MB), and (c) root-to-shoot ratio (R:S) in S. purpurea alpine steppe.
Table 3. Descriptive statistics of above-ground biomass (MA), below-ground biomass (MB), and root-to-shoot (R:S) ratio in S. purpurea alpine steppe.
| MA (g·m−2) | MB (g·m−2) | R:S ratio | |||||||
| Min | Max | Median | Min | Max | Median | Min | Max | Median | |
| Present study | 2.32 | 73.6 | 17.16 | 22.4 | 587.32 | 233 | 6.19 | 29.15 | 11.83 |
| Yang et al. (2009) | 9.8 | 267.4 | 42.8 | 44.6 | 1934.8 | 206 | 0.8 | 13 | 5.2 |
Biomass Allocation for S.purpurea Alpine Steppe
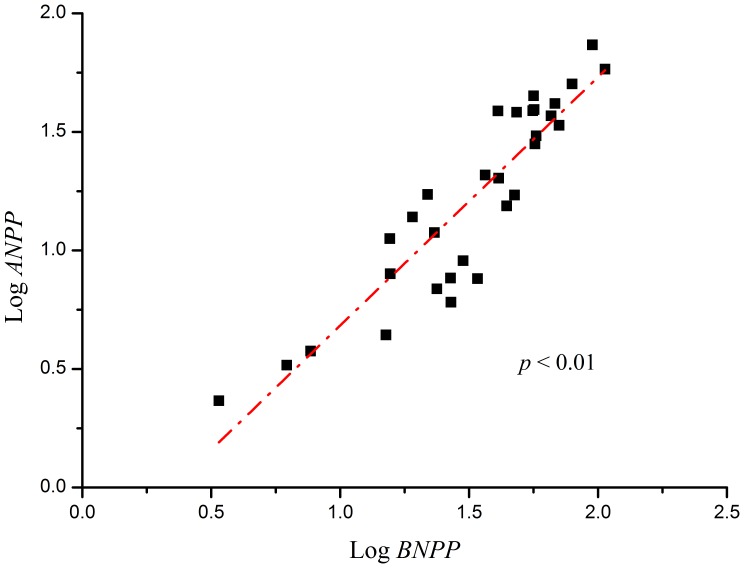
The slope (α) of the plotted relationship between log ANPP and log BNPP of S. purpurea alpine grasslands was 0.87 with 95% confidence intervals of 0.75 and 1.01 (Fig. 3). The slope (α) was significantly different from the slope obtained from SMA analysis when the isometric hypothesis was used.
Figure 3. Relationships between above-ground net primary production (ANPP) and below-ground net primary production (BNPP) in alpine steppe by SMA analysis.
Effects of Soil Nitrogen and Environmental Factors on Biomass and R:S
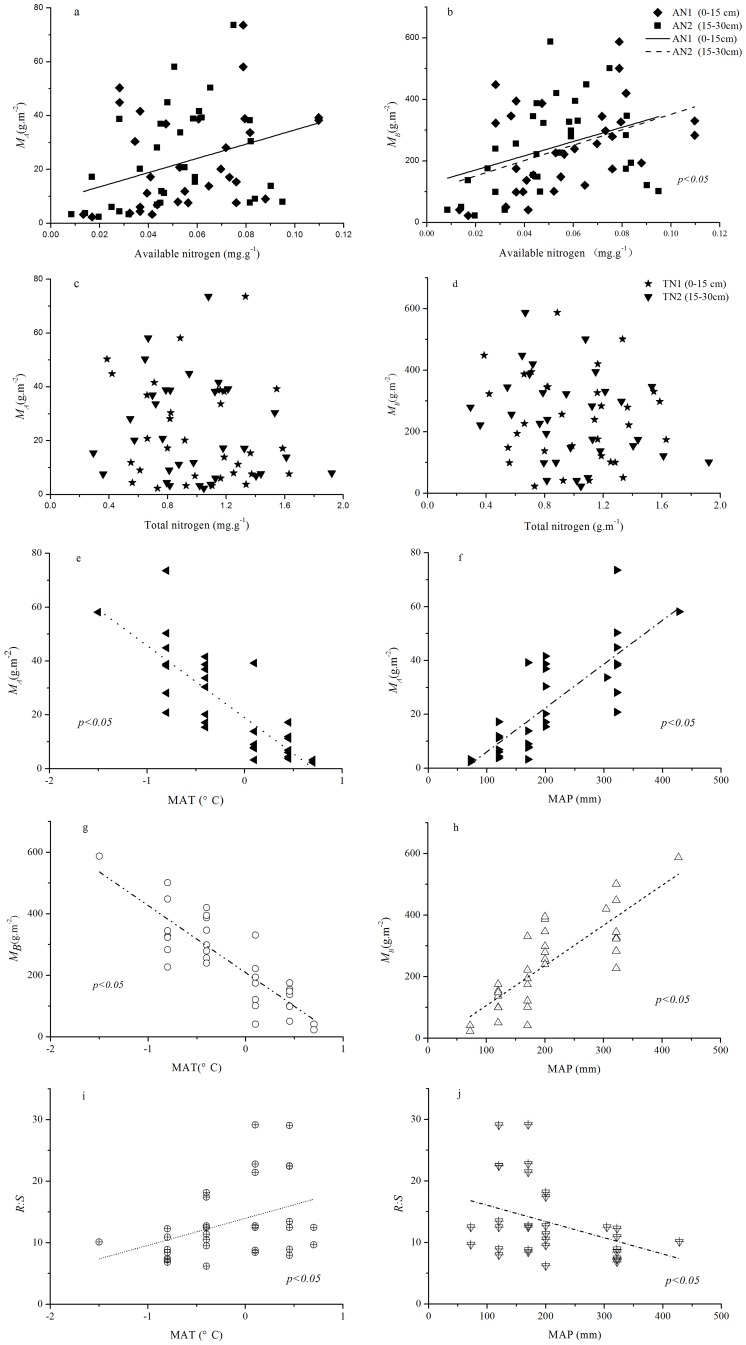
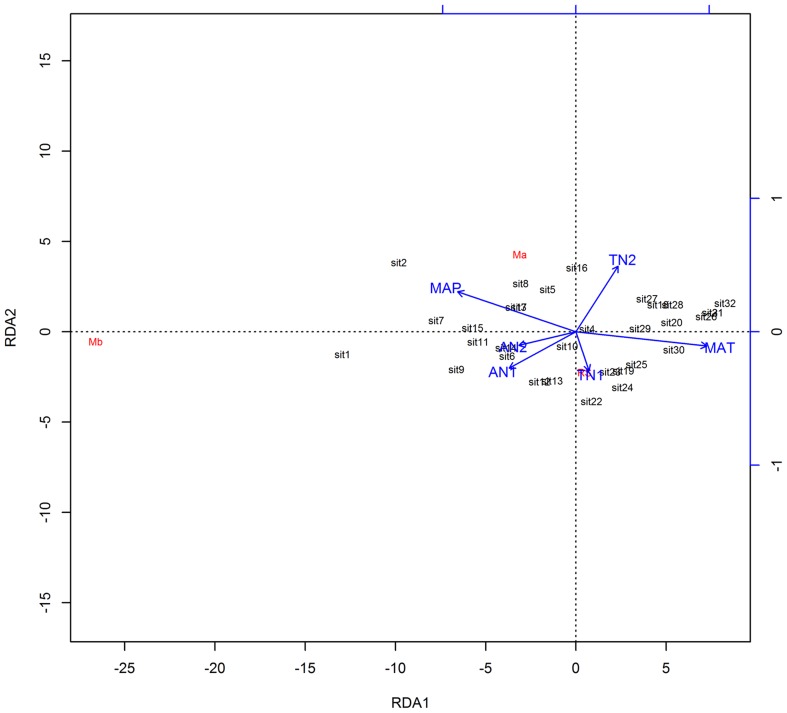
Using the Pearson correlation analysis, we found that MA and MB exhibited a significantly positive correlation with available nitrogen in the soil. However, MA and MB did not exhibit a significant correlation with total nitrogen (Table 4). The R:S ratio also did not exhibit a significant correlation with soil nitrogen (total or available). MA, MB, and R:S did correlate with the MAP of the sampling sites, while these correlations differed from the ones found with MAT (Table 3). In this study, we found that the regression analysis showed the same results as the Pearson correlation analysis (Fig. 4). Using the ordination space partitioning method, we found that MAP was the main factor that affected MA, MB, and R:S (Fig. 5).
Table 4. Pearson’s correlation between MA, MB, and R:S with the environmental factors.
| AN1(0 to 15 cm) | AN2(15 to 30 cm) | TN1(0 to 15 cm) | TN2(15 to 30 cm) | MAT(°C) | MAP(mm) | ||
| MA | 0.351* | 0.317 | –0.126 | –0.165 | –0.809** | 0.791** | |
| MB | 0.429* | 0.372* | –0.088 | –0.285 | –0.853** | 0.817** | |
| R:S | –0.047 | –0.083 | 0.207 | –0.082 | 0.392* | –0.378* | |
Correlation is significant at the 0.01 level (two-tailed).
Correlation is significant at the 0.05 level.
Figure 4. Relationships between biomass allocation (MA, MB, and R:S) and environmental factors in alpine steppe.
Regressions are shown: (a) MA versus available nitrogen, (b) MB versus available nitrogen,(c) MA versus total nitrogen, (d) MB versus total nitrogen,(e) MA versus MAT,(f) MA versus MAP,(g) MB versus MAT, (h) MB versus MAP, (i) R:S ratio versus MAT, and (j) R:S ratio versus MAP.
Figure 5. Analysis of the relationship of above-ground biomass (MA), below-ground biomass (MB), and root-to-shoot ratio (R:S) with the environmental factors by ordination space partitioning method.
Discussion
MA, MB, and R:S in the Alpine Steppe
In the present study, amounts of MA and MB in the alpine steppe (mean = 23.20 g⋅m–2) were found to be lower than those in the alpine meadows [32] and in the temperate grasslands of China [39]. By contrast, R:S in the alpine steppe was found to be higher than it is in China’s alpine meadows [32] and temperate grasslands [39] as well as in temperate grasslands of other regions [1]. These results show that precipitation and temperature affect plant growth and biomass allocation [1], [40]. Slower root turnover in colder environments might also results in higher R:S ratios [41]–[43]. MA, MB, and R:S values found in the present study are not consistent with results reported by Yang et al. (2009a), who performed a field investigation from 2001 to 2004 [32]. R:S values have the potential to vary greatly as a result of climate change and anthropogenic activities [44]–[48].
Mechanism of Biomass Allocation in the Alpine Steppe
Based on the results of our SMA analysis, we found that biomass allocation on the alpine steppe does not fit the isometric hypothesis. By contrast, Yang et al. (2009a) previously reported that biomass allocation on the alpine steppe is supported by the isometric allocation hypothesis [32]. In the harsh alpine ecosystem, scarce precipitation and low temperatures allow plants to allocate more biomass to the roots, which helps plants survive [29]–[31]. Moreover, roots have also been found to store carbohydrates in alpine grasslands [49], [50]. Therefore, biomass allocation in the alpine steppe may reflect the allometric biomass partitioning hypothesis rather than the isometric allocation hypothesis.
Relationships between Environmental Factors and MA, MB, and R:S
Precipitation and temperature are considered to be the limiting factors for the growth and distribution of vegetation over the long term [51], [52]. In the present study, MA, MB, and R:S were mainly affected by the environmental factor of precipitation (MAP), as revealed by ordination space partitioning analysis. These results are consistent with those of other reports about the alpine steppe [46], [53]–[56]. The low temperature in the growing season did not limit the growth of alpine plants because these plants shave evolved to survive in the cold alpine climate [57]. The amounts of aboveground and belowground biomass are higher in sites with higher humidity, but the MAT is also relatively low on the alpine steppe. Precipitation is an essential factor that controls the functions of ecosystems in terrestrial biomes, particularly in arid and semiarid ecosystems [58]. Therefore, precipitation is the main factor that influences amounts of biomass in the alpine steppe.
Moreover, in the present study, we found that amounts of MA and MB on the alpine steppe were affected by the available nitrogen content in the soil but not by the total nitrogen content of the soil. These results are inconsistent with those from previous studies, which have showed that MA and MB are positively related to total nitrogen content [32], [59]. Because available nitrogen can be used to approximate the relative supply of nutrients, nitrogen may be another factor that controls ecosystem processes in regions with abundant water resources [60].
Conclusion
As the climate changes, the degree of aridity has been consistently increasing in northern Tibet [61]. Changes in biomass allocation on the alpine steppe are likely to affect the carbon cycle and the general functioning of the alpine ecosystem. In the present study, we found that the R:S ratio in the alpine steppe was higher than that of other grassland systems. The amounts of aboveground and belowground biomass as well as the R:S ratio were primarily affected by precipitation. The observed biomass allocation was found to follow the allometric biomass partitioning theory rather than the isometric allocation hypothesis. These results suggest that the landscape’s capacity to store carbon will potentially decrease as the degree of aridity in northern Tibet increases.
Acknowledgments
This study was financially supported by the Strategic leading science and technology projects, CAS (XDB03030505), the National science and technology support project (2011BAC09B03), Program of the IMDE, CAS (SDS-135-1203-01), and the Science Foundation for Young Scientists of IMDE, CAS.
Funding Statement
This study was financially supported by the Strategic leading science and technology projects, CAS (XDB03030505), the National science and technology support project (2011BAC09B03), Program of the IMDE, CAS (SDS-135-1203-01), and the Science Foundation for Young Scientists of IMDE, CAS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Global Change Biol 11: 1–13. [Google Scholar]
- 2.Bazzaz FA (1997) Allocation of resources in plants: state of the sciences and critical questions. In: Bazzaz FA, Grace J, editors. Plant Resource Allocation. San Diego: Academic Press. 1–37.
- 3. Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16: 326–331. [Google Scholar]
- 4. Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. J Plant Nutr Soil Sci 163: 421–431. [Google Scholar]
- 5. Litton CM, Raich JW, Ryan MG (2007) Carbon allocation in forest ecosystems. Global Change Biol 13: 2089–2109. [Google Scholar]
- 6. Lacointe A (2000) Carbon allocation among tree organs: a review of basic processes and representation in functional-structural tree models. Ann Forest Sci 57: 521–533. [Google Scholar]
- 7. Müller I, Schmid B, Weiner J (2000) The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect Plant Ecol 3: 115–127. [Google Scholar]
- 8. McCarthy MC, Enquist BJ (2007) Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol 21: 713–720. [Google Scholar]
- 9. Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants an economic analogy. Ann Rev Ecol Syst 16: 363–392. [Google Scholar]
- 10. Chapin FS III, Bloom AJ, Field B, Waring RH (1987) Plant responses to multiple environmental factors. BioScience 37: 49–57. [Google Scholar]
- 11. Scheck HJ, Jackson RB (2005) Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 126(1–2): 129–140. [Google Scholar]
- 12. Mony C, Koschnick TJ, Haller WT, Muller S (2007) Competition between two invasive Hydrocharitaceae (Hydrilla verticillata (L. f.) (Royle) and Egeria densa (Planch)) as influenced by sediment fertility and season. Aquat Bot 86: 236–242. [Google Scholar]
- 13. Enquist BJ, Niklas KJ (2002) Global allocation rules for patterns of biomass partitioning in seed plants. Science 295: 1517–1520. [DOI] [PubMed] [Google Scholar]
- 14. Niklas KJ (2005) Modelling below- and aboveground biomass for non-woody and woody plants. Ann Bot 95: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng DL, Niklas KJ (2007) Above- and belowground biomass relationships across 1534 forested communities. Ann Bot 99: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcelis LFM, Heuvelink E (2007) Concepts of modelling carbon allocation among plant organs. In: Vos J, Marcelis LFM, Visser PHBd, Struik PC, Evers JB, editors. Functional structural plant modelling in crop production. Dordrecht: Springer. 103–111.
- 17. Solow AR (2005) Power laws without complexity. Ecol Lett 8: 361–363. [Google Scholar]
- 18. Génard M, Dauzat J, Franck N, Lescourret F, Moitrier N, et al. (2008) Carbon allocation in fruit trees: from theory to modelling. Trees 22: 269–282. [Google Scholar]
- 19. Han WX, Fang JY (2003) Allometry and its application in ecological scaling. Acta Sci Natur Univ Pekin 39: 583–593 (in Chinese with English abstract).. [Google Scholar]
- 20.Zhou XM (2001) Kobresia Meadow in China. Beijing: Science Press. 136–139 p. [Google Scholar]
- 21. Gugerli F, Bauert MR (2001) Growth and reproduction of Polygonum viviparum show weak responses to experimentally increased temperature at a Swiss alpine site. Bot Helv 111: 169–180. [Google Scholar]
- 22. Warembourg FR, Estelrich HD (2001) Plant phenology and soil fertility effects on below-ground carbon allocation for an annual (Bromus madritensis) and a perennial (Bromus erectus) grass species. Soil Biol Biochem 33(10): 1291–1303. [Google Scholar]
- 23. Cronin G, Lodge DM (2003) Effects of light and nutrient availability on the growth, allocation, carbon/nitrogen balance, phenolic chemistry, and resistance to herbivory of two freshwater macrophytes. Oecologia 137: 32–41. [DOI] [PubMed] [Google Scholar]
- 24. Glynn C, Herms DA, Egawa M, Hansen R, Mattson WJ (2003) Effects of nutrient availability on biomass allocation as well as constitutive and rapid induced herbivore resistance in poplar. Oikos 101: 385–397. [Google Scholar]
- 25. Vanninen P, Makela A (2005) Carbon budget for Scots pine trees: effects of size, competition and site fertility on growth allocation and production. Tree Physiol 25: 17–30. [DOI] [PubMed] [Google Scholar]
- 26. Grechi I, Vivin P, Hilbert G, Milin S, Robert T, et al. (2007) Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ Exp Bot 59: 139–149. [Google Scholar]
- 27. Dukes JS, Chiariello NR, Cleland EE, Moore LA, Shaw MR, et al. (2005) Responses of grassland production to single and multiple global environmental changes. PLoS Biol 3: 1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao YZ, Chen Q, Lin S, Giese M, Brueck H (2011) Resource manipulation effects on net primary production, biomass allocation and rain-use efficiency of two semiarid grassland sites in Inner Mongolia, China. Oecologia 65: 855–864. [DOI] [PubMed] [Google Scholar]
- 29. Körner CH, Renhardt U (1987) Dry matter partitioning and root length/leaf area ratios in herbaceous perennial plants with diverse altitudinal distribution. Oecologia 74: 411–418. [DOI] [PubMed] [Google Scholar]
- 30. Wang CT, Cao GM, Wang QL, Jing ZC, Ding LM, et al. (2008) Changes in plant biomass and species composition of alpine Kobresia meadows along altitudinal gradient on the Qinghai-Tibetan Plateau. Sci China Life Sci 51: 86–94. [DOI] [PubMed] [Google Scholar]
- 31. Fan JW, Wang K, Harris W, Zhong HP, Hu ZM, et al. (2009) Allocation of vegetation biomass across a climate-related gradient in the grassland of Inner Mongolia. J Arid Environ 73: 521–528. [Google Scholar]
- 32. Yang YH, Fang JY, Ji C, W Han (2009a) Above-and belowground biomass allocation in Tibetan grasslands. J Veg Sci 20: 177–184. [Google Scholar]
- 33. Yan Y, Zhang JG, Zhang JH, Fan JR, Li HX (2005) The belowground biomass in alpine grassland in Nakchu Prefecture of Tibet. Acta Ecol Sinica 11: 2818–2823 (in Chinese with English abstract).. [Google Scholar]
- 34. Li X, Zhang X, Wu J, Shen Z, Zhang Y, et al. (2011) Root biomass distribution in alpine ecosystems of the northern Tibetan Plateau. Environ Earth Sci 64(7): 1911–1919. [Google Scholar]
- 35. Gill R A, Kelly RH, Parton WJ, Day KA, Jackson RB, et al. (2002) Using simple environmental variables to estimate below2ground productivity in grasslands. Global Ecol Biogeogr 11: 79–86. [Google Scholar]
- 36. Bradford JB, Lauenroth WK, Burke I C (2005) The impact of cropping on primary production in the US Great Plains. Ecol 86: 1863–1872. [Google Scholar]
- 37.Zhou XM (2001) Chinese Kobresia Meadow. Beijing: Science Press. 158 p. [Google Scholar]
- 38.Falster DS, Warton DI, Wright IJ (2003) (S)MATR: standardized major axis tests and routines. Version 1.0. http://www.bio. mq.edu.au/ecology/SMATR.
- 39. Ma W, Yang Y, He J, Zeng H, Fang J (2008) Above-and belowground biomass in relation to environmental factors in temperate grasslands, Inner Mongolia. Sci China Life Sci 51(3): 263–270. [DOI] [PubMed] [Google Scholar]
- 40. Hui DF, Jackson RB (2005) Geographical and inter-annual variability in biomass partitioning in grassland ecosystems: a synthesis of field data. New Phytol 169: 85–93. [DOI] [PubMed] [Google Scholar]
- 41. Gill RA, Jackson RB (2001) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147: 13–31. [Google Scholar]
- 42. Gao YZ, Giese M, Lin S, Sattelmacher B, Zhao Y, et al. (2008) Belowground net primary productivity and biomass allocation of a grassland in Inner Mongolia is affected by grazing intensity. Plant Soil 307: 41–50. [Google Scholar]
- 43. Giese M, Gao YG, Zhao Y, Pan QM, Lin S, et al. (2009) Effects of grazing and rainfall variability on root and shoot decomposition in a semi-arid grassland. Appl Soil Ecol 41: 8–18. [Google Scholar]
- 44. Wang GX, Hu HC, Wang YB, Chen L (2007) Response of alpine cold ecosystem biomass to climate changes in permafrost regions of the Tibetan Plateau. J Glaciol Geocryol 29: 671–679 (in Chinese with English abstract).. [Google Scholar]
- 45. Yu H, Luedeling E, Xu J (2010) Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc Natl Acad Sci U S A 107: 22151–22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi FS, Chen H, Wu Y, Wu N (2010) Effects of livestock exclusion on vegetation and soil properties under two topographic habitats in an alpine meadow on the Eastern Qinghai-Tibetan Plateau. Polish J Ecol 58: 125–133. [Google Scholar]
- 47. Li W, Huang HZ, Zhang ZN, Wu GL (2011) Effects of grazing on the soil properties and C and N storage in relation to biomass allocation in an alpine meadow. J Soil Sci Plant Nutr 11: 27–39. [Google Scholar]
- 48. Wu JZ, Zhang XZ, Shen ZX, Shi PL, Yu CQ, et al. (2012) Species richness and diversity of alpine grasslands on the Northern Tibetan Plateau: effects of grazing exclusion and growing season precipitation. J Resour Ecol 3: 236–242. [Google Scholar]
- 49. Wang JS, Zhang XZ, Zhao YP, Shen ZX, Shi PL, et al. (2008) Spatio- temporal pattern of climate changes in Northern Tibet’s Qiangtang Plateau. Resour Sci 12: 1852–1859 (in Chinese with English abstract).. [Google Scholar]
- 50. Wang L, Niu KC, Yang YH, Zhou P (2010) Patterns of above- and belowground biomass allocation in China’s grasslands: evidence from individual-level observations. Sci China Life Sci 53: 851–857. [DOI] [PubMed] [Google Scholar]
- 51. Woodward FI, Williams BG (1987) Climate and plant distribution at global and local scales. Plant Ecol 69: 189–197. [Google Scholar]
- 52. Stephenson NL (1990) Climatic control of vegetation distribution: the role of the water balance. Am Nat 5: 649–670. [Google Scholar]
- 53. O′Connor TG, Haines LM, Snyman HA (2001) Influence of precipitation and species composition on phytomass of a semiarid African grassland. J Ecol 89: 850–860. [Google Scholar]
- 54. Huxman TE, Smith MD, Fay PA, Knapp AK, Shaw MR, et al. (2004) Convergence across biomes to a common rain-use efficiency. Nature 429: 651–654. [DOI] [PubMed] [Google Scholar]
- 55. Yang YH, Fang JY, Pan YD, Ji CJ (2009b) Above-ground biomass in Tibetan grasslands. J Arid Environ 73(1): 91–95. [Google Scholar]
- 56. Zhang BC, Cao JJ, Bai YF, Zhou XH, Ning ZG, et al. (2013) Effects of rainfall amount and frequency on vegetation growth in a Tibetan alpine meadow. Clim Chang 118: 197–212. [Google Scholar]
- 57.Körner C (2003) Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Berlin:Springer. 101–111 p. [Google Scholar]
- 58.Reynolds JF, Stafford SDM (2002) Do humans cause deserts? In Reynolds JF, Stafford SDM, editors. Berlin: Dahlem University Press. 1–21.
- 59. Sun J, Cheng GW, Li WP (2013) Meta-analysis of relationships between environmental factors and aboveground biomass in the alpine grassland on the Tibetan Plateau. Biogeosciences 10: 1707–1715. [Google Scholar]
- 60. Burke IC, Lauenroth WK, Parton WJ (1997) Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecol 78(5): 1330–1340. [Google Scholar]
- 61. Liu X, Chen B (2000) Climatic warming in the Tibetan Plateau during recent decades. Int J Climatol 20: 1729–1742. [Google Scholar]