Abstract
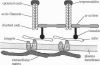
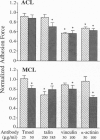
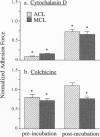
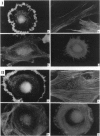
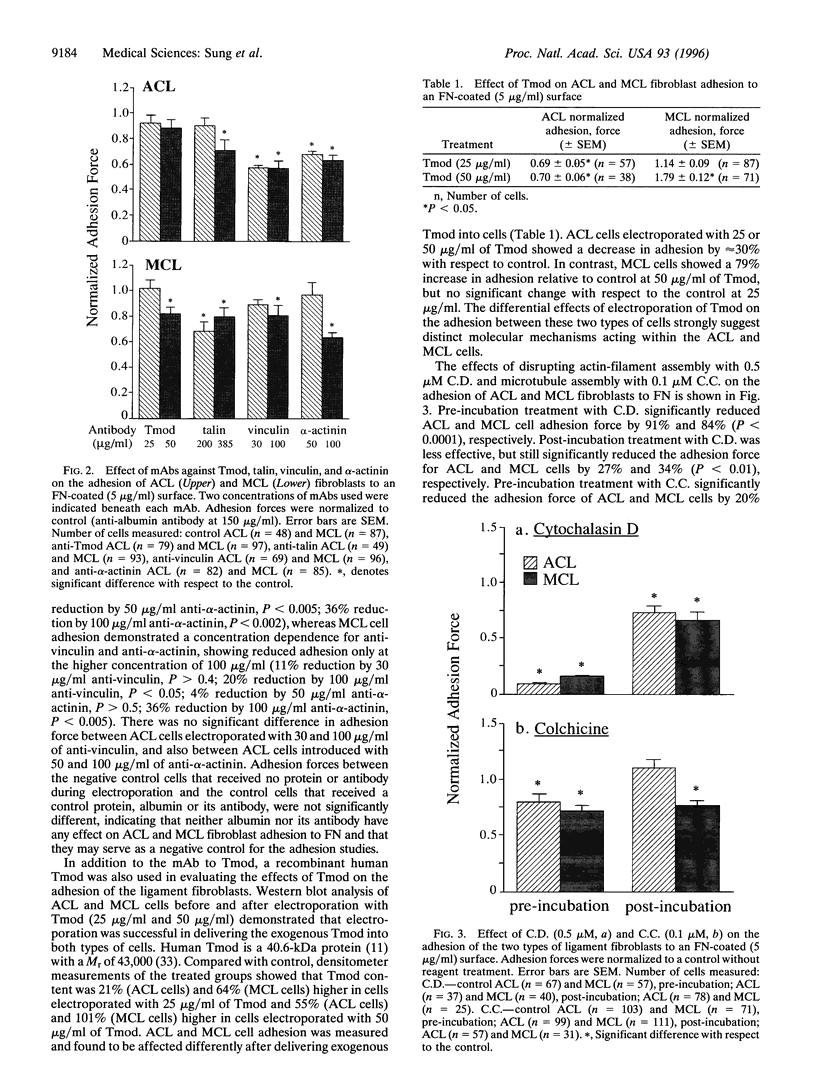
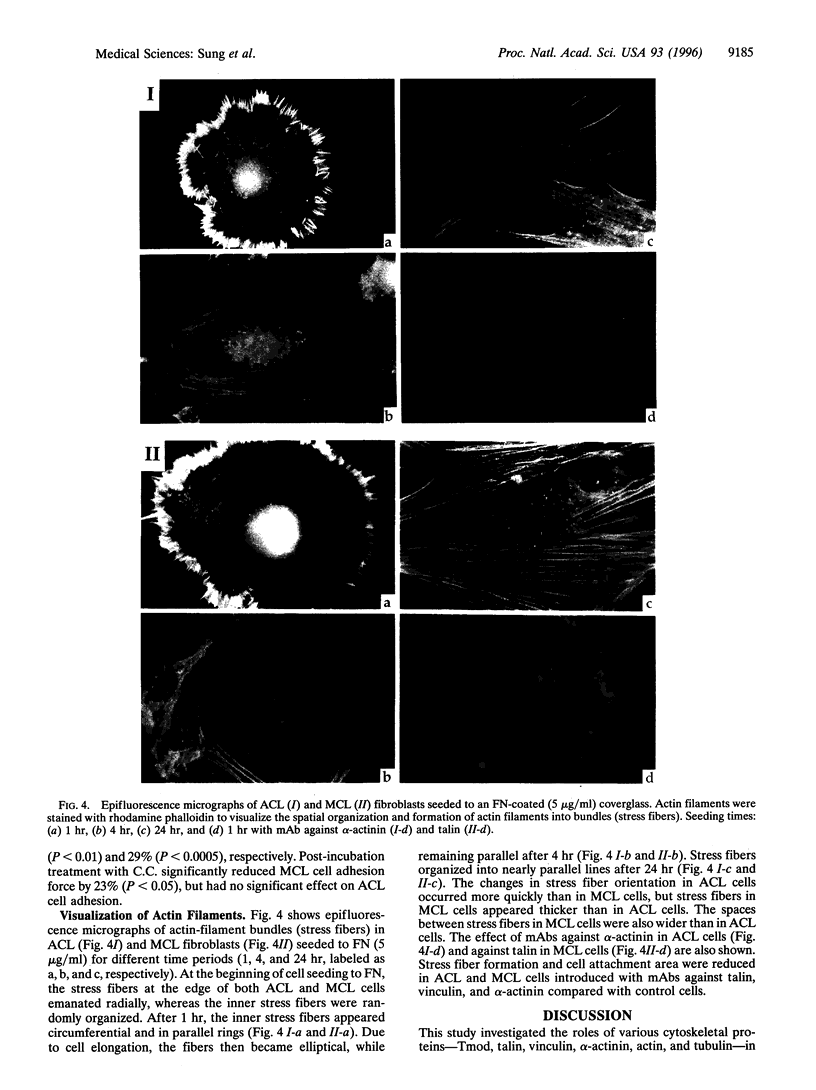
We have determined the effects of tropomodulin (Tmod), talin, vinculin, and alpha-actinin on ligament fibroblast adhesion. The anterior cruciate ligament (ACL), which lacks a functional healing response, and the medial collateral ligament (MCL), a functionally healing ligament, were selected for this study. The micropipette aspiration technique was used to determine the forces needed to separate ACL and MCL cells from a fibronectin-coated surface. Delivery of exogenous tropomodulin, an actin-filament capping protein, into MCL fibroblasts significantly increased adhesion, whereas its monoclonal antibody (mAb) significantly decreased cell adhesiveness. However, for ACL fibroblasts, Tmod significantly reduced adhesion, whereas its mAb had no effect. mAbs to talin, vinculin, and alpha-actinin significantly decreased the adhesion of both ACL and MCL cells with increasing concentrations of antibody, and also reduced stress fiber formation and cell spreading rate as revealed by immunofluorescence microscopy. Disruption of actin filament and microtubule assembly with cytochalasin D and colchicine, respectively, also significantly reduced adhesion in ACL and MCL cells. In conclusion, both ACL and MCL fibroblast adhesion depends on cytoskeletal assembly; however, this dependence differs between ACL and MCL fibroblasts in many ways, especially in the role of Tmod. These results add yet another possible factor in explaining the clinical differences in healing between the ACL and the MCL.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiel D., Frank C., Harwood F., Fronek J., Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1(3):257–265. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R., Wylie D. E., Schuster S. M. Transfer of monoclonal antibodies into mammalian cells by electroporation. J Biol Chem. 1989 Sep 15;264(26):15494–15500. [PubMed] [Google Scholar]
- Cunningham C. C., Gorlin J. B., Kwiatkowski D. J., Hartwig J. H., Janmey P. A., Byers H. R., Stossel T. P. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992 Jan 17;255(5042):325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Fowler V. M. Identification and purification of a novel Mr 43,000 tropomyosin-binding protein from human erythrocyte membranes. J Biol Chem. 1987 Sep 15;262(26):12792–12800. [PubMed] [Google Scholar]
- Fowler V. M. Tropomodulin: a cytoskeletal protein that binds to the end of erythrocyte tropomyosin and inhibits tropomyosin binding to actin. J Cell Biol. 1990 Aug;111(2):471–481. doi: 10.1083/jcb.111.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger M. H., Green M. H., Monosov A., Akeson W. H., Amiel D. An in vitro assay of anterior cruciate ligament (ACL) and medial collateral ligament (MCL) cell migration. Connect Tissue Res. 1994;30(3):215–224. doi: 10.3109/03008209409061973. [DOI] [PubMed] [Google Scholar]
- Glück U., Ben-Ze'ev A. Modulation of alpha-actinin levels affects cell motility and confers tumorigenicity on 3T3 cells. J Cell Sci. 1994 Jul;107(Pt 7):1773–1782. doi: 10.1242/jcs.107.7.1773. [DOI] [PubMed] [Google Scholar]
- Hug C., Jay P. Y., Reddy I., McNally J. G., Bridgman P. C., Elson E. L., Cooper J. A. Capping protein levels influence actin assembly and cell motility in dictyostelium. Cell. 1995 May 19;81(4):591–600. doi: 10.1016/0092-8674(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Kato G., Wakabayashi K. Effect of polylysine-bound laminin on human retinoblastoma cell lines. In Vitro Cell Dev Biol. 1988 Apr;24(4):274–280. doi: 10.1007/BF02628827. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Niggli V. Colchicine-induced stimulation of PMN motility related to cytoskeletal changes in actin, alpha-actinin, and myosin. Cell Motil Cytoskeleton. 1993;25(1):10–18. doi: 10.1002/cm.970250103. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Birchmeier W. Microinjection of fluorescently labeled proteins into living cells with emphasis on cytoskeletal proteins. Int Rev Cytol. 1982;75:209–214. doi: 10.1016/s0074-7696(08)61005-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Wulfkuhle J. D., Otto J. J. Vinculin binding site mapped on talin with an anti-idiotypic antibody. J Biol Chem. 1992 Aug 15;267(23):16355–16358. [PubMed] [Google Scholar]
- Lin J. J., Hegmann T. E., Lin J. L. Differential localization of tropomyosin isoforms in cultured nonmuscle cells. J Cell Biol. 1988 Aug;107(2):563–572. doi: 10.1083/jcb.107.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokeshwar V. B., Fregien N., Bourguignon L. Y. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol. 1994 Aug;126(4):1099–1109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon R. M., Akeson W. H., Amiel D., Kitabayashi L. R., Woo S. L. Ultrastructural differences between the cells of the medical collateral and the anterior cruciate ligaments. Clin Orthop Relat Res. 1991 Nov;(272):279–286. [PubMed] [Google Scholar]
- Mueller S. C., Kelly T., Dai M. Z., Dai H. N., Chen W. T. Dynamic cytoskeleton-integrin associations induced by cell binding to immobilized fibronectin. J Cell Biol. 1989 Dec;109(6 Pt 2):3455–3464. doi: 10.1083/jcb.109.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni C. N., Amiel D., Green M. H., Berchuck M., Akeson W. H. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992 Jul;10(4):465–475. doi: 10.1002/jor.1100100402. [DOI] [PubMed] [Google Scholar]
- Newton P. O., Woo S. L., MacKenna D. A., Akeson W. H. Immobilization of the knee joint alters the mechanical and ultrastructural properties of the rabbit anterior cruciate ligament. J Orthop Res. 1995 Mar;13(2):191–200. doi: 10.1002/jor.1100130207. [DOI] [PubMed] [Google Scholar]
- Nuckolls G. H., Romer L. H., Burridge K. Microinjection of antibodies against talin inhibits the spreading and migration of fibroblasts. J Cell Sci. 1992 Aug;102(Pt 4):753–762. doi: 10.1242/jcs.102.4.753. [DOI] [PubMed] [Google Scholar]
- Pavalko F. M., Schneider G., Burridge K., Lim S. S. Immunodetection of alpha-actinin in focal adhesions is limited by antibody inaccessibility. Exp Cell Res. 1995 Apr;217(2):534–540. doi: 10.1006/excr.1995.1119. [DOI] [PubMed] [Google Scholar]
- Peter K., O'Toole T. E. Modulation of cell adhesion by changes in alpha L beta 2 (LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeleton interaction. J Exp Med. 1995 Jan 1;181(1):315–326. doi: 10.1084/jem.181.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E. D., McKeel M., Hays T. Rapid rate of tubulin dissociation from microtubules in the mitotic spindle in vivo measured by blocking polymerization with colchicine. J Cell Biol. 1984 Sep;99(3):1066–1075. doi: 10.1083/jcb.99.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson S. J., Luther P. W., Pumplin D. W., Bloch R. J. Structures linking microfilament bundles to the membrane at focal contacts. J Cell Biol. 1993 Jul;122(2):485–496. doi: 10.1083/jcb.122.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. C., Georgescu H. I., Kwoh C. K., Blomstrom G. L., Engle C. P., Larkin L. A., Evans C. H., Woo S. L. Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments. J Orthop Res. 1995 Mar;13(2):184–190. doi: 10.1002/jor.1100130206. [DOI] [PubMed] [Google Scholar]
- Schreck P. J., Kitabayashi L. R., Amiel D., Akeson W. H., Woods V. L., Jr Integrin display increases in the wounded rabbit medial collateral ligament but not the wounded anterior cruciate ligament. J Orthop Res. 1995 Mar;13(2):174–183. doi: 10.1002/jor.1100130205. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Sung K. L., Kwan M. K., Maldonado F., Akeson W. H. Adhesion strength of human ligament fibroblasts. J Biomech Eng. 1994 Aug;116(3):237–242. doi: 10.1115/1.2895725. [DOI] [PubMed] [Google Scholar]
- Sung K. L., Sung L. A., Crimmins M., Burakoff S. J., Chien S. Determination of junction avidity of cytolytic T cell and target cell. Science. 1986 Dec 12;234(4782):1405–1408. doi: 10.1126/science.3491426. [DOI] [PubMed] [Google Scholar]
- Sung L. A., Fowler V. M., Lambert K., Sussman M. A., Karr D., Chien S. Molecular cloning and characterization of human fetal liver tropomodulin. A tropomyosin-binding protein. J Biol Chem. 1992 Feb 5;267(4):2616–2621. [PubMed] [Google Scholar]
- Sung L. A., Lin J. J. Erythrocyte tropomodulin binds to the N-terminus of hTM5, a tropomyosin isoform encoded by the gamma-tropomyosin gene. Biochem Biophys Res Commun. 1994 Jun 15;201(2):627–634. doi: 10.1006/bbrc.1994.1747. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Weber A., Pennise C. R., Babcock G. G., Fowler V. M. Tropomodulin caps the pointed ends of actin filaments. J Cell Biol. 1994 Dec;127(6 Pt 1):1627–1635. doi: 10.1083/jcb.127.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig M. E., Amiel D., Ivarsson M., Nagineni C. N., Wallace C. D., Arfors K. E. Type I procollagen gene expression in normal and early healing of the medial collateral and anterior cruciate ligaments in rabbits: an in situ hybridization study. J Orthop Res. 1991 May;9(3):374–382. doi: 10.1002/jor.1100090309. [DOI] [PubMed] [Google Scholar]
- Witke W., Sharpe A. H., Hartwig J. H., Azuma T., Stossel T. P., Kwiatkowski D. J. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995 Apr 7;81(1):41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- Wodnicka M., Pierzchalska M., Bereiter-Hahn J., Kajstura J. Comparative study on effects of cytochalasins B and D on F-actin content in different cell lines and different culture conditions. Folia Histochem Cytobiol. 1992;30(3):107–111. [PubMed] [Google Scholar]