Background: A device of QCM can be used in the transient kinetics of oxidation of a pair of cysteine residues in DsbA by DsbB.
Results: The obtained kinetic parameters indicate that the two pairs of cysteine residues in DsbB are important.
Conclusion: The reaction pathway of almost all DsbA oxidation processes would proceed through the stable intermediate.
Significance: The transient kinetics of the reaction intermediate is important.
Keywords: Enzyme Kinetics, Kinetics, Membrane Lipids, Membrane Proteins, Protein Chemistry, Disulfide Bond Formation Protein, Quartz Crystal Microbalance, Supported Lipid Bilayer, Transient Kinetic Analysis
Abstract
Disulfide bond formation protein B (DsbBS-S,S-S) is an inner membrane protein in Escherichia coli that has two disulfide bonds (S-S, S-S) that play a role in oxidization of a pair of cysteine residues (SH, SH) in disulfide bond formation protein A (DsbASH,SH). The oxidized DsbAS-S, with one disulfide bond (S-S), can oxidize proteins with SH groups for maturation of a folding preprotein. Here, we have described the transient kinetics of the oxidation reaction between DsbASH,SH and DsbBS-S,S-S. We immobilized DsbBS-S,S-S embedded in lipid bilayers on the surface of a 27-MHz quartz crystal microbalance (QCM) device to detect both formation and degradation of the reaction intermediate (DsbA-DsbB), formed via intermolecular disulfide bonds, as a mass change in real time. The obtained kinetic parameters (intermediate formation, reverse, and oxidation rate constants (kf, kr, and kcat, respectively) indicated that the two pairs of cysteine residues in DsbBS-S,S-S were more important for the stability of the DsbA-DsbB intermediate than ubiquinone, an electron acceptor for DsbBS-S,S-S. Our data suggested that the reaction pathway of almost all DsbASH,SH oxidation processes would proceed through this stable intermediate, avoiding the requirement for ubiquinone.
Introduction
Transport reactions that occur inside a cell, such as electron transport reactions, are catalyzed by specific enzymes (1). The mechanism of protein interaction-based transport systems is different from that of electron transport reactions of general chemical compounds. The disulfide bond formation (Dsb) system is a catalytic system that accelerates the oxidative formation of protein disulfide bonds in Escherichia coli and involves a number of protein factors, including the Dsb proteins DsbA,2 DsbB, DsbC, DsbD, and DsbG (2–4). Together, DsbA and DsbB function as a disulfide-introducing unit in the bacterial periplasmic space. DsbA belongs to the thioredoxin superfamily and contains a disulfide bond in the thioredoxin domain (Cys-30–X-X–Cys-33) that is known to act as an acceptor of two electrons (and two protons) from a preprotein containing cysteine residues during the transfer of the disulfide bond from the oxidized DsbAS-S to the preprotein (Fig. 1) (2–5). To repeat this disulfide bond-introducing reaction in the next catalytic cycle, re-oxidation of DsbASH,SH is required. DsbBS-S,S-S is an inner membrane protein in E. coli that has four transmembrane segments containing four cysteine residues that form two disulfide bonds (Cys-41 with Cys-44, and Cys-104 with Cys-130) within the periplasmic space. Reduced DsbASH,SH is reoxidized by the transfer of two electrons (and two protons) to the two thiol groups of the disulfide bonds on DsbBS-S,S-S, resulting in disulfide exchange. Finally, the two electrons (and two protons) are transferred to lipophilic ubiquinone in the inner membrane, where DsbB functions as a quinone reductase, catalyzing the conversion of ubiquinone to ubiquinol (3).
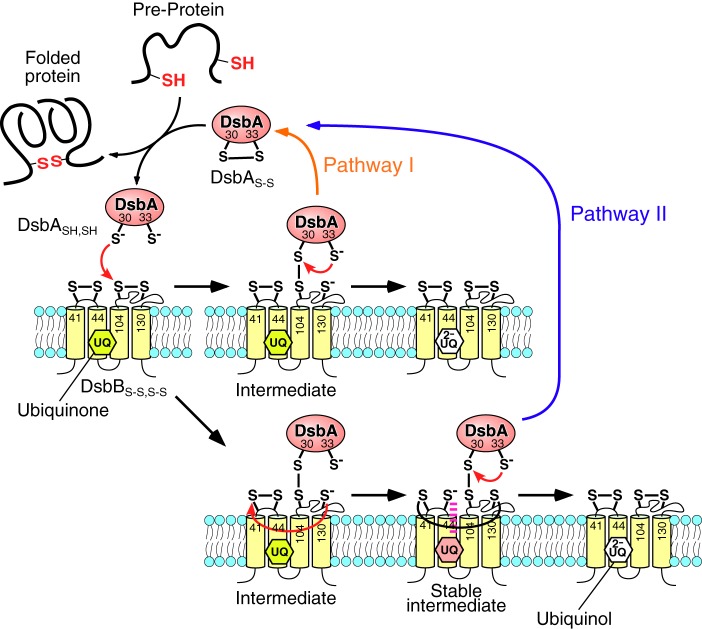
FIGURE 1.
Two potential pathways for the thiol-disulfide exchange reaction of disulfide bond formation protein A (DsbASH,SH) on disulfide bond formation protein B (DsbBS-S,S-S) in the inner membrane of Escherichia coli.
The mechanism and direction of electron transfer between chemical compounds can be explained in terms of its oxidation-reduction potential. In the case of electron transport based on protein factors, such as the DsbA-DsbB system, protein-protein interactions and intermediate complexes are also important for understanding the mechanism of the electron transport reaction. Various methods have been used to investigate the electron transport mechanisms associated with thiol-disulfide exchange in the DsbA-DsbB system. Nonreducing SDS-PAGE analysis has been used to estimate the amounts of reduced DsbASH,SH and oxidized DsbAS-S after modification by a thiol-reacting alkylating agent (6–8). Previous investigations also used x-ray crystal structure analysis to determine the structures of DsbB and the intermediate DsbB-DsbA complex (inactivated with mutation); this analysis revealed the positional relationship of cysteine residues and ubiquinone within the complex (9, 10). Snapshots of nuclear magnetic resonance solution structures of DsbB and its variant have proven useful for understanding electron movement from DsbA to ubiquinone (11). Moreover, fluorescence measurements have enabled the observation of the oxidation reaction of DsbA over time on the basis of the change in the tryptophan fluorescence of DsbA (12). Stopped-flow absorbance measurements have also been conducted to monitor DsbB-ubiquinone complexes in the process of electron transfer from DsbA to ubiquinone (13).
Based on these previous studies, two potential models of the reaction pathway have been proposed (Fig. 1) (3). In the first step the interprotein disulfide bond between Cys-30(DsbA) and Cys-104(DsbB) is formed by the nucleophilic attack of Cys-30 from DsbASH,SH on the Cys-104–Cys-130 disulfide bond of DsbBS-S,S-S. Then, Cys-33(DsbA) intramolecularly attacks Cys-30, and oxidized DsbAS-S is released from the reduced DsbBS-S,SH,SH (pathway I, referred to as the rapid pathway) (6, 7). In this pathway, ubiquinone reduction occurs in the reduced DsbBSH,SH,S-S–ubiquinone complex, independent of DsbA. In the alternative model, after the formation of the Cys-30(DsbA)–Cys-104(DsbB) complex, the newly reduced Cys-130(DsbB) immediately attacks Cys-41(DsbB) in an intramolecular isomerization reaction to form the interloop disulfide bond Cys-41–Cys-130 in DsbB (the stable intermediate). The consequent thiolate of Cys-44(DsbB) interacts with ubiquinone in DsbB to form the electron transfer complex (7, 13, 14). Then, DsbA in the complex is oxidized by the intramolecular attack of Cys-33 on Cys-30 of the Cys-30–Cys-104 disulfide bond and is released (pathway II, referred to as the slow pathway) (8, 13). These reaction models are still currently under discussion (3). Although the structure of the DsbB-DsbA complex is known, the reaction pathway has not yet been elucidated because it involves an intermediate structure that is at the branching point of the two proposed reaction pathways. Thus, identification of the reaction pathway requires quantitative kinetic studies of the behavior of intermediates as key molecules.
Quartz crystal microbalance (QCM) devices can measure nanogram-scale mass changes based on the altered resonance frequency (ΔF) of a quartz-plate oscillator, which indicates mass changes on the plate (15–17). Oscillation of QCMs in aqueous solutions has been applied to nonlabeling biosensors that can detect biomolecular interactions as mass changes on the sensor surface in real time (18). We previously used this technique to detect various biomolecular interactions, such as DNA-DNA, DNA-peptide, and peptide-peptide interactions using a ligand-immobilized quartz plate (19–21). Recently, enzyme reactions, such as DNA polymerase reactions (22), DNA cleavage reactions (23), and protease reactions (24, 25), were carried out on substrate-immobilized QCM plates, allowing the formation and decomposition of the enzyme-substrate complex (intermediate) to be monitored as a detectable mass change. In addition, transient kinetic analysis could be conducted on the basis of the behavior of the intermediate (24, 25).
In this paper we have described the immobilization of a membrane protein, DsbB, embedded in supported lipid bilayers on a QCM plate and the detection of an intermediate complex between DsbASH,SH and DsbBS-S,S-S according to mass changes on the QCM device (Fig. 2). A QCM with a sensor surface consisting of a planar gold electrode was used for immobilization of DsbB membrane proteins in supported lipid bilayers to obtain kinetic parameters under nearly native conditions rather than in surfactant-solubilized DsbB in bulk solutions. Three kinetic parameters were measured, the formation and reverse rate constants (kf and kr, respectively) of the intermolecular disulfide bond in the DsbA-DsbB intermediate and the release rate constant (kcat) of oxidized DsbAS-S from reduced DsbBS-S,SH,SH in the membrane. These kinetic parameters were compared with those of the mutated variants DsbA(C33A)SH, DsbA(C30A)SH, and DsbB(C41A,C44A)S-S in the presence or absence of ubiquinone. Based on the stability of the DsbA-DsbB intermediate estimated by the obtained kinetic parameters, we concluded that DsbBS-S,S-S mainly oxidized DsbASH,SH in a ubiquinone-independent manner through a stable intermediate in which delocalization of the transferred electron on cysteine residues of DsbB was important (modified pathway II).
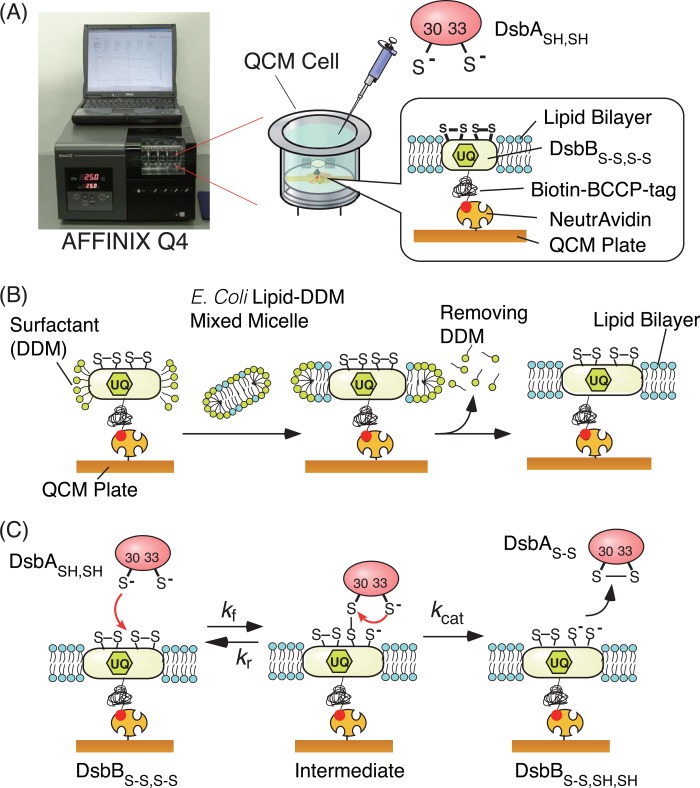
FIGURE 2.
Schematic illustrations of the QCM experimental setup. A, a 27-MHz QCM device (AFFINIX Q4) for observation of the thiol-disulfide exchange reaction of reduced DsbASH,SH with biotin-BCCP-DsbBS-S,S-S embedded in a lipid bilayer on a NeutrAvidin-modified QCM plate. B, preparation of a supported lipid bilayer around biotin-BCCP-DsbBS-S,S-S immobilized on a NeutrAvidin-modified QCM plate. C, kinetic parameters (kf, kr, and kcat) measured in this work.
EXPERIMENTAL PROCEDURES
Materials
PCR primer oligonucleotides were purchased from Operon Biotechnologies (Tokyo, Japan). The pET22b(+) vector for protein expression in E. coli was purchased from TaKaRa Bio Inc. (Shiga, Japan). PinPoint Xa-1 vector was obtained from Promega KK (Tokyo, Japan). n-Dodecyl-β-d-maltoside (DDM) and 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide were purchased from Dojindo Laboratories (Kumamoto, Japan). Isopropyl-β-d-thio-galactopyranoside, N-hydroxysuccinimide, phenylmethylsulfonyl fluoride (PMSF), and ubiquinone-10 were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). NeutrAvidin, Zeba Desalt Spin Column, and tris(2-carboxyethyl)phosphine were obtained from Thermo Fisher Scientific Inc. (Yokohama, Japan). The E. coli total lipid extract was obtained from Avanti Polar Lipids Inc. (Alabaster, AL). 4-Acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) was purchased from Invitrogen. Unless otherwise noted, all other reagents were purchased from Nacalai Tesque Co. (Kyoto, Japan) and used without further purification.
Preparation of DsbASH,SH and Its Variants
A 627-bp fragment encoding DsbA (EC 5.3.4.1) was amplified from the genomic DNA of E. coli JM109 (K12 wild-type strain) by PCR with the primers 5′-GGAGATATACATATGAAAAAGATTTGGCTGGCGCTG-3′ and 5′-GAGCTCGAATTCTTATTTTTTCTCGGACAGATATTTCAC-3′ and cloned into pET22b(+) through the restriction sites NdeI and EcoRI. Two vectors for variant expression of DsbA(C33A)SH and DsbA(C30A)SH were prepared using the QuikChange Site-directed Mutagenesis kit (Agilent Technologies Japan, Tokyo, Japan). The constructed vectors were verified by dideoxy sequencing.
DsbASH,SH, DsbA(C33A)SH, and DsbA(C30A)SH proteins were expressed and purified according to a previously reported method, with minor modifications (26). E. coli JM109 (DE3) harboring each expression vector was grown in 1 liter of LB broth with ampicillin at 26 °C, and then 1 mm isopropyl-β-d-thio-galactopyranoside was added into the medium for protein induction at an A600 of 1.0 followed by additional incubation for 16 h. The cells were harvested and then suspended in 20 ml of a buffer (200 mm boric acid-NaOH, pH 8.0, 160 mm NaCl, 5 mm EDTA) to allow conversion of the cells into spheroplasts. The suspension was stirred on ice for 2 h and centrifuged (48,000 × g, 30 min, 4 °C) to remove the spheroplasts, and the collected supernatant containing the periplasmic contents was repeatedly dialyzed against 2 liters of Buffer A (10 mm MOPS-NaOH, pH 7.0). The solution was added onto a 5-ml DEAE FF anion exchange column (GE Healthcare), and target proteins were obtained by elution with a linear gradient of 0–500 mm NaCl in A buffer. Typically, 10 mg of purified DsbA per 1 liter of LB broth was obtained and stored at −80 °C until use.
Preparation of Biotin-BCCP-DsbBS-S,S-S and Its Variant
An amplified fragment encoding DsbB (EC 1.8.4.2) was obtained in the same manner as DsbASH,SH by PCR with the primers 5′-GGAGATATACATATGTTGCGATTTTTGAACCAATGTTCACAAGGC-3′ and 5′-GAGCTCGAATTCTTAGCGACCGAACAGATCACGTTTTTTCGC-3′ and cloned into pET22b(+) through the restriction sites NdeI and EcoRI. The codons for the two nonessential cysteine residues, Cys-8 and Cys-49, were then replaced by alanine and valine codons, respectively, using the QuikChange method to prevent disulfide-linked oligomerization (7, 8). A fragment encoding the biotin carboxyl carrier protein (BCCP) tag was PCR-amplified from Pinpoint Xa vector and then inserted into the XhoI-HindIII digested vector coding the improved DsbB by using In-Fusion Advantage PCR Cloning Kit (Takara Bio Inc., Shiga, Japan). A vector for the double-mutated DsbB(C41A,C44A)S-S protein was prepared by the QuikChange method.
Biotin-BCCP-DsbBS-S,S-S and biotin-BCCP-DsbB(C41A, C44A)S-S proteins were also expressed and purified as described previously (12, 27). E. coli C41 (DE3) harboring each expression vector was grown in 3 liters of LB broth with ampicillin and biotin at 37 °C, and then 15 μm isopropyl-β-d-thio-galactopyranoside was added into the medium for protein induction at an A600 of 0.6 followed by the additional incubation for 4 h. The cells were harvested and suspended in a buffer (50 mm Tris-HCl, pH 8.0, 300 mm NaCl, 1 mm PMSF) and then passed through a French press. After precentrifugation for 30 min at 10,000 × g to remove untreated cells, the cell lysate was centrifuged for 1 h at 100,000 × g to collect membrane fractions as a pellet. The membrane pellet was homogenized in a Dounce homogenizer and passed through a syringe attached to a needle (0.7 × 50 mm). After the DDM solution was added to the suspension at a final concentration of 1%, the suspension was ultracentrifuged (100,000 × g, 30 min) to remove insoluble membrane fractions. The supernatant was loaded on a 5-ml nickel-nitrilotriacetic acid column (GE Healthcare) equilibrated with a buffer (50 mm phosphate, pH 8.0, 300 mm NaCl, 0.1% DDM), and the target protein was eluted from the column by a 0–0.5 m imidazole gradient at pH 6.0. The collected protein was additionally purified on a gel filtration column (Superdex 200 pg, GE Healthcare) in Buffer B (50 mm phosphate, pH 6.0, 300 mm NaCl, 0.1% DDM) and stored at −80 °C until use.
Setup of 27-MHz QCM and Immobilization of Biotin-BCCP-DsbB on a NeutrAvidin-modified QCM Plate
An AFFINIX Q4 system used as the QCM instrument (Initium Co. Ltd., Tokyo, Japan) is shown in Fig. 2A. The QCM instrument has four 500-μl cells equipped with a 27-MHz QCM plate (60-μm-thick and 8.7-mm-diameter AT-cut shear-mode quartz plate and gold electrode with a 5.7 mm2 area) at the bottom of each cell, a stirring bar, and a temperature controlling system (23–25). NeutrAvidin (60 kDa) was immobilized covalently on the QCM plate as described previously (19, 20, 23). Briefly, 3,3′-dithiodipropionic acid was immobilized on a cleaned bare gold electrode, and then carboxylic acids were activated as N-hydroxysuccinimidyl esters on the surface using 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide. NeutrAvidin was reacted with the activated esters by mounting aqueous solutions on the QCM plate. The binding behaviors of biotin-BCCP-DsbBS-S,S-S to the NeutrAvidin-immobilized QCM were followed in DDM(+) buffer (50 mm HEPES-NaOH, pH 7.5, 100 mm NaCl, 0.1% DDM at 25 °C) as frequency changes (ΔFwater) with time. When the ΔFwater value corresponding to an amount of an anchored biotin-BCCP-DsbBS-S,S-S solubilized with DDM reached −2000 Hz (a predetermined value), 10 μm free biotin was added to regulate the immobilization amount. At −2000 Hz, the amount of biotin-BCCP-DsbBS-S,S-S solubilized with DDM was estimated to be 560 ng·cm−2 (16 pmol cm−2) based on a calibration value of 0.28 ng·cm−2·Hz−1 (28–30). The relationship between frequency changes and mass changes for calibration is described elsewhere (28–30). The coverage of immobilized biotin-BCCP-DsbBS-S,S-S was calculated to be ∼68% with 7.1 nm2 molecule−1 of DsbBS-S,S-S area on the QCM electrode (5.7 mm2).
Preparation of DsbB Embedded in Lipid Bilayers on a QCM Plate
The procedure for DsbB preparation is illustrated in Fig. 2B. A 200-μl chloroform solution of total lipid extracts from E. coli (10 mg/ml) was evaporated in a recovery flask under light shielding. In the preparation of lipid bilayers containing excess ubiquinone-10, a mixed solution of the above-mentioned lipid extracts and a 200-μl chloroform solution of ubiquinone-10 (1.45 mg/ml, corresponding to 11 mol%) was used. A mixed micelle solution of total lipids from E. coli and DDM was prepared by the addition of 398 μl of DDM(+) buffer (50 mm Tris-HCl, pH 7.5, 150 mm KCl, and 0.44% DDM) into the flask. After immobilization of biotin-BCCP-DsbBS-S,S-S in 500 μl of DDM(+) buffer onto a NeutrAvidin-modified QCM plate by the above-mentioned procedure, 450 μl of DDM(+) buffer was replaced by 20 μl of the mixed micelle solution and 430 μl of DDM(−) buffer (50 mm HEPES-NaOH, pH 7.5, 100 mm NaCl), resulting in a final DDM concentration of 0.028%, which was slightly higher than the critical micelle concentration of DDM (0.01%). After incubating for 1 h to form a lipid bilayer around the immobilized DsbBS-S,S-S, the remaining DDM was removed from the QCM cell by replenishing the DDM(−) buffer several times. Finally, the QCM cell was filled with 500 μl of a reaction buffer (50 mm citrate, pH 6.0, with 100 mm NaCl), which was adjusted to the optimum pH of the reaction (12). Immobilization of biotin-BCCP-DsbB(C41A,C44A)S-S was also performed in the same manner.
Observation of the DsbASH,SH Oxidation Reaction on the DsbBS-S,S-S-immobilized QCM Plate
DsbA was reduced with 30 mol eq of tris(2-carboxyethyl)phosphine for 30 min, and reduced DsbASH,SH was then recovered using desalting spin columns just before the QCM experiments. The DsbBS-S,S-S-immobilized QCM was thermally pre-equilibrated at 25 °C until the resonance frequency was held constant (± 1 Hz over 15 min), and frequency changes in response to the addition of DsbASH,SH or DsbA variants were then measured over time.
Observation of the DsbASH,SH Oxidation Reaction on DsbBS-S,S-S in the Absence of Endogenous Ubiquinone on a QCM Plate
The DsbBS-S,S-S-immobilized QCM cell was prepared according to the above-mentioned procedure and was filled with 500 μl of a reaction buffer (50 mm citrate, pH 6.0, 100 mm NaCl) at 25 °C. An excess amount of reduced DsbASH,SH (400 nm) was added into the DsbBS-S,S-S-immobilized QCM cell and incubated for 30 min to completely reduce DsbBS-S,S-S and endogenous ubiquinone. Then the QCM plate was incubated for 2 h with redox buffer (50 mm phosphate, pH 8.0, 100 mm NaCl, 100 mm oxidized DTT, and 1 μm reduced DTT) that had a redox potential (−162 mV) between that of DsbBS-S,S-S (−224 to −207 mV) and ubiquinone (+110 mV) to oxidize only DsbBS-S,SH,SH but not ubiquinol (UQ2−) (31). The QCM cell containing oxidative DsbBS-S,S-S and ubiquinol was filled with 500 μl of reaction buffer, and frequency changes in response to the addition of DsbASH,SH or the DsbA(C33A)SH variant were followed over time.
Quantification of Oxidized DsbAS-S by SDS-PAGE
In a 0.5-ml tube, 17 μm tris(2-carboxyethyl)phosphine-reduced DsbASH,SH was incubated with 34 μm surfactant-solubilized DsbBS-S,S-S or the DsbB(C41A,C44A)S-S variant in 100 mm citrate, pH 6.0, with 0.1% DDM for 3 min at room temperature. DsbA was recovered by trichloroacetic acid precipitation into a pellet, which was then dissolved with 50 mm Tris-HCl, pH 8.0, containing 1% SDS. The free thiol of the unreacted DsbASH,SH was modified with AMS (32). Oxidized DsbAS-S (21 kDa) and AMS-derivatized DsbA (22 kDa, the unreacted DsbA) were separated on 12% nonreducing gels by SDS-PAGE and identified using the Gel Doc EZ System (Bio-Rad) after staining with Coomassie Brilliant Blue.
Kinetic Analysis of DsbA-DsbB Reactions on Supported Bilayers
The thiol-disulfide exchange reaction of DsbASH,SH with DsbBS-S,S-S is described by Equation 1. At time t, the mass of the reaction intermediate of the DsbA-DsbB complex is given by Equations 2 and 3, where [DsbA]0 is the initial concentration of reduced DsbASH,SH, [DsbB]0 is the initial concentration of oxidized DsbBS-S,S-S, [DsbA-DsbB] is the concentration of the DsbA-DsbB intermediate, Mw is the molecular weight of DsbASH,SH, and [DsbA]0 ≫ [DsbB]0,
 |
where
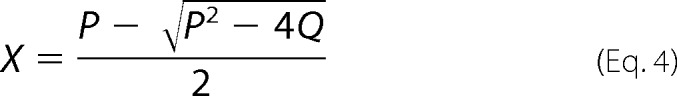 |
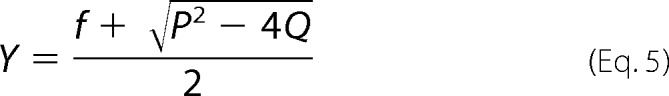 |
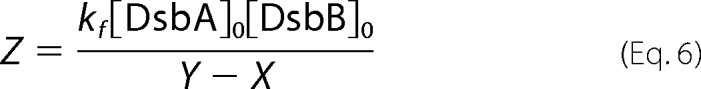 |
We followed the formation and degradation of the DsbA-DsbB intermediate on the QCM as frequency (mass) changes over time (curve a in Fig. 3A). Then we excluded nonspecific binding (curve b in Fig. 3A) from the ladle-shaped curve before curve-fitting. The formation and reverse rate constants (kf and kr, respectively) of the intermediate and the release rate constant (kcat) were obtained from curve fittings on the basis of Equation 3. More detailed information about transient kinetic analysis on a QCM has been described in previous studies (24, 25).
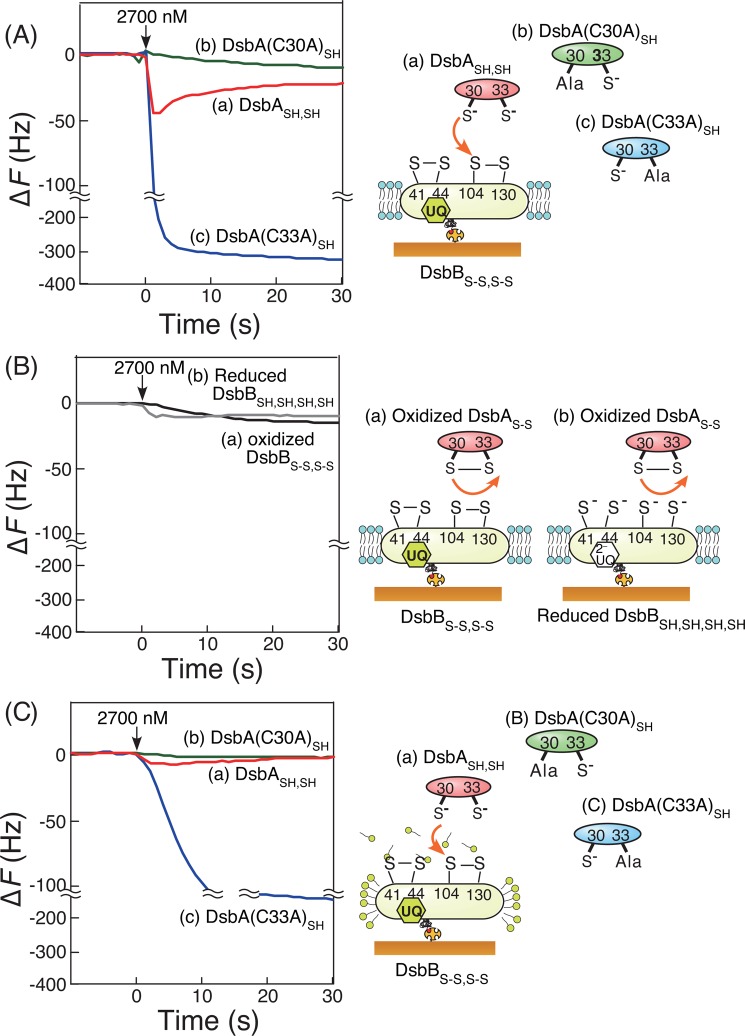
FIGURE 3.
Typical time courses of frequency decreases (mass increases) in QCM experiments. Shown are the reaction of DsbBS-S,S-S embedded in supported bilayers (A), reaction of DsbBS-S,S-S (a) and the reduced DsbBSH,SH,SH,SH (b) embedded in supported bilayers in response to the addition of the oxidized DsbAS-S (B), and DsbBS-S,S-S covered with DDM micelles, responding to the addition of DsbASH,SH (a), DsbA(C30A)SH (b), and DsbA(C33A)SH (c), respectively (C). All experiments were carried out under the same conditions (50 mm citrate, pH 6.0, and 100 mm NaCl at 25 °C, [DsbA] = 2700 nm in 500 μl, and [DsbB] = 16 pmol/cm2 on the QCM plate).
The DsbA(C33A)SH variant could form the DsbA-DsbB intermediate with DsbBS-S,S-S but could not proceed to the oxidization step. The formation of an intermediate between the DsbA(C33A)SH variant and DsbBS-S,S-S is described by Equation 9. At time t the mass of the intermediate is given by Equations 10–12, as previously described (20).
 |
By following the formation of the DsbA(C33A)SH-DsbB intermediate over time on the QCM, the relaxation time (τ) was obtained from curve fittings of the simple QCM frequency decrease (mass increase) on the basis of Equation 11. When binding experiments were carried out at various concentrations of the DsbA(C33A)SH variant to obtain each relaxation time, the formation and reverse rate constants (kf and kr, respectively) of the DsbA(C33A)SH variant for DsbBS-S,S-S were obtained from the slope and intercept of the plot for Equation 12, respectively. The parameter corresponding to the dissociation constant (Kd) was calculated from the ratio of kr to kf. Kinetic analysis of DsbA(C33A)SH with DsbB(C41A,C44A)S-S variants was also carried out in the same manner.
RESULTS
Construction of DsbB-embedded Supported Lipid Bilayers on QCM
In vitro experiments on membrane proteins, the activities of hydrophobic membrane proteins are usually measured in a surfactant-solubilized form (6, 11, 13, 33). In the presence of a surfactant, however, enzymatic activity may be reduced, due to the creation of an unnatural environment for the membrane protein. Although using a proteoliposome is another method for solubilizing membrane proteins in solution, it is unsuitable for quantitative measurements due to the small and unclear amounts of active membrane proteins in bulk solutions. To observe and quantify thiol-disulfide exchange reactions between soluble DsbASH,SH and membrane-embedded DsbBS-S,S-S, we examined the construction of DsbBS-S,S-S-embedded supported lipid bilayers on a QCM plate (Fig. 2). We first prepared biotin-BCCP-DsbB using an E. coli protein expression system as a fusion protein consisting of the BCCP tag, which included a region biotinylated by an endogenous biotin ligase in E. coli. Biotin-BCCP-DsbB was immobilized on a NeutrAvidin-modified QCM plate through biotin-avidin linking in the presence of DDM. This immobilization method has the advantage of regulating the amount of protein bound onto the QCM and the unidirectional orientation of membrane proteins (Fig. 2B). Next, lipid bilayers were formed around the biotin-BCCP-DsbB immobilized on the QCM plate by the addition of lipid-surfactant-mixed micelles followed by slow removal of the surfactants. We observed frequency decreases (mass increases) due to the binding of the mixed micelle around the DsbB (data not shown). After saturation of the frequency decrease, the buffer was replaced by a buffer without DDM several times to achieve a lower DDM concentration than the critical micelle concentration of DDM. To confirm the formation of lipid bilayers on the QCM plate, we also prepared a lipid bilayer on the QCM plate in the same manner by using a mixture of E. coli lipid extracts, DDM, and biotinylated lipid (5 mol%), and we subsequently observed the binding of avidin onto the biotinylated lipid bilayer (data not shown). The amount of bound avidin was consistent with the amount of avidin adsorbed onto a planar substrate, such as the gold electrode of a QCM, indicating that planar lipid bilayers around DsbB were formed on the QCM plate.
Observation of Reactions between DsbA and DsbB
Fig. 3A indicates typical frequency changes as a function of time for 27-MHz QCM on which DsbBS-S,S-S embedded in lipid bilayers was immobilized, in response to the addition of native DsbASH,SH, DsbA(C30A)SH, and DsbA(C33A)SH variants. After the addition of DsbASH,SH, the frequency decreased (i.e. the mass increased) rapidly and then increased gradually to return to the original frequency (curve a in Fig. 3A). This behavior corresponds to the formation of the DsbA-DsbB intermediate followed by the release of oxidized DsbAS-S from reduced DsbBS-S,SH,SH in the membrane (Fig. 2C). When the DsbA(C30A)SH variant was added onto the DsbBS-S,S-S-immobilized QCM plate, only a very slight change was observed (curve b in Fig. 3A), indicating that Cys-30 of DsbA was essential for the formation of the DsbA-DsbB intermediate. This is consistent with x-ray crystallography reports demonstrating that a disulfide bond is formed between Cys-30 of DsbASH,SH and Cys-104 of DsbBS-S,S-S (Fig. 2C) (3). We also confirmed that oxidized DsbAS-S could not interact with DsbBS-S,S-S or reduced DsbBSH,SH,SH,SH on the QCM (curves a and b in Fig. 3B). When the DsbA(C33A)SH variant was added, it could bind to, but not be released from DsbBS-S,S-S (curve c in Fig. 3A). This indicated that Cys-30 of DsbASH,SH formed an interprotein disulfide bond with Cys-104 of DsbBS-S,S-S and that Cys-33 of DsbASH,SH was essential for the oxidation of DsbASH,SH to allow it to be released from reduced DsbBS-S,SH,SH (Fig. 2C). Thus, we could detect the intermediate of the covalently bonded DsbA-DsbB complex on a DsbB-immobilized QCM.
We also confirmed that supported bilayer structures are important for formation of DsbA-DsbB intermediates. When DsbBS-S,S-S was covered with DDM micelles, the amounts and formation rates of DsbA-DsbB intermediates produced by the addition of DsbASH,SH (curve a in Fig. 3C) and DsbA(C33A)SH (curve c) were very small and slow compared with those shown in Fig. 3A. This clearly indicated that DDM inhibited interactions between DsbASH,SH and DsbBS-S,S-S and/or that lipid bilayers were required to activate DsbBS-S,S-S.
Importance of the Two Cysteine Residues (Cys-41 and Cys-44) of DsbBS-S,S-S for the Reaction with DsbASH,SH
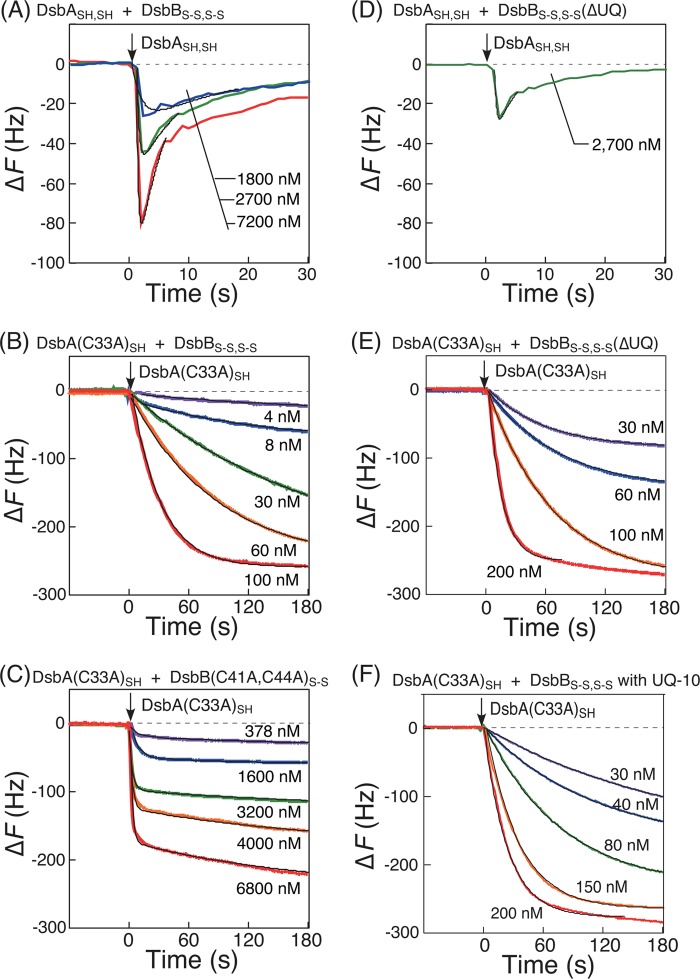
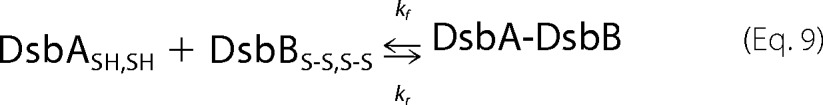
Previously, two potential models were proposed to represent the reaction pathway of the oxidation mechanism of DsbASH,SH by DsbBS-S,S-S as follows. 1) Oxidized DsbAS-S is released from the DsbA-DsbB intermediate before intraprotein isomerization in DsbB (attack of the reduced Cys-130 on Cys-41 of DsbB, pathway I in Fig. 1), or 2) oxidized DsbAS-S is released after the formation of the interloop Cys-41–Cys-130 disulfide bond of DsbB (pathway II). In the case of pathway I, only two cysteine residues (Cys-104 and Cys-130) of DsbB are involved in the formation of the DsbA-DsbB intermediate. For pathway II, four cysteine residues (Cys-41, Cys-44, Cys-104, and Cys-130) of DsbB are required for the formation of the DsbA-DsbB intermediate. To investigate the importance of these cysteine residues, we used the DsbB(C41A,C44A)S-S variant, which has only two cysteine residues (Cys-104 and Cys-130). As shown in Fig. 4A, both DsbASH,SH and the DsbA(C33A)SH variant formed only small amounts of the DsbA-DsbB intermediate when reacted with the DsbB(C41A,C44A)S-S variant under the same conditions as used in Fig. 3A. Meanwhile, when using a high concentration of the DsbA(C33A)SH variant, we found that the amount of the interprotein disulfide-bonded intermediate formed by DsbA(C33A)SH with DsbB(C41A,C44A)S-S was equal to that with DsbBS-S,S-S (Fig. 4B). These data indicated that the DsbB(C41A,C44A)S-S variant was not inactivated by Ala mutations and that the stability of the DsbA-DsbB(C41A,C44A)S-S intermediate was clearly lower than that of the DsbA-DsbB intermediate, which included all four DsbB cysteine residues.
FIGURE 4.
Effects of the two cysteine residues (Cys-41 and Cys-44) of DsbBS-S,S-S for the reaction with DsbASH,SH. A, typical time courses of frequency changes in the QCM on which DsbB(C41A,C44A)S-S embedded in supported bilayers was immobilized in response to the addition of DsbASH,SH (a) and DsbA(C33A)SH (b). The experimental condition was the same as that of reactions between DsbASH,SH and DsbBS-S,S-S in Fig. 3A. B, saturation curves of mass increases due to the intermediate formation between DsbA(C33A)SH and DsbBS-S,S-S (a) or DsbB(C41A,C44A)S-S (b) immobilized on a QCM. Maximum binding amounts (−ΔFmax) were calculated to be 321 ± 12 Hz for DsbBS-S,S-S and 392 ± 78 Hz for DsbB(C41A,C44A)S-S with experimental errors, respectively.
Effects of Ubiquinone in DsbBS-S,S-S on the Reaction with DsbASH,SH
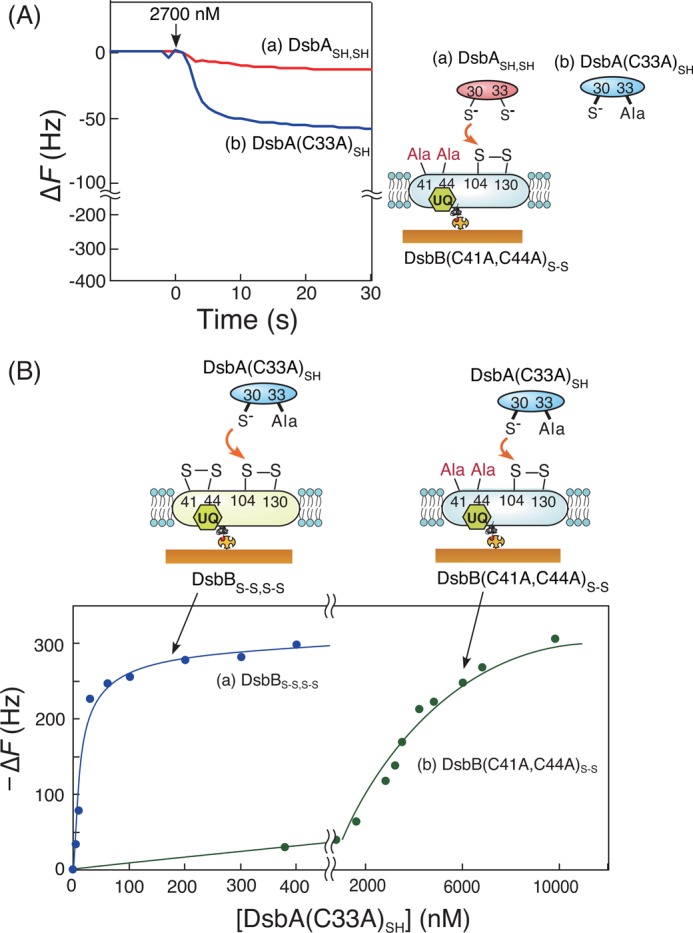
The intermediates of the covalently bonded DsbA-DsbB complex detected by QCM included electron-transfer complexes between DsbASH,SH and DsbBS-S,S-S. We predicted that ubiquinone, an acceptor of two electrons (and two protons) from reduced DsbBS-S,SH,SH in the DsbA-DsbB system, may play a key role in the formation of the stable intermediate as a one-electron-transfer complex. Ala mutations in the unstable DsbA-DsbB(C41A,C44A)S-S intermediate seemed to block electron transfer from DsbASH,SH to ubiquinone via DsbBS-S,S-S. To investigate whether ubiquinone contributes to the stability of the DsbA-DsbB intermediate, we observed the formation of the DsbA-DsbB intermediate in the presence of excessive ubiquinone-10 (UQ) that was added in the supported lipid bilayers (Fig. 5A) and in the absence of endogenous ubiquinone (Fig. 5B) using DsbBS-S,S-S with reduced ubiquinol (UQ2−). DsbBS-S,S-S(ΔUQ) (with UQ2−) was prepared as described under “Experimental Procedures.” Surprisingly, the formation curve of the DsbA-DsbB intermediate from the reaction of DsbA(C33A)SH and DsbBS-S,S-S was almost the same, independent of the redox state of ubiquinone (UQ or UQ2−; Fig. 5, A and B). Similar results were obtained using native DsbASH,SH. The plateau phase (3–10 s) found in curve a in Fig. 5A in the presence of excess UQ can be explained by the turnover of DsbASH,SH to DsbBS-S,S-S, recycled by UQ in supported lipid bilayers. These results implied that the formation of the DsbA-DsbB intermediate and the oxidation of DsbA should occur nearly independently of ubiquinone and that ubiquinone functions in the re-oxidation of DsbBSH,SH,S-S rather than DsbA.
FIGURE 5.
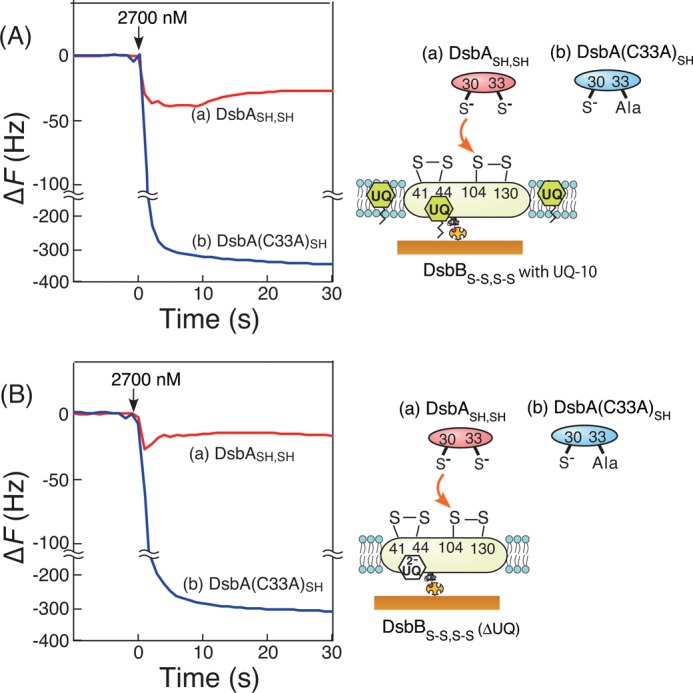
Effects of ubiquinone on time courses of frequency changes in the QCM experiments. Shown are reactions of DsbBS-S,S-S embedded in supported lipid bilayers including additional excessive UQ (A) and reactions of DsbBS-S,S-S that were coexistent with ubiquinol (UQ2−), responding to the addition of DsbASH,SH (a) and DsbA(C33A)SH (b), respectively (B). All experiments were carried out under the same conditions in Fig. 3A.
SDS-PAGE Analysis of Oxidation Products from Reactions between DsbA and DsbB in the Bulk Solution
Although we could follow the oxidation reaction of DsbASH,SH with DsbBS-S,S-S embedded in supported bilayers on the QCM as changes in frequency, we needed to confirm the actual products formed in this reaction. Therefore, we next performed nonreducing SDS-PAGE analysis for the reaction between DsbASH,SH and DsbBS-S,S-S in the DDM micellar solution. After reactions of DsbASH,SH with DsbBS-S,S-S or DsbB(C41A,C44A)SH in the bulk solution, AMS was added to modify the free thiol of the unreacted DsbASH,SH, as described in previous reports (32). As shown in lane 1 in Fig. 6, we confirmed that oxidized DsbAS-S appeared as the reaction product (reaction time = 3 min) at 21 kDa together with unreacted DsbASH,SH appearing as a reaction product with AMS (22 kDa). The oxidation was completed within 30 min (data not shown). In addition, very little oxidized DsbAS-S was found in the reaction with DsbB(C41A,C44A)S-S as compared with that found in the reaction with DsbBS-S,S-S (lane 2). This also suggested that the two additional cysteine residues (Cys-41 and Cys-44) in DsbBS-S,S-S were essential for achieving a good yield of oxidized DsbAS-S.
FIGURE 6.
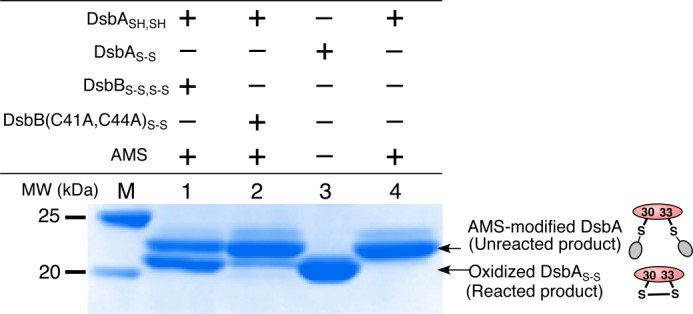
SDS-PAGE of DsbASH,SH and DsbBS-S,S-S or DsbB(C41A,C44A)S-S reaction products in the bulk solution with DDM micelles. Reactions were carried out in 100 mm citrate, pH 6.0, with 0.1% DDM for 3 min and terminated by the addition of trichloroacetic acid. Oxidized DsbAS-S (21 kDa) and AMS-modified DsbA (22 kDa) were identified on 12% nonreducing gels by SDS-PAGE after staining with Coomassie Brilliant blue. Lane M, molecular weight marker; lane 1, DsbASH,SH and DsbBS-S,S-S reaction products after AMS treatment; lane 2, DsbASH,SH and DsbB(C41A,C44A)S-S reaction products after AMS treatment; lane 3, oxidized DsbAS-S as a reference; lane 4, the product of DsbASH,SH reacted with AMS as a reference.
Kinetic Analysis of Reactions between DsbA and DsbB
To assess the dynamic behavior of the formation of the DsbA-DsbB intermediate, we performed kinetic analysis of the time dependences of the ΔF curves. The ladle-shaped curve of the reaction between DsbASH,SH and DsbBS-S,S-S (curve a in Fig. 3A) is described by Equations 1–3 under “Experimental Procedures” (24, 25). Kinetic parameters, i.e. the formation and reverse rate constants (kf and kr, respectively) and the oxidation rate constant (kcat), were obtained from curve-fittings as optimized coefficients in these equations. Experimental curves, generated using different concentrations of DsbASH,SH (Fig. 7A) were fitted well with theoretical curves described by Equations 2 and 3. The kf and kcat values were obtained from one experimental curve, and the average of values at three different concentrations of DsbASH,SH in Fig. 7A were calculated. The obtained binding rate constants (kf = 5.9 × 105 m−1 s−1) and catalytic rate constants (kcat = 0.12 s−1) are summarized in Table 1. The kr value could not be accurately measured because it was negligibly smaller than the kcat value.
FIGURE 7.
Kinetic analyses of DsbA-DsbB reactions. A, curve fitting of experimental curves with different concentrations of DsbASH,SH (colored lines) and the calculated time dependence of the reaction between DsbASH,SH and DsbBS-S,S-S (black smooth lines) as shown in Equation 3 under “Experimental Procedures.” B, binding of DsbA(C33A)SH to DsbBS-S,S-S as a function of DsbA(C33A)SH concentration (4–100 nm). C, binding of DsbA(C33A)SH to DsbB(C41A,C44A)S-S as a function of DsbA(C33A)SH concentration (378–6,800 nm). D, curve fitting of an experimental curve with the calculated time dependence of the reaction between DsbASH,SH and DsbBS-S,S-S(ΔUQ) excluding endogenous ubiquinone. E, binding of DsbA(C33A)SH onto DsbBS-S,S-S(ΔUQ) excluding endogenous ubiquinone as a function of the DsbA(C33A)SH concentration (30–200 nm). F, binding behaviors of DsbA(C33A)SH onto DsbBS-S,S-S in the presence of excessive ubiquinone-10, depending on the DsbA(C33A)SH concentrations (30–200 nm). Fitted curves of experimental curves (colored lines) with the calculated time dependence of the reaction are indicated as black smooth lines. Reactions were performed in 50 mm citrate, pH 6.0, 100 mm NaCl at 25 °C.
TABLE 1.
Kinetic parameters of the thiol-disulfide exchange reaction between DsbA and DsbB embedded in supported bilayers on the 27-MHz QCM
The experimental conditions were 50 mm citrate, pH 6.0, 100 mm NaCl, 25 °C. Each kinetic parameter was obtained from Equations 2 and 3 and from 4–6 and is reported with experimental errors.
| DsbA | DsbB | kf | kr | Kd = kr/kf | kcat |
|---|---|---|---|---|---|
| 105m−1 s−1 | s−1 | nm | s−1 | ||
| DsbASH,SH | DsbBS-S,S-S | 5.9 ± 1.8 | 0.12 ± 0.04 | ||
| DsbA(C33A)SH | DsbBS-S,S-S | 2.3 ± 0.2 | 0.0020 ± 0.004 | 9 ± 18 | |
| DsbA(C33A)SH | DsbB(C41A,C44A)S-S | 0.5 ± 0.05 | 0.12 ± 0.02 | 2300 ± 447 | |
| DsbASH,SH | DsbBS-S,S-S(ΔUQ) | 7.8 ± 2.6 | 0.15 ± 0.06 | ||
| DsbA(C33A)SH | DsbBS-S,S-S(ΔUQ) | 3.6 ± 0.2 | 0.0012 ± 0.004 | 3 ± 10 | |
| DsbA(C33A)SH | DsbBS-S,S-S with UQ-10 | 1.8 ± 0.08 | 0.0024 ± 0.002 | 13 ± 11 |
The reaction between DsbA(C33A)SH and DsbBS-S,S-S or the DsbB(C41A,C44A)S-S variant showed simple binding behaviors (Figs. 3A and 4A) and is described by Equations 9–12 under “Experimental Procedures.” The relaxation time (τ) was calculated from curve-fittings of frequency changes at various concentrations, as shown in Fig. 7, B and C. The formation and reverse rate constants (kf and kr, respectively) for the intermediate were calculated from the slope and intercept of Equation 12 (Fig. 8). The parameter corresponding to the dissociation constants (Kd) was obtained as the ratio of kr to kf. The obtained kinetic parameters are summarized in Table 1. The kf value (2.3 × 105 m−1 s−1) obtained from the reaction of the DsbA(C33A)SH variant and DsbBS-S,S-S was close to kf = 5.9 × 105 m−1 s−1, which was obtained from the reaction of DsbASH,SH and DsbBS-S,S-S, suggesting that the DsbA(C33A)SH variant maintained almost the same activity as DsbASH,SH. Because the kf values of DsbASH,SH and the DsbA(C33A)SH variant were close, the kr value for DsbASH,SH, which could not be obtained (Table 1), was estimated to be similar to kr = 0.0020 s−1 for the DsbA(C33A)SH variant. The kf value of the reaction between DsbA(C33A)SH and the DsbB(C41A, C44A)S-S variant (0.5 × 105 m−1 s−1) was lower than the kf value of the reaction between the DsbA(C33A)SH variant and DsbBS-S,S-S (2.3 × 105 m−1 s−1; see Table 1). This may be explained by the unexpected effect of C41A and C44A mutations on the attack of DsbA on DsbB.
FIGURE 8.
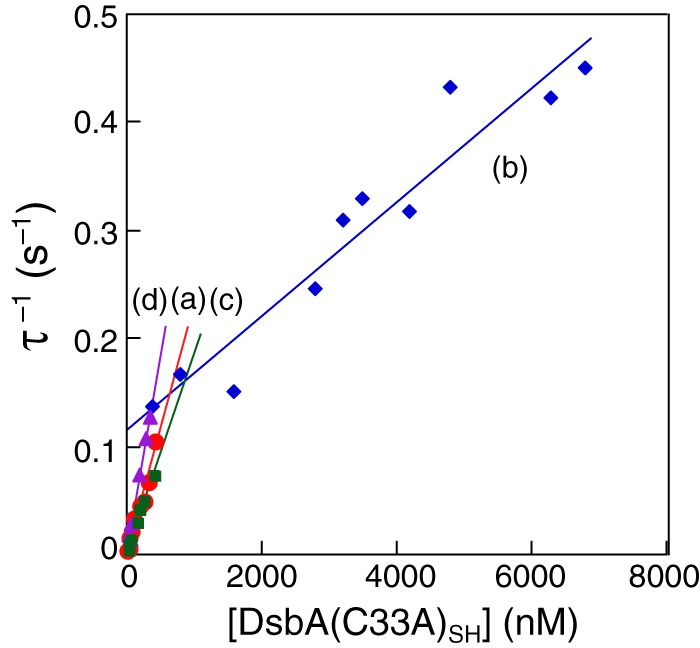
Liner reciprocal plots of the relaxation time (τ) versus DsbA(C33A)SH concentration according to Equation 12 in reactions with DsbBS-S,S-S including endogenous ubiquinone (from Fig. 7B) (a), DsbB(C41A,C44A)S-S (from Fig. 7C) (b), DsbBS-S,S-S including excessive ubiquinone-10 (from Fig. 7F) (c), and DsbBS-S,S-S(ΔUQ) excluding endogenous ubiquinone (from Fig. 7E) (d). Reactions were performed in 50 mm citrate, pH 6.0, with 100 mm NaCl at 25 °C.
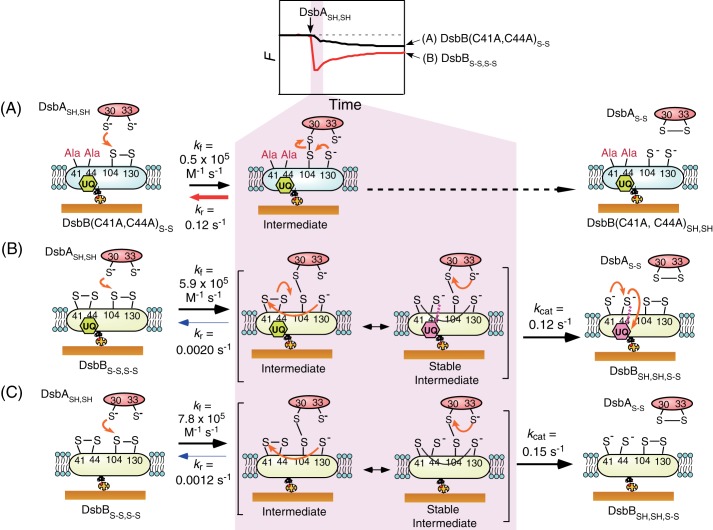
In addition, we also performed kinetic analysis of the reactions of DsbASH,SH or the DsbA(C33A)SH variant with DsbBS-S,S-S in the absence of endogenous ubiquinone (Fig. 7, D and E) and in the presence of excess ubiquinone-10 (Fig. 7F) in the same manner. The obtained kinetic parameters are also summarized in Table 1. Despite variance in the formation rate of the DsbA-DsbB intermediate (kf), these parameters indicated that kf values and the reaction rates of DsbASH,SH oxidation (kcat values) were mostly independent of ubiquinone, as compared with the effects of Ala mutations at Cys-41 and Cys-44 in DsbB on the reduction of kr values (Fig. 8). These kinetic values are also shown in the reaction scheme in Fig. 9.
FIGURE 9.
Reaction schematic and kinetic parameters. A, DsbASH,SH oxidation on DsbB(C41A,C44A)S-S. B, DsbASH,SH oxidation on DsbBS-S,S-S including endogenous ubiquinone. C, DsbASH,SH oxidation on DsbBS-S,S-S excluding ubiquinone.
DISCUSSION
In the thiol-disulfide exchange reactions of DsbASH,SH on DsbBS-S,S-S embedded in supported lipid bilayers bound to a QCM plate, we could observe the covalently bonded DsbA-DsbB intermediate in real time. This allowed us to obtain kinetic parameters of the reaction, including the formation and reverse rate constants (kf and kr, respectively) of the intermediate and the DsbA oxidation rate constant (kcat). Absorbance measurements based on the electronic states of ubiquinone in DsbB have previously been used to monitor reactions involving sequential electron transfers from DsbBS-S,S-S to ubiquinone. In contrast, the kinetic parameters obtained using our QCM method reflected electron transfer from DsbASH,SH to DsbBS-S,S-S, thereby providing additional information on this reaction step.
Comparison of Previous Absorbance Measurements with the Kinetic Parameters Obtained in the Current Study
Previous kinetic characterization of the oxidation reaction of DsbA with DsbB solubilized by DDM micelles was performed by stopped-flow absorbance measurements (13). Despite different experimental conditions, the kf value (5.9 × 105 m−1 s−1) obtained showing purple color, see Stable intermediate in Fig. 9B from the formation of the covalent DsbA-DsbB complex in this study was similar to the rate constant (∼5 × 105 m−1 s−1) of formation of the DsbB-ubiquinone complex (purple complex) in the process of electron transfer from DsbA to ubiquinone reported in the previous study (reaction conditions: 50 mm sodium phosphate, pH 8.0, 300 mm NaCl, 0.04% DDM at 10 °C) (13). This suggests that the rate-limiting step is likely the covalent binding of DsbA to DsbB. On the other hand, the rate of DsbA oxidation (∼2 s−1 at pH 8.0), which was found to be the same as the rate of quinone reduction in the previous work, is larger than the kcat value (0.12 s−1 at pH 6.0) obtained in the current work. This may be due to the higher pH used in the previous work, as the presence of the thiolate anion at Cys-33(DsbA) increases with increasing pH. We confirmed the similar kf value and increased kcat value at pH 8.0 in our other studies (34). Unlike absorbance measurements, where the solution condition requires a pH greater than 8.0 to obtain the purple complex of DsbB-ubiquinone by deprotonation of Cys-44(DsbB) (14), the current method allows us to perform experiments at the optimum pH for the specific reaction (here, pH 6.0) (12) (Fig. 9B).
The Mechanism of DsbA/DsbB Reaction Based on Kinetic Parameters
The destiny of the DsbA-DsbB intermediate can be determined by both the kr and kcat values (Fig. 8B). On comparing the first-order rate constants, kr and kcat, between DsbASH,SH and DsbBS-S,S-S, we found that the kcat value (0.12 s−1) was 60 times larger than the estimated kr value (0.0020 s−1). This suggested that the reaction of DsbASH,SH with DsbBS-S,S-S was a consecutive reaction. Moreover, the kr value in the reaction between DsbASH,SH and the DsbB(C41A,C44A)S-S variant (0.12 s−1) was also 60 times larger than the kr value in the reaction between DsbASH,SH and DsbBS-S,S-S (0.0020 s−1; Fig. 9A). These results clearly suggested that the stability of the DsbA-DsbB(C41A,C44A)S-S intermediate was largely reduced due to the lack of these two cysteine residues.
In the thiol-disulfide exchange reaction between DsbASH,SH and DsbBS-S,S-S, the interprotein disulfide bond of Cys-30 with Cys-104 is first formed by nucleophilic attack of Cys-30 of DsbASH,SH on Cys-104 of DsbBS-S,S-S. Then, reduced Cys-130 attacks Cys-104 or Cys-41 of the Cys-41–Cys-44 disulfide bond of DsbB. The attack on Cys-104 results in the reverse reaction, whereas the attack on Cys-41 results in the formation of the interloop Cys-41–Cys-130 disulfide bond in pathway II. The formation of the interloop Cys-41–Cys-130 prevents the reverse reaction caused by the attack of Cys-130 on Cys-104 as a stable intermediate. We found that the formation of the stable intermediate reduced the rate of the reverse reaction from kr = 0.12 s−1 to kr = 0.0020 s−1. This allowed the forward reaction to proceed (Fig. 9B). Thus, our data demonstrated that the thiol-disulfide exchange reaction between DsbASH,SH and DsbBS-S,S-S could occur efficiently through the stable intermediate, as indicated by pathway II.
Ubiquinone-independent Oxidation of DsbA on DsbB
We expected that ubiquinone in DsbB could contribute to the reduction of the reverse rate constant (kr) in the stable intermediate due to delocalization of the electrons transferred from DsbA because ubiquinone can interact with the Cys-44 thiolate anion produced by the cleavage of the Cys-41–Cys-44 disulfide bond of DsbB (14). However, we obtained kinetic parameters (kf, kr, and kcat) that were virtually unchanged in the presence or absence of ubiquinone. These data indicated that a stable intermediate could form between DsbASH,SH and DsbBS-S,S-S without ubiquinone and that the reaction step involving two electron transfers from DsbASH,SH to DsbBS-S,S-S could proceed even in the absence of ubiquinone. In fact, it has been reported that quinone-free DsbBS-S,S-S can produce oxidative DsbASH,SH (31). In the stable intermediate in pathway II, the interloop Cys-41–Cys-130, which prevents the reverse reaction, may be retained due to the reduced nucleophilicity of the thiolate anion at Cys-44(DsbB) by protonation below pH 6.6, the reported pKa of the thiol group of Cys-44(DsbB) (14) instead of electron transfer to ubiquinone. In other words, the stable separation of a transferred electron from DsbASH,SH to Cys-44(DsbB) instead of ubiquinone should contribute to the stability of the DsbA-DsbB intermediate, resulting in the efficient oxidation of DsbASH,SH. Thus, DsbB has two functions in the DsbA-DsbB system; that is, DsbASH,SH oxidation and ubiquinone reduction. The optimum pH for the DsbASH,SH oxidation reaction by DsbBS-S,S-S is reported to be 5.5–6.0 (12). We expect that the oxidation of DsbASH,SH at the proper pH (i.e. pH 6.0) can proceed independently of the reduction of ubiquinone. We present this reaction model, referred to as modified pathway II, in Fig. 9C.
Pathway of the Oxidation Reaction of DsbA and DsbB
Next, we examined the possibility that the oxidation reaction proceeds through pathway I (Fig. 1). A small amount of oxidized DsbAS-S was found in the reaction between DsbASH,SH and the DsbB(C41A,C44A)S-S variant in SDS-PAGE experiments (Fig. 6), indicating that the oxidation reaction occurred without passage through the stable intermediate. This result also supported the hypothesis that DsbA oxidation can occur independent of ubiquinone (31). Given the kinetic parameters obtained in our current study, the forward reaction should become kinetically competitive with the reverse reaction (kr = 0.12 s−1 and kcat = 0.12 s−1) in the reaction mechanism. The ratio of the amount of product generated through pathways II and I can be estimated from a comparison of the rates of product formation (=kcat[DsbA-DsbB]). Because the existence of the stable intermediate reduced the kr value by 60-fold, we estimated that the ratio of the pathway I intermediate to the pathway II stable intermediate would be 1:60 (Fig. 1). This suggested that pathway II occurs ∼98% of the time, whereas pathway I occurs only ∼2% of the time. Therefore, we concluded that the thiol-disulfide exchange reaction between DsbASH,SH and DsbBS-S,S-S proceeds mainly through pathway II, which involves formation of the stable intermediate, and that pathway I may sometimes occur due to the more rapid attack of Cys-33 on Cys-30 than that of Cys-130 on Cys-104 in the intermediate step.
Driving Force of the Disulfide Bond Exchange Reaction between DsbA and DsbB
The thiol-disulfide bond exchange reaction between DsbASH,SH and DsbBS-S,S-S involves the transfer of two electrons (and two protons) from DsbASH,SH to DsbBS-S,S-S. The driving force of the reaction can be explained by differences between the redox potential values of DsbASH,SH (−120 mV) and ubiquinone (+110 mV) (6, 31, 33). Because the redox potentials of DsbBS-S,S-S (Cys-41–Cys-44: −207 mV, Cys-104–Cys-130 and Cys-41–Cys-130: −224 mV) are reductive toward DsbASH,SH, the oxidative power of ubiquinone was previously thought to be essential for the oxidation of DsbA. In our results, however, the effects of ubiquinone on the kinetic parameters of the formation of the DsbA-DsbB intermediate (one-electron transfer complex) and the oxidation of DsbA (two-electron transfer reaction) were not apparent. In addition, the DsbB(C41A,C44A)S-S variant lacking two cysteine residues to transfer the electron to ubiquinone could form the DsbA-DsbB intermediate (one-electron transfer complex) and could oxidize DsbASH,SH, although the reaction efficiency was low. These results imply that the oxidative power of ubiquinone is not a prerequisite for DsbA oxidation. This surprising consequence is consistent with a previous report demonstrating that quinone-free DsbBS-S,S-S and the DsbB(C41S,C44S)S-S variant could oxidize DsbASH,SH (31). To interpret the mechanism involved in ubiquinone-independent oxidation of DsbA, we present two possibilities; 1) the redox potentials of the disulfide bonds of DsbBS-S,S-S in the DsbA-DsbB complex are oxidative in comparison with DsbASH,SH, and 2) the electron transfer reaction from DsbASH,SH to DsbBS-S,S-S proceeds through a kinetically controlled pathway. We confirmed that reverse electron-transfer from reduced DsbBSH,SH,SH,SH to oxidized DsbAS-S could not occur on the QCM despite a favorable reaction in terms of the redox potential (curve b in Fig. 3B). Thus, our results supported that the thiol-disulfide bond exchange reaction between DsbASH,SH and DsbBS-S,S-S should proceed in a kinetically favorable direction.
Innovation
We demonstrated that the DsbA-DsbB intermediate could be directly detected using a QCM device on which DsbBS-S,S-S was embedded in supported lipid bilayers according to the ladle-type frequency curve of the thiol-disulfide exchange reaction between DsbASH,SH and DsbBS-S,S-S. The kinetic parameters (kf, kr, and kcat) obtained from in situ monitoring of the DsbA-DsbB complex intermediate allowed us to examine the reaction mechanism kinetically. Biomolecular reactions in vivo may be controlled not only thermodynamically but also kinetically. Thus, elucidation of the transient kinetics of this reaction intermediate broadens our understanding of the kinetics of biomolecular reaction mechanisms.
This work was supported by a grant-in-aid from the Japan Society for the Promotion of Science.
- DsbA
- disulfide bond formation protein A
- DsbB
- disulfide bond formation protein B
- AMS
- 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid
- BCCP
- biotin carboxyl carrier protein
- DDM
- n-dodecyl-β-d-maltose
- QCM
- quartz crystal microbalance
- UQ
- ubiquinone-10
- UQ−2
- ubiquinol.
REFERENCES
- 1. Watson J. D., Baker T. A., Bell S. P., Gann A., Levine M., Losick R. (2003) Molecular Biology of the Gene, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 2. Inaba K. (2009) Disulfide bond formation system in Escherichia coli. J. Biochem. 146, 591–597 [DOI] [PubMed] [Google Scholar]
- 3. Kadokura H., Beckwith J. (2010) Mechanisms of oxidative protein folding in the bacterial cell envelope. Antioxid. Redox Signal. 13, 1231–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sevier C. S., Kaiser C. A. (2002) Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 3, 836–847 [DOI] [PubMed] [Google Scholar]
- 5. Inaba K., Ito K. (2008) Structure and mechanisms of the DsbB-DsbA disulfide bond generation machine. Biochim. Biophys. Acta 1783, 520–529 [DOI] [PubMed] [Google Scholar]
- 6. Grauschopf U., Fritz A., Glockshuber R. (2003) Mechanism of the electron transfer catalyst DsbB from Escherichia coli. EMBO J. 22, 3503–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inaba K., Takahashi Y. H., Fujieda N., Kano K., Miyoshi H., Ito K. (2004) DsbB elicits a red shift of bound ubiquinone during the catalysis of DsbA oxidation. J. Biol. Chem. 279, 6761–6768 [DOI] [PubMed] [Google Scholar]
- 8. Kadokura H., Beckwith J. (2002) Four cysteines of the membrane protein DsbB act in concert to oxidize its substrate DsbA. EMBO J. 21, 2354–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inaba K., Murakami S., Suzuki M., Nakagawa A., Yamashita E., Okada K., Ito K. (2006) Crystal structure of the DsbB-DsbA complex reveals a mechanism of disulfide bond generation. Cell 127, 789–801 [DOI] [PubMed] [Google Scholar]
- 10. Malojcić G., Owen R. L., Grimshaw J. P., Glockshuber R. (2008) Preparation and structure of the charge-transfer intermediate of the transmembrane redox catalyst DsbB. FEBS Lett. 582, 3301–3307 [DOI] [PubMed] [Google Scholar]
- 11. Zhou Y., Cierpicki T., Jimenez R. H., Lukasik S. M., Ellena J. F., Cafiso D. S., Kadokura H., Beckwith J., Bushweller J. H. (2008) NMR solution structure of the integral membrane enzyme DsbB. Functional insights into DsbB-catalyzed disulfide bond formation. Mol. Cell 31, 896–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bader M., Muse W., Zander T., Bardwell J. (1998) Reconstitution of a protein disulfide catalytic system. J. Biol. Chem. 273, 10302–10307 [DOI] [PubMed] [Google Scholar]
- 13. Tapley T. L., Eichner T., Gleiter S., Ballou D. P., Bardwell J. C. (2007) Kinetic characterization of the disulfide bond-forming enzyme DsbB. J. Biol. Chem. 282, 10263–10271 [DOI] [PubMed] [Google Scholar]
- 14. Tang M., Sperling L. J., Berthold D. A., Nesbitt A. E., Gennis R. B., Rienstra C. M. (2011) Solid-state NMR study of the charge-transfer complex between ubiquinone-8 and disulfide bond generating membrane protein DsbB. J. Am. Chem. Soc. 133, 4359–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buttry D. A., Ward M. D. (1992) Measurement of interfacial processes at electrode surfaces with the electrochemical quartz crystal microbalance. Chem. Rev. 92, 1355–1379 [Google Scholar]
- 16. Janshoff A., Galla H. J., Steinem C. (2000) Piezoelectric mass-sensing devices as biosensors. An alternative to optical biosensors? Angew. Chem. Int. Ed. 39, 4004–4032 [DOI] [PubMed] [Google Scholar]
- 17. Marx K. A. (2003) Quartz crystal microbalance. A useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromolecules 4, 1099–1120 [DOI] [PubMed] [Google Scholar]
- 18. Becker B., Cooper M. A. (2011) A survey of the 2006–2009 quartz crystal microbalance biosensor literature. J. Mol. Recognit. 24, 754–787 [DOI] [PubMed] [Google Scholar]
- 19. Matsuno H., Furusawa H., Okahata Y. (2004) Kinetic study of phosphorylation-dependent complex formation between the kinase-inducible domain (KID) of CREB and the KIX domain of CBP on a quartz crystal microbalance. Chemistry 10, 6172–6178 [DOI] [PubMed] [Google Scholar]
- 20. Okahata Y., Kawase M., Niikura K., Ohtake F., Furusawa H., Ebara Y. (1998) Kinetic measurements of DNA hybridization on an oligonucleotide-immobilized 27-MHz quartz crystal microbalance. Anal. Chem. 70, 1288–1296 [DOI] [PubMed] [Google Scholar]
- 21. Okahata Y., Niikura K., Furusawa H., Matsuno H. (2000) A highly sensitive 27 MHz quartz-crystal microbalance as a device for kinetic measurements of molecular recognition on DNA strands. Anal. Sci. 16, 1113–1119 [Google Scholar]
- 22. Niikura K., Matsuno H., Okahata Y. (1998) Direct monitoring of DNA polymerase reactions on a quartz-crystal microbalance. J. Am. Chem. Soc. 120, 8537–8538 [DOI] [PubMed] [Google Scholar]
- 23. Takahashi S., Matsuno H., Furusawa H., Okahata Y. (2007) Kinetic analyses of divalent cation-dependent EcoRV digestions on a DNA-immobilized quartz crystal microbalance. Anal. Biochem. 361, 210–217 [DOI] [PubMed] [Google Scholar]
- 24. Furusawa H., Takano H., Okahata Y. (2008) Transient kinetic studies of pH-dependent hydrolyses by exo-type carboxypeptidase P on a 27-MHz quartz crystal microbalance. Anal. Chem. 80, 1005–1011 [DOI] [PubMed] [Google Scholar]
- 25. Furusawa H., Takano H., Okahata Y. (2008) Transient kinetic studies of protein hydrolyses by endo- and exo-proteases on a 27 MHz quartz-crystal microbalance. Org. Biomol. Chem. 6, 727–731 [DOI] [PubMed] [Google Scholar]
- 26. Wunderlich M., Glockshuber R. (1993) Redox properties of protein disulfide isomerase (DsbA) from Escherichia coli. Protein Sci. 2, 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bader M., Muse W., Ballou D. P., Gassner C., Bardwell J. C. (1999) Oxidative protein folding is driven by the electron transport system. Cell 98, 217–227 [DOI] [PubMed] [Google Scholar]
- 28. Furusawa H., Komatsu M., Okahata Y. (2009) In situ monitoring of conformational changes of and peptide bindings to calmodulin on a 27 MHz quartz-crystal microbalance. Anal. Chem. 81, 1841–1847 [DOI] [PubMed] [Google Scholar]
- 29. Furusawa H., Ozeki T., Morita M., Okahata Y. (2009) Added mass effect on immobilizations of proteins on a 27 MHz quartz crystal microbalance in aqueous solution. Anal. Chem. 81, 2268–2273 [DOI] [PubMed] [Google Scholar]
- 30. Ozeki T., Morita M., Yoshimine H., Furusawa H., Okahata Y. (2007) Hydration and energy dissipation measurements of biomolecules on a piezoelectric quartz oscillator by admittance analyses. Anal. Chem. 79, 79–88 [DOI] [PubMed] [Google Scholar]
- 31. Inaba K., Takahashi Y. H., Ito K. (2005) Reactivities of quinone-free DsbB from Escherichia coli. J. Biol. Chem. 280, 33035–33044 [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi T., Kishigami S., Sone M., Inokuchi H., Mogi T., Ito K. (1997) Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc. Natl. Acad. Sci. U.S.A. 94, 11857–11862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inaba K., Ito K. (2002) Paradoxical redox properties of DsbB and DsbA in the protein disulfide-introducing reaction cascade. EMBO J. 21, 2646–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yazawa K., Furusawa H., Okahata Y. (2013) Mechanism of thiol-disulfide exchange reactions between DsbA and DsbB over a wide pH range. Chem. Lett. 42, 241–243 [Google Scholar]