Abstract
Biological invasions often cause major perturbations in the environment and are well studied among macroorganisms. Less is known about invasion by free-living microbes. Gonyostomum semen (Raphidophyceae) is a freshwater phytoplankton species that has increased in abundance in Northern Europe since the 1980's and has expanded its habitat range. In this study, we aimed to determine the genetic population structure of G. semen in Northern Europe and to what extent it reflects the species' recent expansion. We sampled lakes from 12 locations (11 lakes) in Norway, Sweden and Finland. Multiple strains from each location were genotyped using Amplified Fragment Length Polymorphism (AFLP). We found low differentiation between locations, and low gene diversity within each location. Moreover, there was an absence of genetic isolation with distance (Mantel test, p = 0.50). According to a Bayesian clustering method all the isolates belonged to the same genetic population. Together our data suggest the presence of one metapopulation and an overall low diversity, which is coherent with a recent expansion of G. semen.
Introduction
Invasive species can pose a significant threat to ecosystems with a wide range of effects on the newly colonized environment such as loss of biodiversity, disappearance of native species, shift in species dominance in the community, and alteration of ecosystem function [1]. While biological invasions in terrestrial and aquatic systems have been widely studied for a large range of macroorganisms, few studies focus on invasions of free-living aquatic microbes [2]. The lack of studies on microbial invasions may be partly explained by the fact that microbial invasion are difficult to detect in the environment [2], as the individuals are small and initially low in abundance. Furthermore, microbes were for a long time considered to be cosmopolitan due to their high dispersal capacity and large population sizes [3], and thus not viewed as potential invaders. Although some species are cosmopolitan, it is now known that many have a more restricted geographical distribution [4].
In aquatic systems, microbial planktonic algae (phytoplankton) are key players as primary producers that form the base of the food web in aquatic systems. Studies concerning invasive phytoplankton are scarce, and only a handful of microalgal species are currently described as invasive. These include the marine dinoflagellate Alexandrium tamarense [5], the freshwater cyanobacteria Aphanizomenon ovalisporum and Cylindrospermopsis raciborskii [6], [7], and the freshwater raphidophyte Gonyostomum semen [8], [9].
The present study focuses on the raphidophyte species G. semen, which is considered a nuisance species and invasive. This species can form dense blooms over extended periods of time reaching cell abundances of 1.5 millions cells per liter, despite very low growth rates [10]. Moreover, G. semen expels slimy threads upon mechanical stress which can cause skin irritation on bathers [11], [12]. The abundance and occurrence of this species has increased in Northern Europe during the last four decades [9], [12], [13], and more recently in Poland [14]. In a recent study based on data from the Swedish National Lake Monitoring program, Rengefors et al. [9] showed that blooms of G. semen have appeared in new lakes during the past twenty years. These findings are in line with the results of a previous study in Finland [13], also showing an expansion of G. semen into new lakes. Thus, the species is now considered invasive in Northern Europe. It has been proposed that an invasive species colonizes new habitats, spread quickly, and forms dominant populations [15].
With the expansion of G. semen in Northern European lakes, studies exploring the environmental conditions of G. semen lakes and describing the species ecological characteristics have been undertaken [8], [12], [16]. For instance, G. semen growth has been shown to be favored by humic substances [16]. Hence, the species blooms primarily in brown-water lakes with high concentrations of dissolved organic carbon [12], although it has also be observed in clear-water lakes [17]. In addition, the formation of dense population by G. semen has been suggested to be favored by a reduced grazing pressure by zooplankton [8], and its ability to migrate in the water column to acquire nutrients during night and light during day [12]. However, little has been done to explore the population genetic structure of G. semen populations, and no study yet attempted to explain the invasion pattern of the species. Presumably, a recent (decades rather than thousands of years) invasion should be reflected in the species population genetic structure. Several studies on phytoplankton population genetics have shown that populations are typically highly differentiated with low gene flow even between neighboring populations [18], [19], [20], [21], [22]. However, most of the studies focused on marine systems and little has been done in limnic systems, which are more isolated, thus more likely to show restricted gene flow. Rengefors et al. [21] showed that lakes can act as islands according to the island biogeography theory [23], [24], with the presence of highly differentiated populations on small geographical scale. For invasive phytoplankton, we can expect the populations to show little differentiation due to the short time since separation and low genetic diversity following the bottleneck occurring during colonization and establishment of individuals.
In this study, we aimed at characterizing the population genetic structure and diversity of G. semen in Northern Europe to get a better understanding of the species expansion. To this end, we used the DNA fingerprinting method Amplified Fragment Length Polymorphism (AFLP) to determine the population structure and genetic diversity of G. semen in 11 lakes spread across Sweden, Finland and Norway.
Methods
Study sites and Sampling
During summer 2010 (July-August), 12 stations from 11 lakes were sampled across Sweden (7 lakes), Norway (3 lakes), and Finland (1 lake) (Fig. 1, and Table 1). The sampling scheme was designed to cover two major axes: a North-South axis within Sweden; and a West-East axis with samples from Norway, Sweden and Finland. The locations were chosen to obtain a nested design with a wide range of geographical distances, from a few km to more than a thousand km between lakes (Table 2). The largest lake, Helgasjön was sampled at two different sites 10 km apart to determine if different populations co-exist in larger lakes. The selection of the lakes was done using databases from monitoring programs (SYKE for Finland, NIVA for Norway and SLU for Sweden) or from published data. The lakes were known to have recurrent G. semen blooms. At each sampling station G. semen cells were collected from the shore in the surface water using a 20-µm mesh-size plankton net. Large zooplankton which might feed on G. semen, were removed from the sample using a 150-µm net. Water from each lake was collected for preparation of culture medium for the G. semen isolates.
Figure 1. Map representing the sampled lakes.
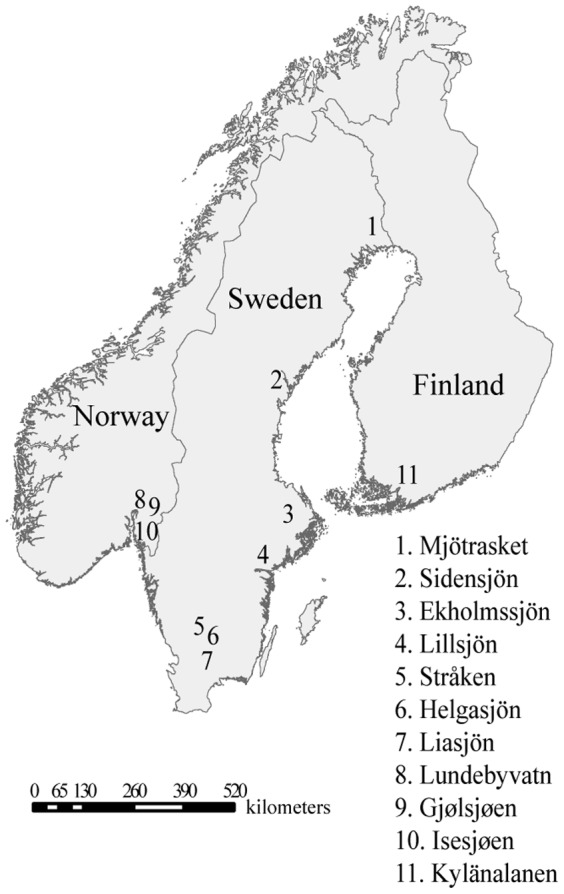
Table 1. Summary of lake data for each samples lakes (-: data not known).
| Lakes | Country | Sampling date | Coordinates | Area (km2) | G. semen abundance (cells L−1) | Monitoring period | Known G. semen occurrence years |
| Mjöträsket | Sweden | 6 July | 65°55′55″N 23°05′50″E | 2.56 | 4200 | 2006 | 2006 |
| Sidensjön | 8 July | 63°53′12″N 19°48′53″E | 0.089 | 117200 | 2004–2010 | 2004–2010 | |
| Ekholmssjön | 11 Aug | 59°52′19″N 17°03′36″E | 0.057 | 34700 | 1995–2010 | 1995–2010 | |
| Lillsjön | 10 Aug | 58°48′06″N 17°26′37″E | - | 30000 | - | - | |
| Stråken | 5 Aug | 57°07′11″N 14°34′10″E | 7.7 | 26000 | - | 1948 | |
| Helgasjön Station 2 | 4 Aug | 56°58′42″N 14°42′21″E | 50.2 | 3200 | - | - | |
| Helgasjön Station 1 | 4 Aug | 56°55′26″N 14°49′24″E | 50.2 | 1800 | - | 1948 | |
| Liasjön | 23 July | 56°26′78″N 13°59′56″E | 0.12 | 408000 | - | - | |
| Lundebyvatn | Norway | 28 July | 59°32′48″N 11°28′57″E | 0.42 | 286300 | 2005–2010 | 2005–2010 |
| Gjølsjøen | 28 July | 59°26′24″N 11°41′01″E | 0.98 | 14800 | - | - | |
| Isesjøen | 28 July | 59°17′42″N 11°14′40″E | 6.35 | 60200 | 2005–2010 | 2005–2010 | |
| Kylänalanen | Finland | 20 July | 60°24′21″N 23°45′01″E | - | 5100 | - | 2004 |
Table 2. Geographic distances in km between sampling locations.
| Mjöträsket | Sidensjön | Ekholmssjön | Lillsjön | Stråken | Helgasjön Stat. 2 | Helgasjön Stat. 1 | Liasjön | Lundebyvatn | Gjølsjøen | Isesjøen | Kylänalanen | |
| Mjöträsket | 371 | 841 | 949 | 1177 | 1189 | 1193 | 1259 | 1004 | 1006 | 1035 | 727 | |
| Sidensjön | 470 | 580 | 806 | 818 | 822 | 888 | 652 | 651 | 682 | 438 | ||
| Ekholmssjön | 121 | 339 | 350 | 353 | 422 | 315 | 300 | 334 | 375 | |||
| Lillsjön | 253 | 260 | 260 | 333 | 350 | 331 | 359 | 397 | ||||
| Stråken | 18 | 27 | 83 | 325 | 305 | 311 | 643 | |||||
| Helgasjön Stat. 2 | 9 | 74 | 343 | 323 | 328 | 647 | ||||||
| Helgasjön Stat. 1 | 74 | 351 | 332 | 337 | 645 | |||||||
| Liasjön | 375 | 358 | 356 | 718 | ||||||||
| Lundebyvatn | 21 | 31 | 690 | |||||||||
| Gjølsjøen | 35 | 675 | ||||||||||
| Isesjøen | 709 |
Ethics statement
No specific ethical permits were required for the specific study according to the Swedish, Finnish, and Norwegian laws. The locations are not privately-owned or protected in any way.
Isolation, culture and harvesting of G. semen cells
Single cell isolations were performed to obtain a final number of approximately 20 clonal cultures per sampling location (Table 3). The isolations and culture of the strains were performed according to Lebret et al. [10]. The strains of each lake were cultivated in a culture medium, composed of 50% of modified Wright's cryptophyte medium (MWC) [25] with an addition of selenium to a final concentration of 2.5 µg L−1 and 50% sterile-filtered water from the respective lakes to increase the isolation success. Survival rates of the strains for each population (in%) were calculated after two months of culture, by dividing the number of strains alive by the number of cells isolated. When the cell concentration of the cultures had reached approximately 2000 cells mL−1, the cells were harvested by centrifugation according to Lebret et al. [10] and the pellets were frozen at −80°C until DNA extraction.
Table 3. Number of strains genotyped, survival rate, Nei's gene diversity within locations and percentage of polymorphic loci for each location.
| Sampling stations | Number of genotyped strains | Survival rates (%) | Nei's gene diversity | Percentage of polymorphic loci | |
| 1 | Mjöträsket | 22 | 43 | 0.043 | 22.1 |
| 2 | Sidensjön | 16 | 29 | 0.050 | 21.0 |
| 3 | Ekholmssjön | 17 | 51 | 0.029 | 12.4 |
| 4 | Lillsjön | 15 | 35 | 0.022 | 8.9 |
| 5 | Stråken | 18 | 45 | 0.054 | 25.4 |
| 6a | Helgasjön Stat. 1 | 8 | 22 | 0.049 | 12.9 |
| 6b | Helgasjön Stat. 2 | 19 | 50 | 0.041 | 19.2 |
| 7 | Liasjön | 13 | 25 | 0.023 | 7.8 |
| 8 | Lundebyvatn | 18 | 41 | 0.038 | 17.9 |
| 9 | Gjølsjøen | 17 | 36 | 0.077 | 32.6 |
| 10 | Isesjøen | 17 | 42 | 0.040 | 16.9 |
| 11 | Kylänalanen | 14 | 41 | 0.040 | 16.0 |
DNA extraction
DNA was extracted using a CTAB-based protocol described by Lebret et al. [10]. The DNA concentration of the samples was estimated by measuring the absorbance of a subsample diluted ten times at 260 nm using a spectrophotometer (Ultraspec 3000, Pharmacia biotech). For each sample, the quality of the DNA was determined using the 260/280 ratio. Only samples of high DNA quality, i.e. with a 260/280 ratio of 2.0, were used for downstream analyses. The DNA samples were stored at −80°C until genotyping.
Genotyping by AFLP analysis
Amplified fragment length polymorphism (AFLP) analyses were performed on the samples as described by Lebret et al. [10]. For the selective amplification, the M and E-primers were 5′-GACTGCGTACCAATTCNNN-3′, and 5′-GATGAGTCCTGAGTAANNN-3′ respectively. Specifically, the following six primer combinations were used: ETCT x MCGA, ETCT x MCCG, ETAG x MCGG, ETCG x MCAG, ETCG x MCGG and ETCG x MCGA. PCR products from three primer combinations labeled with the different dyes (Ned, Fam and Hex) were combined in single wells of a 96-well plate (Applied Biosystems). All samples were analyzed by ABI37730XL capillary electrophoresis using a MapMarker 1000 bp size standard at the Uppsala Genome Center, Sweden.
AFLP data analyses
The raw data was analyzed with Genemapper (Version 4.0, Applied Biosystems) and AFLPscore version 1.4 [26] was used to score the data. Fragments between 50 to 1000 bp were sized and scored. The error rate between replicates was minimized to less than 2.5% for each primer combination based on duplicates of 20 randomly chosen strains according to Whitlock et al. [26]. After the scoring, a data set based on presence/absence of fragments was generated using AFLPscore. The data were checked manually to identify identical clones (genotypic diversity). The Nei's gene diversity [27] and the percentage of polymorphic loci were determined for each sampling date using the R script AFLPdat [28]. The data file was converted into input files compatible for Arlequin and STRUCTURE using AFLPdat [28]. Arlequin version 3.5.1.2 [29] was used to calculate pair-wise FST values to estimate genetic differentiation between sampling locations, the p-values were determined using 1000 permutations, and the FST were considered significant for p≤0.05. Genetic differentiation was also estimated with Jost's D distance [30] between each location using the program Spade [31]. Confidence intervals of the Jost's D values were calculated with a bootstrap of 1000. The geographic distances between lakes were calculated using the GPS coordinates using the R script AFLPdat [28]. A Mantel test was performed using Arlequin with 1000 iterations to determine the presence of isolation by distance. An analysis of the molecular variance (AMOVA) was performed using Arlequin [29]. Arlequin was also used to determine the presence of loci under selection in the data set, a hierarchical island model was used with 10,000 iterations, loci with p≤0.01 were defined as outliers, meaning potential loci under selection. A principal component analysis based on genetic distances at the population level was performed using the package GENALEX 6.41 in Excel [32] using the binary model for diploid organisms based on the method of Huff et al. [33]. The data were not checked for multivariate normality. The number of genetic populations were determined using the software STRUCTURE 2.3.3 [34] without prior information on the sampling location. All the combinations of model settings, admixture or no-admixture ancestry models with either correlated or independent allele frequency, were tested (four models in total). Each run had a burn-in of 50,000 iterations followed by 50,000 iterations of data collections. We tested up to 16 populations (K) with 10 iterations at each level. The results were analyzed according to Evanno et al. [35] to identify the number of populations that best fits our dataset.
Results
A total of 194 strains from 12 sampling stations (11 lakes) across Sweden, Norway and Finland (Fig. 1 and Table 3), were successfully isolated, cultivated, and genotyped (Table 3). The survival rates of the isolates ranged from 22 to 51%, (Table 3). 614 AFLP loci were retained for the population genetic analyses. No identical genotypes were observed in the entire dataset, i.e. all the AFLP profiles were different. Nei's gene diversity ranged from 0.022 (Lillsjön) to 0.077 (Gjølsjøen; Table 3). The percentage of polymorphic loci within location varied between 7.8% (Liasjön) and 32.6% (Gjølsjøen; Table 3).
To analyze the STRUCTURE results, the number of populations (K) was determined using two approaches. Based on the calculated lnP(K) value (Fig. 2A), the smallest K on the plateau is the correct number, i.e. K = 1 in this case. According to the method recommended by Evanno et al. [35], two or three populations could be identified because of the presence of a peak of ΔK (23.3) at K = 3, and a high ΔK (8.8) at K equal 2 (Fig. 2A). For the simulations of K = 2 and K = 3, the strains were assigned to the same population (>95% of assignment, Fig. 2B and C). Only Gjølsjøen had strains that were assigned to more than one population, suggesting a more diverse population within this lake. Thus, the STRUCTURE results best support the presence of one dominant genetic population across all our sampling points. Analysis of molecular variance (AMOVA) showed that 81% of the variation was observed within locations, and only 19% by variation among locations.
Figure 2. Summary of the results from the STRUCTURE analysis.
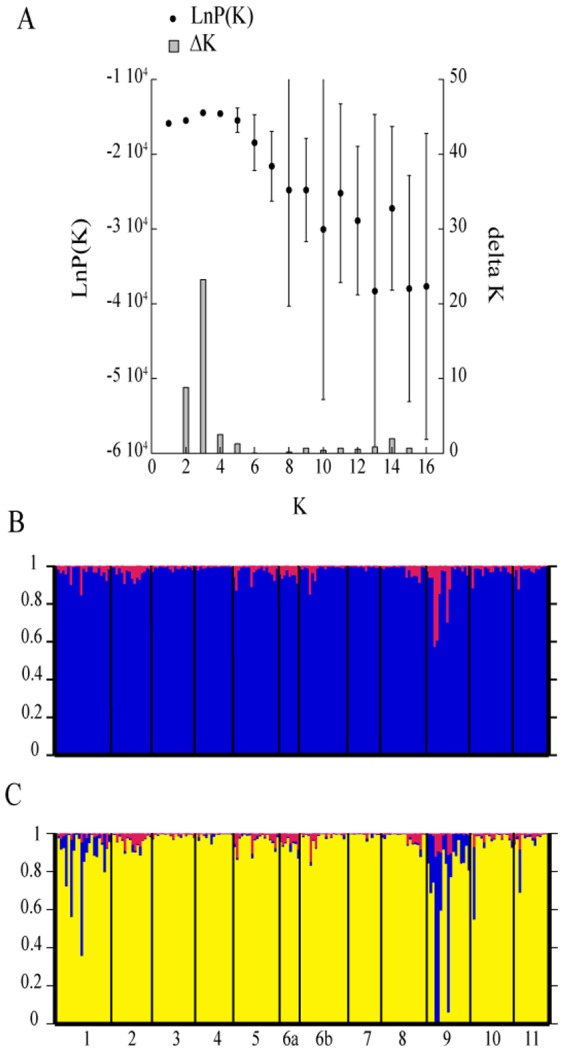
The analysis was performed using the admixture model allowing for correlated frequencies: Graph of LnP(K) (black dots) and ΔK (bars) for the different K population assumptions (A). Bar plots representing the population assignment of the individuals for the assumption of K = 2 (B), K = 3 (C).
All the pairwise FST between sampling locations were statistically highly significant, although low. The Mantel test showed that the differentiation between the lakes could not be explained by genetic isolation due to geographic distances (P = 0.50). The highest FST was observed between the samples collected in Helgasjön 1 and Lillsjön (FST = 0.306; Table 4). The lowest value was observed between Helgasjön 2 and Stråken with FST equal to 0.048 (Table 4). In general, Stråken showed the lowest FST values, and Helgasjön station 1 the highest ones, although the sample size of Helgasjön station 1 was low, thus this result should be interpreted with caution. The genetic differentiation between sampling locations was also estimated using Jost's D distances. All Jost's D distances were equal to zero or low between the locations (Table 4). The highest differentiation was observed between Gjølsjøen and Lillsjön (D = 0.058). The confidence intervals include zero for all the pairwise comparisons at the exception of the D values between Gjølsjøen-Lillsjön and Gjølsjøen-Mjöträsket, suggesting an absence of genetic differentiation between most of the sampling locations.
Table 4. Genetic differentiation expressed as pair-wised FST (below the diagonal; ** denotes p<0.01, *** denotes p<0.001), and Jost's D (above the diagonal; with confidence intervals between brackets) between sampling locations.
| Mjöträsket | Sidensjön | Ekholmssjön | Lillsjön | Stråken | Helgasjön Stat. 2 | Helgasjön Stat. 1 | Liasjön | Lundebyvatn | Gjølsjøen | Isesjøen | Kylänalanen | |
| Mjöträsket | 0.010 (0–0.041) | 0.008 (0–0.039) | 0 (0–0.029) | 0 (0–0.021) | 0 (0–0.017) | 0.007 (0–0.061) | 0 (0–0.019) | 0.003 (0–0.034) | 0.039 (0.005–0.074) | 0.012 (0–0.045) | 0 (0–0.012) | |
| Sidensjön | 0.155*** | 0 (0–0.022) | 0 (0–0.030) | 0 (0–0) | 0 (0–0.002) | 0 (0–0.032) | 0 (0–0.029) | 0 (0–0.013) | 0.002 (0–0.037) | 0 (0–0.028) | 0 (0–0.004) | |
| Ekholmssjön | 0.182*** | 0.136*** | 0 (0–0.009) | 0 (0–0.018) | 0 (0–0) | 0.001 (0–0.052) | 0 (0–0.010) | 0 (0–0.024) | 0.030 (0–0.068) | 0 (0–0.020) | 0 (0–0.010) | |
| Lillsjön | 0.167*** | 0.165*** | 0.181*** | 0.001 (0–0.034) | 0 (0–0.012) | 0.006 (0–0.057) | 0 (0–0.003) | 0.006 (0–0.043) | 0.058 (0.022–0.099) | 0.012 (0–0.048) | 0 (0–0.003) | |
| Stråken | 0.101*** | 0.068*** | 0.114*** | 0.150*** | 0 (0–0) | 0 (0–0.022) | 0 (0–0.019) | 0 (0–0.004) | 0 (0–0.026) | 0 (0–0.025) | 0 (0–0) | |
| Helgasjön Stat. 2 | 0.109*** | 0.082*** | 0.095*** | 0.150*** | 0.048*** | 0 (0–0.012) | 0 (0–0.003) | 0 (0–0) | 0 (0–0.035) | 0 (0–0.013) | 0 (0–0) | |
| Helgasjön Stat. 1 | 0.220*** | 0.166*** | 0.271*** | 0.306*** | 0.134*** | 0.140*** | 0.005 (0–0.061) | 0 (0–0.053) | 0 (0–0.046) | 0.006 (0–0.065) | 0 (0–0.031) | |
| Liasjön | 0.139*** | 0.165*** | 0.185*** | 0.205*** | 0.118*** | 0.124*** | 0.301*** | 0 (0–0.013) | 0.030 (0–0.074) | 0 (0–0.021) | 0 (0–0) | |
| Lundebyvatn | 0.153*** | 0.111*** | 0.178*** | 0.233*** | 0.075*** | 0.093*** | 0.237*** | 0.159*** | 0.006 (0–0.041) | 0 (0–0.019) | 0 (0–0.004) | |
| Gjølsjøen | 0.157*** | 0.112*** | 0.169*** | 0.209*** | 0.087*** | 0.104*** | 0.132** | 0.162*** | 0.124*** | 0.011 (0–0.045) | 0 (0–0.034) | |
| Isesjøen | 0.167*** | 0.134*** | 0.158*** | 0.241*** | 0.114*** | 0.113*** | 0.234*** | 0.172*** | 0.139*** | 0.116*** | 0 (0–0.015) | |
| Kylänalanen | 0.115*** | 0.103*** | 0.150*** | 0.144*** | 0.071*** | 0.064*** | 0.192*** | 0.132*** | 0.115*** | 0.110*** | 0.142*** |
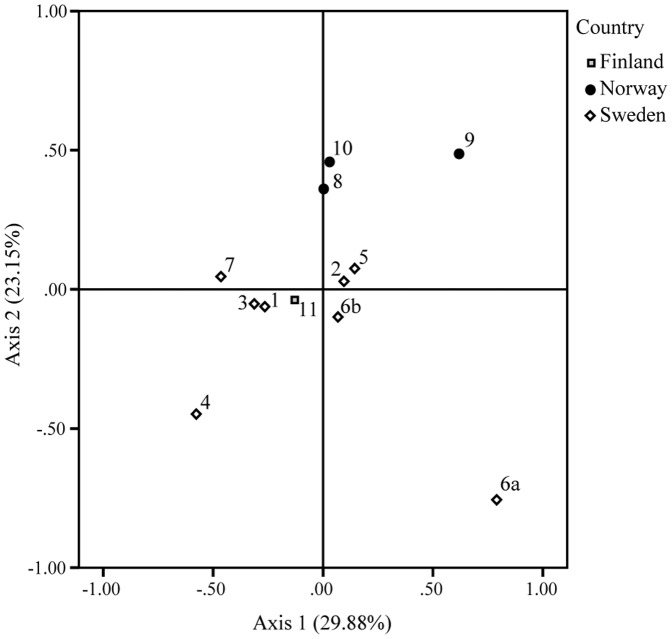
The results of the principal component analysis (PCA) based on genetic distances, showed that the first axis explained 30% of the observed variation, the second 23% and the third 15% (Fig. 3). Although, the efficiency of the PCA was low, a pattern can be observed. Hence, along the 1st axis, Lillsjön, Gjølsjøen and Helgasjön 1 clustered separately from all the other lakes. The Norwegian lakes were separated from the other lakes along the 2nd axis. These results suggest the presence of a weak east-west geographic pattern of differentiation. However, the PCA was not highly supported, as the first two axes explaining only 53% of the observed pattern. Moreover, Lillsjön and Helgasjön station 1 were genetically separated from the other lakes. Helgasjön station 1 was the most genetically distant lake, in accordance with the differentiation observed with the FST values. The other Swedish and Finnish lakes grouped together, indicating that the lakes were not genetically distant from each other (Fig. 3). Along the 3rd axis, all the lakes grouped together except for Mjöträsket in Northern Sweden.
Figure 3. Principal component analysis (PCA) of the genetic distances of the sampled locations.
The axes 1 and 2 explained 53% of the variance in distribution of the lake populations (1: Mjöträsket; 2: Sidensjön; 3: Ekholmssjön; 4: Lillsjön; 5: Stråken; 6a: Helgasjön Stat. 1; 6b: Helgasjön Stat. 2; 7: Liasjön; 8: Lundebyvatn; 9: Gjølsjøen; 10: Isesjøen; 11: Kylänalanen).
We performed an outlier analysis in Arlequin to determine if our dataset contained loci that might be under selection. The outlier analysis showed that 8 loci were potentially under selection pressure. Four of these loci were only present in the strains collected from Helgasjön station 1. Single unique loci were only observed in the Mjöträsket, Lillsjön, Isesjøen, and Gjølsjøen strains respectively.
Discussion
Previous studies have revealed that G. semen shows signs of range expansion in Northern Europe, and the species has been described as invasive [9], [13]. In this study we have used a population genetic approach to characterize the population structure of G. semen, to gain a better understanding of patterns of invasion. Our results showed the presence of a single metapopulation (STRUCTURE and AMOVA analyses) in Northern Europe, with low differentiation between sampling locations. The results are discussed below in light of the recent expansion (during the last decades) of the species in Northern Europe.
According to population genetic theory, a recently established population is expected to have low diversity because most likely only a fraction of the source population would have dispersed, generating a diversity bottleneck in the new population [36]. In addition, invasive species are expected to show weak or low population structure [37]. This is because dispersed individuals that have established and formed the invasive populations likely originate from the same population or from closely related populations. Previous studies on invasive species showed that genetic variation was mostly explained by within population variation in the invaded areas, showing a lack of population structure in the invasive range [38], [39]. The results from our study on G. semen are consistent with these patterns.
The presence of one single metapopulation (STRUCTURE and AMOVA analyses) over large distances, most likely reflect the recent invasion, as more differentiated populations could be expected from non-invasive phytoplankton populations. For instance, FST values between two phytoplankton populations identified by STRUCTURE typically range ≥0.22–0.4 in other studies [21], [40], which is despite some overlap overall slightly higher than found in the present study (FST ranged between 0.048 and 0.306). Similarly, Logares et al. [41] showed the presence of two distinct populations (using STRUCTURE analysis on AFLP data) of the dinoflagellate Peridinium aciculiferum in two Swedish lakes.
Although the STRUCTURE analysis suggests the presence of a single population in Northern Europe, there was still a significant but low genetic differentiation (based on FST) among the lakes. This discrepancy can be explained by the different approaches and assumptions of FST and STRUCTURE to analyze population structure. The STRUCTURE analysis assigns individuals to potential populations assuming that the populations are in Hardy-Weinberg equilibrium, whereas the FST analyzes the heterozygosity between pre-defined populations. Thus, the STRUCTURE analysis is often considered more conservative, and might detect higher level of population structure. However, the statistical significance observed for our FST values between locations might result from low within location variation, rather than reflecting strong differentiation. The statistical differentiation observed for the FST analysis can also result from the presence of a few loci only observed in specific sampling locations (loci under selection analysis). In fact, our data does show the presence of several loci that may be under selection, which are only present in specific lakes. These results need to be interpreted with caution, as they can result from false-positive or hitchhiking phenomenon, where alleles appear to be under selection due to linkage to loci under true selection [42]. Further investigations are needed to understand the role of neutral versus selected loci in phytoplankton populations. To complement the FST and STRUCTURE analyses, we also determined Jost's D distance as a measure of population differentiation [30]. Previous studies have suggested that Jost's D distance might have less limitation than FST analyses, thus being more relevant to study population differentiation [30], [43]. We found pairwise Jost's D distances equal to zero or low, indicating an absence of differentiation of the population of G. semen between the different locations. The combination of low FST values and the absence of differentiation according to Jost's D distance suggest that there is little or no differentiation between the sampling locations, thereby supporting the results of the STRUCTURE analysis.
The lack of genetic differentiation among lakes and low genetic diversity within lakes are best explained by a recent colonization of the lakes, which has likely occurred during the last decades or century as suggested by the monitoring data [9], [13]. Nevertheless, it is very challenging with the data at hand to determine with certainty when the colonization has occurred. If G. semen had colonized the lakes following the last glaciation period (several thousands to 10,000 years ago), we would have expected to find high differentiation of populations with a pattern of isolation by distance [44], [45]. In this scenario, G. semen would have colonized the lakes gradually from South to North following the receding of the ice cap. Our data does not support the latter hypothesis, thus we conclude that our results reflect the recent (past decades) invasion of lakes.
There are, however, alternative explanations for our results without invoking a recent invasion. A single metapopulation could also have resulted by high gene flow among lakes in G. semen even if the lakes were colonized thousands of years ago. However, that explanation seems less likely as monitoring data suggest that G. semen has colonized new lakes [9], [13] and that we are observing a true expansion rather than an increase in population sizes. Nevertheless, the possibility that G. semen's presence was previously undetected due to very low abundance in cell numbers remains. For example, a recent study showed that the freshwater diatom Stephanodiscus binderanus, which was described as an exotic species in the Great lakes since the mid-20th century, was already present in these lakes since a least three centuries [46]. That study shows that a species might be native although other evidence suggested that it was introduced recently. In our study, while the results of our genetic analyses are coherent with a species under expansion, and support the results from the monitoring studies, a recent increase of abundance of a population that colonized long ago cannot be completely ruled out.
Invasive species are expected to present lower diversity in the invasive range than in the native range [47], [48], [49]. This theory is called the paradox of invasion biology, and suggests that invasive populations should have lower genetic diversity, and consequently have lower capacity to adapt to new conditions than source populations. G. semen showed low gene diversity compared to other phytoplankton population studies. For example, freshwater dinoflagellates had a Nei's gene diversity between 0.07 and 0.37 [41], compared to 0.02–0.08 in this study. However, to date, there are few studies on population genetics in phytoplankton species and the level of diversity that can be expected in phytoplankton populations is still unclear. In addition, phytoplankton typically reproduce asexually during their active stage, but sexual events also occur regularly. While asexual reproduction will not alter the genetic diversity, sexual events are important in creating diversity through recombination and by allowing the spreading of favorable alleles [50]. G. semen alternates between asexual and sexual reproduction, with a mainly asexual phase during the growing season followed by sexual reproduction at the termination of the bloom [51]. In G. semen, the low gene diversity observed in the different populations might be explained by a bottleneck effect resulting from colonization by few individuals, which was maintained in the populations due to the life cycle characteristics involving vegetative growth. However, the gene diversity data alone cannot rule out that the observed diversity is not due to an increase in population sizes of populations that were already present. Nor do we have data from a native range to compare with that of the current range.
In our data set, the lakes Stråken and Helgasjön (Station 1) are the lakes with the oldest known G. semen blooms (first reported in 1948, by Sörensen [11]). However, the genetic diversity of these lakes is not very different from that in the other lakes although they are towards the higher end. This phenomenon cannot be explained with the current data set, since phytoplankton data is lacking from this time period (1940's) in the other lakes.
A further complication is that the measured diversities are most likely underestimates of the real diversity as it was calculated using the strains that were able to grow in culture. Isolation and culture techniques are known to be a form of selection of strains [52]. In order to minimize the selection effect and increase our isolation success we used a mix of artificial culture medium and sterile lake water from the respective lakes. Yet, the culture bias cannot be circumvented, as culture of isolates is currently necessary to perform population genetic studies of microorganisms.
In conclusion, our results showed the presence of a single metapopulation in Northern Europe with low differentiation of G. semen populations from the different locations, and relatively low gene diversity. We suggest that the genetic pattern observed in this study might reflect the recent expansion of G. semen (during the last decades), and the colonization of new lakes.
Acknowledgments
We thank Katharina Luehrig for helping during sampling and Marie Svensson for laboratory assistance. We also would like to thank Mattias Ekvall and Johan Ahlgren for the sampling of Liasjön. We thank the Plankton Ecology Group of Lund University for the constructive comments on an early version of the manuscript.
Funding Statement
The Swedish Council for Environment, Agricultural Sciences and Spatial Planning (Formas, # 2007-548) and the Crafoord Foundation (# 20061089) provided financial support. The funders had no roles in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Davis MA (2009) Invasion biology: Oxford University Press. i-xiv, 1–244 p. [Google Scholar]
- 2. Litchman E (2010) Invisible invaders: non-pathogenic invasive microbes in aquatic and terrestrial ecosystems. Ecology Letters 13: 1560–1572. [DOI] [PubMed] [Google Scholar]
- 3. Finlay BJ (2002) Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 4. Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, et al. (2006) Microbial biogeography: putting miroorganisms on the map. Nature Reviews 4: 102–112. [DOI] [PubMed] [Google Scholar]
- 5. Lilly EL, Kulis DM, Gentien P, Anderson DM (2002) Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from the western Pacific: Evidence from DNA and toxin analysis. Journal of Plankton Research 24: 443–452. [Google Scholar]
- 6. Neilan BA, Saker ML, Fastner J, Törökne A, Burns BP (2003) Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii . Molecular Ecology 12: 133–140. [DOI] [PubMed] [Google Scholar]
- 7.Sukenik A, Hadas O, Kaplan A, Quesada A (2012) Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes? Physiological, regional and global driving forces. Frontiers in Microbiology 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lebret K, Fernández MF, Hagman CHC, Rengefors K, Hansson L-A (2012) Grazing resistance allows bloom formation and may explain invasion success of Gonyostomum semen . Limnology and Oceanography 57: 727–734. [Google Scholar]
- 9. Rengefors K, Weyhenmeyer GA, Bloch I (2012) Temperature as a driver for the expansion of the microalga Gonyostomum semen in Swedish lakes. Harmful Algae 18: 65–73. [Google Scholar]
- 10. Lebret K, Kritzberg ES, Figueroa RI, Rengefors K (2012) Genetic diversity within and genetic differentiation between blooms of a microalgal species. Environmental Microbiology 14: 2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sörensen I (1954) Gonyostomum semen (Ehrenb.) Diesing - en vattenorganism av teoretiskt och praktiskt intresse. Svensk Faunistisk Revy 2: 47–52. [Google Scholar]
- 12. Cronberg G, Lindmark G, Björk S (1988) Mass development of the flagellate Gonyostomum semen (Raphidophyta) in swedish forest lake - an effect of acidification? Hydrobiologia 161: 217–236. [Google Scholar]
- 13. Lepistö L, Antikainen S, Kivinen J (1994) The occurence of Gonyostomum semen (Ehr) Diesing in Finnish lakes. Hydrobiologia 273: 1–8. [Google Scholar]
- 14. Poniewozik M, Wojciechowska W, Solis M (2011) Dystrophy or eutrophy: phytoplankton and physicochemical parameters in the functioning of humic lakes. Oceanological and Hydrobiological Studies 40: 22–29. [Google Scholar]
- 15. Valery L, Fritz H, Lefeuvre JC, Simberloff D (2008) In search of a real definition of the biological invasion phenomenon itself. Biological Invasions 10: 1345–1351. [Google Scholar]
- 16. Rengefors K, Pålsson C, Hansson L-A, Heiberg L (2008) Cell lysis of competitors and osmotrophy enhance growth of the bloom-forming alga Gonyostomum semen . Aquatic Microbial Ecology 51: 87–96. [Google Scholar]
- 17. Findlay DL, Paterson MJ, Hendzel LL, Kling HJ (2005) Factors influencing Gonyostomum semen blooms in a small boreal reservoir lake. Hydrobiologia 533: 243–252. [Google Scholar]
- 18. Rynearson TA, Armbrust EV (2004) Genetic differentiation among populations of the planktonic marine diatom Ditylum brightwellii (Bacillariophyceae). Journal of Phycology 40: 34–43. [Google Scholar]
- 19. Evans KM, Chepurnov VA, Sluiman HJ, Thomas SJ, Spears BM, et al. (2009) Highly differentiated populations of the freshwater diatom Sellaphora capitata suggest limited dispersal and opportunities for allopatric speciation. Protist 160: 386–396. [DOI] [PubMed] [Google Scholar]
- 20. Nagai S, Lian C, Yamaguchi S, Hamaguchi M, Matsuyama Y, et al. (2007) Microsatellite markers reveal population genetic structure of the toxic dinoflagellate Alexandrium tamarense (Dinophyceae) in Japanese coastal waters. Journal of Phycology 43: 43–54. [Google Scholar]
- 21. Rengefors K, Logares R, Laybourn-Parry J (2012) Polar lakes may act as ecological islands to aquatic protists. Molecular Ecology 21: 3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tahvanainen P, Alpermann TJ, Figueroa RI, John U, Hakanen P, et al. (2012) Patterns of Post-Glacial Genetic Differentiation in Marginal Populations of a Marine Microalga. Plos One 7: e53602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dolan JR (2005) An introduction to the biogeography of aquatic microbes. Aquatic Microbial Ecology 41: 39–48. [Google Scholar]
- 24.MacArthur RH, Wilson EO (1967) The theory of island biogeography: Princeton University Press. pp. 198 p. [Google Scholar]
- 25. Guillard RRL, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide C. Journal of Phycology 8: 10–14. [Google Scholar]
- 26. Whitlock R, Hipperson H, Mannarelli M, Butlin RK, Burke T (2008) An objective, rapid and reproducible method for scoring AFLP peak-height data that minimizes genotyping error. Molecular Ecology Resources 8: 725–735. [DOI] [PubMed] [Google Scholar]
- 27.Nei M (1987) Molecular evolutionary genetics. 1–512 p. [Google Scholar]
- 28. Ehrich D (2006) AFLPDAT: a collection of R functions for convenient handling of AFLP data. Molecular Ecology Notes 6: 603–604. [Google Scholar]
- 29. Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evolutionary Bioinformatics 1: 47–50. [PMC free article] [PubMed] [Google Scholar]
- 30. Jost L (2008) G(ST) and its relatives do not measure differentiation. Molecular Ecology 17: 4015–4026. [DOI] [PubMed] [Google Scholar]
- 31.Chao A, Shen T-J (2003) Program SPADE (Species Prediction and Diversity Estimation). Available at: http://chao.stat.nthu.edu.tw. Accessed 06 august 2013.
- 32. Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huff DR, Peakall R, Smouse PE (1993) RAPD variation within and among natural-populations of outcrossing Buffalograss Buchloe-dactyloides (Nutt) Engelm. Theoretical and Applied Genetics 86: 927–934. [DOI] [PubMed] [Google Scholar]
- 34. Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- 36.Excoffier L, Foll M, Petit RJ (2009) Genetic Consequences of Range Expansions. Annual Review of Ecology Evolution and Systematics. pp. 481–501.
- 37. Hutchison DW, Templeton AR (1999) Correlation of pairwise genetic and geographic distance measures: Inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution 53: 1898–1914. [DOI] [PubMed] [Google Scholar]
- 38. Lachmuth S, Durka W, Schurr FM (2010) The making of a rapid plant invader: genetic diversity and differentiation in the native and invaded range of Senecio inaequidens . Molecular Ecology 19: 3952–3967. [DOI] [PubMed] [Google Scholar]
- 39. Huotari T, Korpelainen H, Leskinen E, Kostamo K (2011) Population genetics of the invasive water weed Elodea canadensis in Finnish waterways. Plant Systematics and Evolution 294: 27–37. [Google Scholar]
- 40. Härnström K, Ellegaard M, Andersen TJ, Godhe A (2011) Hundred years of genetic structure in a sediment revived diatom population. Proceedings of the National Academy of Sciences of the United States of America 108: 4252–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Logares R, Boltovskoy A, Bensch S, Laybourn-Parry J, Rengefors K (2009) Genetic diversity patterns in five protist species occurring in lakes. Protist 160: 301–317. [DOI] [PubMed] [Google Scholar]
- 42. Nosil P, Funk DJ, Ortiz-Barrientos D (2009) Divergent selection and heterogeneous genomic divergence. Molecular Ecology 18: 375–402. [DOI] [PubMed] [Google Scholar]
- 43. Heller R, Siegismund HR (2009) Relationship between three measures of genetic differentiation G(ST), D(EST) and G'(ST): how wrong have we been? Molecular Ecology 18: 2080–2083. [DOI] [PubMed] [Google Scholar]
- 44. Coyer JA, Peters AF, Stam WT, Olsen JL (2003) Post-ice age recolonization and differentiation of Fucus serratus L. (Phaeophyceae; Fucaceae) populations in Northern Europe. Molecular Ecology 12: 1817–1829. [DOI] [PubMed] [Google Scholar]
- 45. Vainio JK, Väinölä R (2003) Refugial races and postglacial colonization history of the freshwater amphipod Gammarus lacustris in Northern Europe. Biological Journal of the Linnean Society 79: 523–542. [Google Scholar]
- 46. Hawryshyn J, Ruehland KM, Julius M, Smol JP (2012) Absence of evidence is not evidence of absence: is Stephanodiscus binderanus (Bacillariophyceae) an exotic species in the great lakes region? Journal of phycology 48: 270–274. [DOI] [PubMed] [Google Scholar]
- 47. Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends in Ecology & Evolution 22: 454–464. [DOI] [PubMed] [Google Scholar]
- 48. Hardesty BD, Le Roux JJ, Rocha OJ, Meyer J-Y, Westcott D, et al. (2012) Getting here from there: testing the genetic paradigm underpinning introduction histories and invasion success. Diversity and Distributions 18: 147–157. [Google Scholar]
- 49. Grapputo A, Boman S, Lindstrom L, Lyytinen A, Mappes J (2005) The voyage of an invasive species across continents: genetic diversity of North American and European Colorado potato beetle populations. Molecular Ecology 14: 4207–4219. [DOI] [PubMed] [Google Scholar]
- 50. Halkett F, Simon JC, Balloux F (2005) Tackling the population genetics of clonal and partially clonal organisms. Trends in Ecology & Evolution 20: 194–201. [DOI] [PubMed] [Google Scholar]
- 51. Figueroa RI, Rengefors K (2006) Life cyle and sexuality of the freshwater raphidophyte Gonyostomum semen (Raphidophyceae). Journal of Phycology 42: 859–871. [Google Scholar]
- 52. Lakeman MB, von Dassow P, Cattolico RA (2009) The strain concept in phytoplankton ecology. Harmful Algae 8: 746–758. [Google Scholar]