β-Cell dysfunction contributes to diabetes mellitus. Puri et al. show that deletion of the von Hippel-Lindau (Vhlh) gene is deleterious to canonical β-cell gene expression. Vhlh loss triggers erroneous expression of factors normally active in progenitor cells, including Sox9. β-Cell-specific expression of Sox9 results in diabetes mellitus. This study reveals that perturbed β-cell identity contributes to diabetes mellitus.
Keywords: β cell, dedifferentiation, diabetes mellitus, von-Hippel Lindau, hypoxia
Abstract
Precise functioning of the pancreatic β cell is paramount to whole-body glucose homeostasis, and β-cell dysfunction contributes significantly to diabetes mellitus. Using transgenic mouse models, we demonstrate that deletion of the von Hippel-Lindau (Vhlh) gene (encoding an E3 ubiquitin ligase implicated in, among other functions, oxygen sensing in pancreatic β cells) is deleterious to canonical β-cell gene expression. This triggers erroneous expression of factors normally active in progenitor cells, including effectors of the Notch, Wnt, and Hedgehog signaling cascades. Significantly, an up-regulation of the transcription factor Sox9, normally excluded from functional β cells, occurs upon deletion of Vhlh. Sox9 plays important roles during pancreas development but does not have a described role in the adult β cell. β-Cell-specific ectopic expression of Sox9 results in diabetes mellitus from similar perturbations in β-cell identity. These findings reveal that assaults on the β cell that impact the differentiation state of the cell have clear implications toward our understanding of diabetes mellitus.
The pancreatic β cell secretes insulin in response to elevated blood glucose levels. Insulin then serves as a signal to other tissues in the body to use glucose as a source of energy and clear it from the blood, and persistently high blood sugar levels are detrimental to organ health. Diabetes mellitus, an ever-growing disease, results from sustained elevated levels of glucose in the blood. Either as a result of an autoimmune assault or in combination with peripheral insulin resistance, it is now clear that defects in β-cell function are an integral part of diabetes mellitus (Kahn 2003; Prentki and Nolan 2006).
The concept of altered β-cell fate, or dedifferentiation, as a cause of diabetes mellitus is gaining prominence. β-Cell dedifferentiation is a known consequence of glucotoxicity (Jonas et al. 1999) and in mouse models is thought to occur secondary to insulin resistance, when the body fails to sufficiently compensate for the increased insulin demand. In a transgenic mouse model that ectopically activates the Hedgehog (Hh) signaling pathway in the β cell, cells appear to dedifferentiate, leading to glucose intolerance (Landsman et al. 2011). Depletion of a key transcription factor, Foxo1, in β cells also appears to impact β-cell fate upon exposure to an additional stress such as pregnancy or age, leading to diabetic phenotypes due to reduced insulin in the β cells (Talchai et al. 2012). These studies suggest the possibility that specific types of genetic or environmental stresses might perturb the identity of the β cell sufficiently to cause loss of insulin expression but not cell death. It is thus tempting to speculate that at least some forms of diabetes mellitus may result from such a loss of β-cell identity.
All metazoan cells require oxygen to survive, and the β cell is no exception. Thus, cells have in place a tightly regulated signaling pathway to adapt to hypoxia (reduced oxygen in the cellular environment) to allow the cells to survive until oxygen tension is restored to physiological levels. Interestingly, hypoxia is increasingly implicated as a contributor in the etiology of diabetes mellitus (Dotsch et al. 2006). Cellular response to hypoxia is regulated by the von Hippel-Lindau/hypoxia-inducible factor (VHL/HIF) axis (Kaelin 2008; Semenza 2013). Normoxia causes degradation of the HIF transcription complex via the proteasome in a VHL-dependent manner (Maxwell et al. 1999; Ivan et al. 2001; Kaelin 2005). Decreased oxygen causes stabilization of HIF-α, which dimerizes with HIF-β (aryl hydrocarbon receptor nuclear translocator [ARNT]) to activate numerous hypoxia-inducible genes, including vascular endothelial growth factor (Vegf), glucose transporter 1 (Glut-1), and erythropoietin (Epo). Deletion of murine Vhlh in pancreatic β cells perturbs glucose homeostasis, in which a switch from oxidative phosphorylation to glycolytic metabolism is manifested as severe glucose intolerance in young adult mice (Zehetner et al. 2008; Cantley et al. 2009; Puri et al. 2009). Further involvement of the hypoxia pathway in β-cell dysfunction is illustrated by abnormal expression of VHL/HIF components in prediabetic Zucker diabetic fatty (ZDF) rats and diabetic Goto-Kakizaki (GK) rats (Li et al. 2006; Lacraz et al. 2009; Puri et al. 2013). Down-regulation of the HIF pathway also appears to be detrimental to β-cell function (Cheng et al. 2010). Decreased Hif1β/ARNT was reported in islets obtained from type 2 diabetes (T2D) patients (Gunton et al. 2005). Furthermore, mice with β-cell-specific deletion of ARNT display abnormal glucose tolerance. Altogether, these observations clearly indicate a requirement for strict regulation of VHL/HIF signaling for normal β-cell function. We report that Vhlh deletion in pancreatic β cells adversely affects cellular identity, with the consequential inability of β cells to maintain systemic glucose homeostasis resulting in diabetes mellitus in aged animals. β Cells in diabetic Vhlh-deficient mice not only suppress expression of key β-cell markers but also express factors normally active in embryonic precursor cells. In fact, ectopic activation of one such factor, Sox9, in the β cell is sufficient to modify cellular identity. Sox9 has been shown to play an instrumental role during pancreas organogenesis and marks the multipotent progenitor population of cells that give rise to the pancreatic organ (Seymour et al. 2007). However, it is unclear what the role of this transcription factor is in the adult β cell. Our data provide support for the notion that perturbed β-cell identity contributes to disease and implicate novel roles for Vhlh and Sox9 in the adult β cell.
Results
Deletion of Vhlh in pancreatic β cells results in diabetes mellitus due to reduced insulin in islets
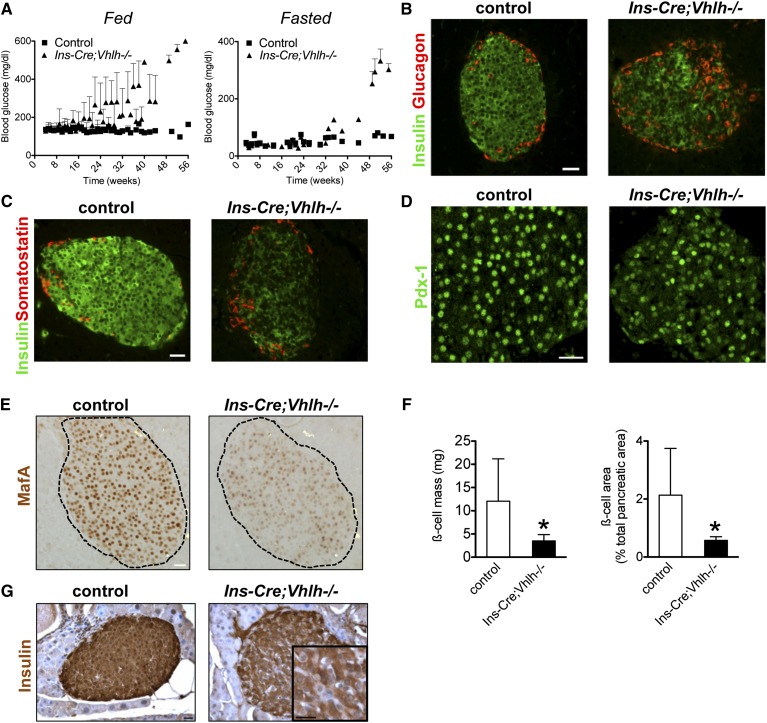
Previous research has established a role for Vhlh in the insulin secretory response of pancreatic β cells in young adult mice (Zehetner et al. 2008; Cantley et al. 2009; Puri et al. 2009). Significantly, glucose homeostasis in older transgenic mice with a β-cell-specific deletion of Vhlh deteriorated with age. A temporal analysis of fed and fasted blood glucose in Ins-Cre;Vhlh−/− (mice expressing Cre recombinase in β cells during embryogenesis) and control littermates revealed an exacerbation of the glucose intolerance in transgenic animals that was evident at 2–4 mo of age (Fig. 1A). With elevated blood glucose at 20 wk during the fed state that progressively increased to overt hyperglycemia, the Ins-Cre;Vhlh−/− model is reminiscent of the progression of T2D in patients. Fasted blood glucose levels were higher in Ins-Cre;Vhlh−/− animals after 32 wk, further illustrating that early episodes of hyperglycemia preceded full-blown disease (Fig. 1A). In two distinct transgenic mouse models, Ins-Cre;Vhlh−/− and Pdx-1-CreER;Vhlh−/− (for deletion in the adult β cells upon administration of tamoxifen at 8 wk of age), β-cell-Vhlh−/− mice older than 8 mo had significantly elevated blood glucose under fed and fasted conditions, indicative of frank diabetes mellitus (Supplemental Fig. S1A). As expected, hyperglycemic Ins-Cre;Vhlh−/− mice failed to respond to a glucose challenge, were consistently leaner than control littermates, and displayed insulin sensitivity comparable with control mice (Supplemental Fig. S1B–D). The absence of a compensatory response to the hyperglycemia was evidenced by diminished plasma insulin in Cre;Vhlh−/− animals (Supplemental Fig. S1E). Collectively, these data demonstrate that Vhlh deficiency in β cells results in diabetes mellitus due to reduced insulin.
Figure 1.
Vhlh loss in β cells leads to diabetes mellitus due to insufficient insulin. (A) Temporal analysis of fed and fasted blood glucose levels in control and Ins-Cre;Vhlh−/− animals. (B,C) Insulin immunoreactivity appears reduced in islets of Ins-Cre;Vhlh−/− mice as compared with control mice (green), while glucagon (B, red) and somatostatin (C, red) appear to maintain normal expression in both groups. The level of mature β-cell markers Pdx-1 (D) and MafA (E) was lower in Ins-Cre;Vhlh−/− islets, while robust expression was observed in control islets. (F) β-Cell mass and area in control (open bars; n = 5) and Ins-Cre;Vhlh−/− (black bars; n = 6) tissue at >10 mo. (*) P < 0.05. (G) Insulin immunostaining reveals nonstaining cells in Ins-Cre;Vhlh−/− tissue. The inset shows a higher-magnification image, with cells showing weak immunoreactivity to insulin. Bars, 20 μm (unless noted otherwise).
Examination of pancreatic islets from diabetic animals revealed a striking reduction in insulin immunoreactivity (Fig. 1B,C). Glucagon and somatostatin, although expressed, exhibited a disrupted organization distinct from the classic murine islet structure with a β-cell core surrounded by a mantle of other hormone-producing cells (Fig. 1B,C). Immunocytochemistry on tissue from diabetic animals further highlighted the stark reduction of mature β-cell markers Pdx-1 (Fig. 1D), MafA (Fig. 1E), Glut-2 (Supplemental Fig. S2A), and Nkx6.1 (Supplemental Fig. S2B) in Ins-Cre;Vhlh−/− islets. Dramatic reduction in the expression of canonical β-cell genes prompted quantification of β-cell mass in animals between 10 mo and 1 yr of age with overt hyperglycemia. β-Cell mass and area were significantly reduced in the Ins-Cre;Vhlh−/− samples (Fig. 1F). On closer examination, islets in diabetic animals had not only lower insulin reactivity as described above (Fig. 1G) but also increased numbers of insulin-negative cells (Fig. 1G, inset). Thus, it is possible that the true values for β-cell mass and area in the Ins-Cre;Vhlh−/− samples are substantially lower than our measurements indicate due to the quantification procedure, in which cells embedded within the islet that lacked insulin staining were included in calculation of the total islet area. The persistence of “empty,” insulin-negative cells and the broad disruption of gene expression observed in Ins-Cre;Vhlh−/− mice consequently suggests a loss of β-cell identity and not mass as the underlying cause of insulin insufficiency. The loss of mature markers in the β-cell lineage may signal the onset of cell death that would provide an explanation for the development of diabetes mellitus. Immunostaining for cleaved caspase-3, however, demonstrated no significant difference between Ins-Cre;Vhlh−/− and control tissue (Supplemental Fig. S2C), indicating that loss of insulin content is not due to sustained cell death in the diabetic islets.
Loss of β-cell identity in Vhlh−/− cells is concomitant with an activated progenitor program
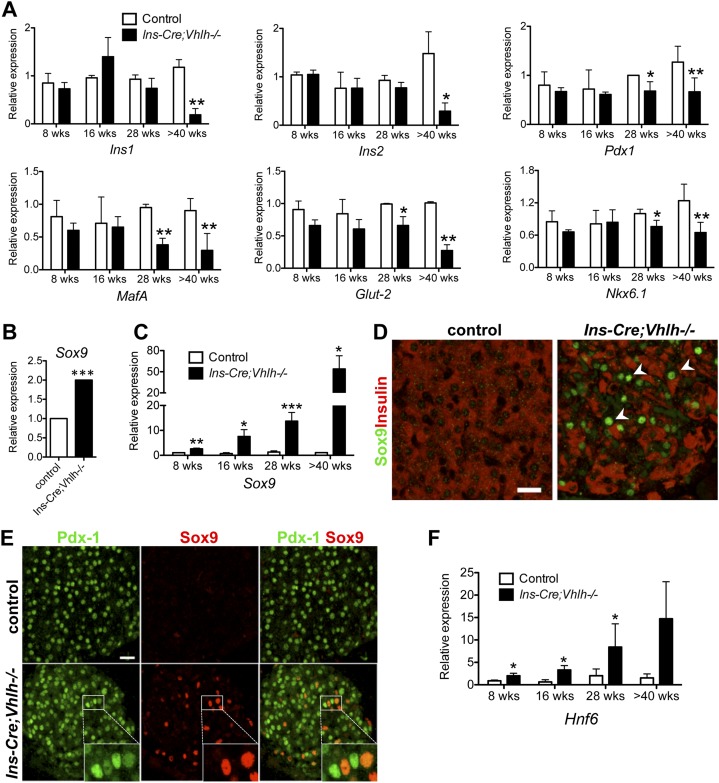
The depletion of β-cell markers in tissue from diabetic animals in the absence of cell death suggests that β cells are dedifferentiating into non-β cells. T2D is a progressive disease, with early episodes of unstable glycemic control that culminates into full-blown sustained hyperglycemia. If the loss of β-cell identity in the Vhlh−/− transgenic mouse model mimics the human condition, then a gradual loss of cellular function is expected. To determine whether this is indeed true, a temporal analysis of gene expression in isolated islets was conducted. A progressive reduction of β-cell-specific gene expression, including Ins1, Ins2, Pdx-1, MafA, Glut-2, and Nkx6.1, was detected, with several genes significantly reduced as early as 28 wk (Fig. 2A). Validating previously published data from 8- to 16-wk-old mice, significant up-regulation of HIF1α and the HIF target genes Glut-1, glyceradehyde phosphate dehydrogenase (Gapdh), lactate dehydrogenase A (Ldha), and monocarboxylate transporter 4 (Mct4) was detected in islets from aged diabetic animals (Supplemental Fig. S3A,B). Interestingly, MafB, a transcription factor expressed in β-cell progenitors but excluded from mature insulin-producing cells, had increased expression over time (Supplemental Fig. S3C).
Figure 2.
Vhlh loss in β cells results in dedifferentiation and activation of progenitor genes. (A) Quantitative PCR analysis of isolated islets confirmed reduced expression of β-cell markers in the Ins-Cre;Vhlh−/− (black bars; n = 2-6) compared with control (open bars; n = 2-7) animals. (*) P < 0.05; (**) P < 0.005. (B) Affymetrix gene array reveals up-regulation of Sox9 in islets isolated from Ins-Cre;Vhlh−/− animals as compared with controls (n = 3 for each group). (C) Progenitor marker Sox9 is up-regulated over time in diabetic Ins-Cre;Vhlh−/− islets (black bars, n = 3) as compared with controls (open bars, n = 3) as determined by quantitative PCR. (*) P < 0.05; (**) P < 0.005; (***) P < 10-4. (D) Immunofluorescence analysis of cells coexpressing Sox9 (green) and insulin (red) in Ins-Cre;Vhlh−/− islets clearly shows down-regulation of insulin in cells with nuclear accumulation of Sox9 (arrowheads). Control samples had no Sox9 staining, and insulin expression was unperturbed. (E) Colocalization of Pdx-1 (green) and Sox9 (red) indicates that in islets from Ins-Cre;Vhlh−/− animals, Sox9-positive cells (inset) have reduced intensity of Pdx-1 staining as compared with neighboring cells that do not express Sox9 (inset). (F) Hnf6 is progressively up-regulated in transgenic islets (black bars; n = 3) as compared with control samples (open bars; n = 3) as determined by quantitative PCR. (*) P < 0.05; (**) P < 0.005.
To assess gene dysregulation in the diabetic animals, differential gene expression arrays were quantified using RNA isolated from islets of diabetic and nondiabetic age-matched animals. Not surprisingly, the gene ontology (GO) analysis showed the most significant changes in metabolic enzymes that are targets of the hypoxia response pathway (Supplemental Tables S1, S3). Surprisingly, included in the next group of genes to be significantly dysregulated was the pancreatic progenitor marker Sox9 (Fig. 2B). Sox9 is expressed during embryonic pancreas formation but is restricted to ducts in the adult organ, with no detectable staining in islets (Lynn et al. 2007; Seymour et al. 2007). Quantitative PCR and immunostaining analyses validated the array results. A temporal increase in Sox9 expression was quantified in islets isolated from Ins-Cre;Vhlh−/− animals, with significantly increased expression as early as 8 wk of age (Fig. 2C). Immunostaining confirmed marked Sox9 up-regulation in pancreatic islets (Fig. 2D). Furthermore, costaining analysis revealed that cells with nuclear accumulation of Sox9 displayed a dramatic reduction in insulin (Fig. 2D, arrowheads; Supplemental Fig. S4A). Sox9-positive cells also had detectable yet reduced Pdx-1 staining (Fig. 2E). As expected, nuclear Sox9 was absent from control islets (Lynn et al. 2007; Seymour et al. 2007). In addition to costaining with Pdx-1, Sox9-positive cells also stained with E-cadherin, ruling out a mesenchymal nature of these cells (Supplemental Fig. S4A). In cell culture, Sox9 binds to a conserved upstream sequence of the Hnf6 gene to activate expression (Lynn et al. 2007), and a similar temporal trend of increased expression was observed in Hnf6 in islets isolated at distinct stages (Fig. 2F). Sox9 and Hnf6 are expressed in the multipotent progenitor cells of the expanding pancreatic epithelium during embryogenesis (between embryonic day 9 [E9] and E9.5) but become progressively restricted to the ductal compartment, with exclusion from adult islets (Rausa et al. 1997; Seymour et al. 2007). Sox9 expression (regulated by a gradient of Notch signaling) in bipotential duct/endocrine progenitor cells appears to be required for the specification of the endocrine lineage through an Ngn3-dependent step (Shih et al. 2012). Detectable expression of Sox9 and Hnf6 in islets of glucose-intolerant, but not yet diabetic, Ins-Cre;Vhlh−/− mice at 8 and 16 wk of age suggests that ectopic expression of Sox9 is initiated well before hyperglycemia onset. To further rule out a contribution from nonendocrine/ductal cells that may have migrated into the islet, pancreata from diabetic animals were stained with anti-synaptophysin (Supplemental Fig. S4B). Cells with Sox9 staining were positive for synaptophysin and showed reduced insulin staining, strengthening our conclusion that these cells are β-cell-derived. Interestingly, no change was detected in Ngn3 expression, a marker for embryonic endocrine progenitors (data not shown). Collectively, our results show aberrant activation of specific embryonic progenitor markers in β cells of diabetic Ins-Cre;Vhlh−/− mice.
Activation of embryonic genes within the islets of diabetic animals led us to examine whether an “embryonic program” exists in these islets. During embryogenesis, endocrine identity is regulated by concerted modulation of signaling pathways, including the Notch, Wnt, and Hh cascades (Puri and Hebrok 2010), and erroneous activation of these pathways impacts endocrine fate. In the adult pancreas, active Notch signaling is believed to be restricted to centro-acinar cells, although it has been postulated to be a mediator of β-cell dedifferentiation (Darville and Eizirik 2006). By quantitative PCR, Notch pathway components including Hes1, Hey1, Hey2, and HeyL were increased in Ins-Cre;Vhlh−/− islets, suggesting activation of the pathway (Fig. 3A; Supplemental Fig. S5A). Transcript up-regulation of canonical target genes Patched (Ptc) and Gli1 (Fig. 3A; Supplemental Fig. S5A) revealed dysregulated Hh signaling in islets from Ins-Cre;Vhlh−/− mice. Similarly, target genes of Wnt/β-catenin signaling, including Axin2, were up-regulated in Ins-Cre;Vhlh−/− islets (Fig. 3A). Perturbed immunostaining for β-catenin was observed in the Vhlh−/− tissue, the signal transducing protein in the canonical Wnt pathway (Fig. 3B). Control tissue displayed strong membranous β-catenin staining, while β cells in Vhlh−/− tissue exhibited dispersed cytoplasmic staining commonly associated with activation of the pathway (Fig. 3B; Morris et al. 2010). These data demonstrate erroneous activation of multiple developmental signaling pathways in the Vhlh−/− β cells that are normally strictly regulated.
Figure 3.
Embryonic signaling pathways are activated in Vhlh−/− islets. (A) Components of the Notch, Hh, and Wnt pathways are dysregulated in diabetic Ins-Cre;Vhlh−/− islets (black bars; n = 3) when compared with controls (open bars; n = 3). (*) P < 0.05; (**) P < 0.005. (B) Perturbed β-catenin staining in transgenic islets correlates with nuclear Sox9 accumulation. (C) Costaining for Sox9 (green) and somatostatin (red) reveals double-positive cells in Ins-Cre;Vhlh−/− islets that are absent in the control tissue. Bars, 20 μm (unless noted otherwise).
Recent literature hints toward the pliability of pancreatic endocrine cells to change fate into other hormone-expressing cell types (Collombat et al. 2009; Thorel et al. 2010; Yang et al. 2011). Thus, it was appropriate to ask whether β cells undergoing dedifferentiation were expressing other hormones. Sox9 was used as a marker for dedifferentiated β cells in Vhlh−/− tissues. No Sox9–glucagon or Sox9–PP double-positive cells were detected; however, we clearly observed somatostatin in a subset of Sox9-staining islet cells (Fig. 3C). Notably, Sox9–somatostatin-copositive cells were not observed in the control tissues. Costaining analysis demonstrated that Sox9–somatostatin-copositive cells were negative for insulin or glucagon (Supplemental Fig. S5B; data not shown). The mutually exclusive staining pattern of insulin and somatostatin further suggests that β-cell genes need to be suppressed in order for δ-cell marker expression to emerge. Although lineage-tracing analyses need to be conducted to conclusively prove the origin of these cells, current data support the hypothesis that sustained hypoxic stimulus converts β cells into a Sox9-expressing cell that can express somatostatin.
Acute hypoxia causes down-regulation of β-cell-specific genes in β-cell lines and isolated islets
To eliminate potential secondary influences present in whole animals, the effects of hypoxia were evaluated in rat and mouse insulinoma cell lines Ins-1 (832/13) and MIN6, respectively. Incubation in 2% oxygen for 24 h induced a robust hypoxic response, as evidenced by activation of canonical HIF target genes, including genes comprising the glycolytic pathway (Fig. 4A,B). Mimicking the in vivo situation, β-cell genes Ins-1 and Pdx-1 were significantly reduced in Ins-1 cells (Fig. 4C; Supplemental Fig. S6). Similarly, Ins-1, Pdx-1, Mafa, and Neurod1 were significantly diminished in MIN6 cells under hypoxia (Fig. 4D). In both cell lines, embryonic progenitor markers Sox9 and Hnf6 were up-regulated during hypoxia (Fig. 4C,D; Supplemental Fig. S6). Nuclear accumulation of Sox9 was detectable by immunostaining in Ins-1 cells (Fig. 4E, arrowheads).
Figure 4.
Acute hypoxia perturbs expression of important β-cell genes. (A,B) Quantitative analysis of gene expression after hypoxic exposure revealed significant changes in the hypoxia-regulated targets in Ins-1 and MIN6 cells. (*) P < 0.05; (**) P < 0.005; (***) P < 0.0005. (C,D) The expression of several β-cell genes and transcription factors Sox9 and Hnf6 was also perturbed in Ins-1 and MIN6 cells cultured under hypoxia. (*) P < 0.05; (**) P < 0.005; (***) P < 0.0005. (E) Ins-1 cells cultured in 20% or 2% oxygen and stained for the β-cell marker Nkx6.1 (red) and Sox9 (green) showed nuclear accumulation of Sox9 (arrowheads). Bar, 20 μm. (F,G) Wild-type mouse islets activated canonical HIF targets upon hypoxic incubation, with a significant down-regulation of β-cell genes. (**) P < 0.005; (***) P < 0.0005. (H) Expression analysis of hypoxia targets in human islets subjected to hypoxia. (*) P < 0.05; (**) P < 0.005. (I) Evaluation of β-cell genes, including the progenitor marker SOX9, in human islets cultured in 2% oxygen. (*) P < 0.05; (***) P < 0.0005. (J) Temporal analysis of gene expression in mouse islets under hypoxia. n = 4 for control and hypoxia samples at 4 h and 8 h, n = 4 for control samples at 16 h, and n = 6 for hypoxia samples at 16 h. (*) P < 0.05; (**) P < 0.005.
Having determined the effect of hypoxia on β-cell lines, wild-type mouse islets were incubated under the same conditions. Importantly, a similar gene expression pattern was seen in islets at 2% oxygen. Up-regulation of Glut1, Ldha, and Vegfa and glycolytic enzymes Pdk1, Pgm2, and Gpi was accompanied by down-regulation of Ins1, Pdx-1, Nkx6.1, MafA, Neurod1, and Glut-2 expression (Fig. 4F,G). Although not statistically significant, Sox9 expression demonstrated a trend toward up-regulation (Fig. 4G). The difference in the significant up-regulation of Sox9 in vivo (Fig. 2C) and ex vivo is likely due to the cell-autonomous nature of the manipulation in the mouse model that is not as effectively mimicked during the acute hypoxic incubation. The Hnf6 message was not detected in either control or hypoxic samples. In summary, acute hypoxia in β-cell lines and mouse islets leads to reduced β-cell-specific gene expression, mimicking a dedifferentiated state of the mature β cell observed under Vhlh−/− conditions in vivo.
Our results clearly demonstrate that an acute exposure to hypoxia is sufficient to modulate gene expression in β cells. To investigate whether such a response to hypoxia occurs in humans, islets from nondiabetic individuals were cultured in 20% or 2% oxygen. Gene expression analysis subsequent to hypoxic incubation revealed up-regulation of GLUT1, LDHA, and ALDOA (Fig. 4H). Significantly, down-regulation of PDX1, MAFA, and NKX6-1 was also detected in the hypoxic human samples (Fig. 4I), indicating that such gene dysregulation occurs across species. SOX9 expression was significantly increased in hypoxic islets, further supporting the murine in vivo and in vitro data. Although down-regulation of INS transcript was not detected (data not shown), these data strongly suggest that β-cell identity can be perturbed by hypoxia in human islets. A time-course analysis of gene expression using mouse islets revealed that key regulatory factors, including Pdx-1, Nkx6.1, MafA, Nkx2.2, and NeuroD1, were down-regulated prior to any changes in Ins1 or Ins2 transcript levels (Fig. 4J). Glut-2 also appeared down-regulated at these early time points of hypoxic exposure (Fig. 4J). MafB expression was unchanged by 16 h of hypoxia; however, a clear increase in Sox9 could be detected over time that correlated with an increase in Ldha and Glut-1 (Fig. 4J). Summarily, these data replicate the in vivo observations of a modified β-cell transcriptome upon exposure to hypoxia in mouse and human islets.
Ectopic expression of Sox9 in β cells leads to diabetes mellitus
The unexpected appearance of Sox9 upon Vhlh elimination encouraged a direct test of the consequence of ectopic expression of Sox9 in β cells. Ins-Cre animals were mated with CAG-Sox9/HA mice that express a hemagglutinin-tagged Sox9 protein upon Cre recombination (Kim et al. 2011) in β cells. Between 6 and 10 mo of age, InsCre;Sox9 mice were overtly hyperglycemic under randomly fed conditions, with no evidence of obesity, similar to what was seen in the Vhlh-depleted animals. (Fig. 5A). Histological analysis confirmed robust accumulation of the HA tag in islets, a marker for the Sox9/HA fusion protein (Fig. 5B). Our analyses of the Ins-Cre;Vhlh mice prompted us to further evaluate the differentiation state of the β cells in the Sox9-overexpressing mice. Indeed, immunostaining analysis confirmed reduced expression of insulin and Pdx-1 in the Sox9-overexpressing islets (Fig. 5B,D). Gene expression analysis from the InsCre;Sox9 islets demonstrated a down-regulation of β-cell-specific genes (Fig. 5E). Although the Glut-2 message did not appear to be significantly reduced in the compound transgenic samples, immunostaining revealed a clear perturbation of the membrane association of this transporter (Fig. 5F). As expected, the human Sox9 message was undetectable in the control samples and was detected at a Ct value of 26 by quantitative PCR in the transgenic islets. The mouse endogenous Sox9 gene was not significantly changed (data not shown). Gene expression analysis of the glycolytic enzymes and hypoxia-responsive genes that are up-regulated in the Vhlh-null islets were unchanged in the Sox9 up-regulation model (Supplemental Fig. S7A). Previous in vitro work has placed Hnf6 gene expression downstream from Sox9, and thus we evaluated the gene expression of Hnf6 in islets from InsCre;Sox9 animals. Although an increase was observed in the compound transgenic samples, this change was not statistically significant (Supplemental Fig. S7B). Thus, it remains unclear whether Sox9 is acting partly through Hnf6 in these animals. Interestingly, Sox9-overexpressing mice did not present with somatostatin/Sox9 double-positive cells by 10 mo of age, suggesting a distinct mechanism for erroneous activation of the somatostatin gene (data not shown). In summary, our data indicate that ectopic expression of Sox9 in β cells is sufficient to erode the functional state of the cells, resulting in diabetes mellitus in vivo. Importantly, the data also illustrate that Sox9 does not regulate the changes in cell metabolism observed under hypoxic conditions.
Figure 5.
Ectopic expression of Sox9 in β cells leads to perturbed function and cellular identity. (A) Blood glucose levels under fed conditions reveal hyperglycemia in the Ins-Cre;Sox9 animals (n = 12) between 6 and 10 mo of age as compared with control littermates (n = 11). There is a significant reduction in body weight of the Ins-Cre;Sox9 animals as compared with the control littermates (n = 10 for each group). (*) P < 0.05; (**) P < 0.005. (B) HA staining represents ectopic Sox9 expression in Cre-positive islets. Insulin staining is clearly perturbed in Ins-Cre;Sox9 islets. (C) Cells positive for HA and Sox9 expression show diminished insulin in Cre-positive islets. Bar, 20 μm. (D) Pdx-1 and insulin staining is reduced in Ins-Cre;Sox9 islets and coincides with HA staining. Bar, 20 μm. (E) Quantitative PCR analysis on islets isolated from 6- to 10-mo-old Ins-Cre;Sox9 animals shows reduced expression of canonical β-cell genes. (*) P < 0.05; (**) P < 0.005; (***) P < 0.0005. (F) Glut-2 staining in Ins-Cre;Sox9 islets reveals a redistribution of the protein from the typical cell surface staining seen in control islets. Bar, 100 μm.
Discussion
The main conclusion of our studies is that mature β cells can undergo a sustained process of dedifferentiation that results in the loss of glucose control. Using two independent mouse models, we demonstrate that β cells under hypoxia have the ability to dedifferentiate into non-β cells that causes perturbations in glucose homeostasis. Adult β-cell function has strict oxygen requirements, and mimicking molecular hypoxia leads to erroneous glucose sensing and perturbed insulin secretion (Zehetner et al. 2008; Cantley et al. 2009; Puri et al. 2009). The β cell, like every cell in the body, has adaptive mechanisms to sense reduced molecular oxygen and respond acutely by activating the hypoxia-sensing pathway. We demonstrate for the first time that in vivo, β cells experiencing chronic hypoxia undergo dedifferentiation that manifests physiologically as diabetes mellitus, with fasted blood glucose between 200 and 400 mg/dL. Islets from diabetic animals are populated with cells with diminished insulin expression. Critical transcription factors, including Pdx-1, Nkx6.1, and MafA, instruct β-cell formation and function from multipotent progenitors during embryogenesis into adulthood (Matsuoka et al. 2003; Puri and Hebrok 2010). Reduced expression of Pdx-1, MafA, Nkx6.1, NeuroD1, and Glut-2 in the Vhlh−/− islets collectively indicates loss of β-cell identity and perturbed function.
Surprisingly, reduced insulin expression correlated with increased Sox9 expression. During pancreas organogenesis, Sox9 costains with Pdx-1 in the epithelium between E9 and E12.5, and Sox9-positive cells contribute to endocrine and exocrine lineages (Seymour et al. 2007; Kopp et al. 2011). However, during endocrine specification, Sox9 expression decreases concomitant with Ngn3 activation (Shih et al. 2012). The absence of Ngn3 expression in diabetic islets might be because the dedifferentiation process does not mimic the state of a general endocrine progenitor. Alternatively, although transient expression or activation below detection cannot be ruled out, persistent levels of Sox9 may be inhibitory for Ngn3 expression in the diabetic islets. Staining for synaptophysin confirms that the Sox9-positive cells are endocrine and not ductal cells that have infiltrated the islets. Finally, islets from diabetic animals have activated Notch signaling and increased levels of Hes-1, which is known to repress Ngn3.
Within the embryonic pancreas, Sox9 costains with Hes-1, an effector of the Notch signaling pathway (Seymour et al. 2007). Notch signaling maintains progenitor pools in the developing epithelium and inhibits endocrine development (Hald et al. 2003; Murtaugh et al. 2003). During development, Notch signaling in the pancreas may be regulated in part by HIF (Chen et al. 2010). In addition, human islet dedifferentiation in culture correlates with activated Notch signaling (Bar et al. 2008). Altogether, existing data and our observations support the nonpermissive role of Notch for maintenance of β-cell identity. Similarly, strict regulation of Hh signaling appears necessary for accurate β-cell function. Ptc and Smoothened (Smo) are expressed in adult rat and mouse β cells and contribute to β-cell function (Thomas et al. 2000; Lau and Hebrok 2010). Interestingly, a mouse model that activates the Hh pathway in the β cell leads to dedifferentiation concomitant with a glucose intolerance phenotype, indicating that dysregulation of Hh signaling modifies β-cell fate and function (Landsman et al. 2011). Although Hh activation may occur in direct response to hypoxia (Onishi et al. 2011), cross-talk between signaling pathways, including Notch and Wnt, may also lead to pathway activation (Carlson et al. 2008). The role of Wnt signaling in the adult β cell is evidenced from expression of stabilized β-catenin in β cells that causes loss of insulin expression and cellular dedifferentiation in aged mice (Heiser et al. 2006). Thus, the chronic state of disease development in the Vhlh−/− mice may result from a combined dysregulation of the Notch, Hh, and Wnt pathways within the β cell.
Sox9 marks hypoxic cells for two reasons: First, nuclear Sox9 protein is absent from normal, adult pancreatic β cells, and second, Sox9 is directly activated by HIF (Zhang et al. 2011). Ductal or exocrine markers do not stain the diabetic islets (data not shown), thus ruling out a nonendocrine fate for the Sox9-positive cells. A subset of Sox9-positive cells, however, does express somatostatin. These cells are depleted of insulin and do not express glucagon. Promiscuous expression of the Ins-Cre line in somatostatin-producing cells is unlikely, as extensive lineage tracing did not indicate expression in δ cells (Herrera 2000; Thorel et al. 2010). During embryonic development, β and δ cells are predicted to have common progenitors; thus, one possible explanation could be that the insulin-negative cells in the diabetic islets have dedifferentiated just enough to activate the δ program in a subset of cells. Further differentiation toward other endocrine cell types or even outside the endocrine realm, as observed in the context of oncogenic transformation (Gidekel Friedlander et al. 2009), would require additional changes not promoted upon hypoxia.
Aberrant expression of Sox9 in Vhlh-deficient β cells suggested the possibility of a functional link between Sox9 up-regulation and β-cell dysfunction. Activation of Hnf6 in the β cell leads to decreased expression of Glut-2 and MafA and diabetes mellitus (Tweedie et al. 2006). Our data revealed aberrant glucose homeostasis in Ins-Cre;Sox9 mice. Ectopic expression of Sox9 led to β-cell dedifferentiation, further indicating that Sox9 alone can lead to perturbation of β-cell identity. Sox9–somatostatin-colabeled cells were not observed in the Ins-Cre;Sox9 mice, suggesting that a Sox9-independent mechanism perturbs that path of fate modulation in the Vhlh−/− β cells. Furthermore, the observation that glycolytic enzymes are not derepressed in Ins-Cre;Sox9 mice further indicates that at least aspects of β-cell dedifferentiation can occur in the absence of a fully blown hypoxic response.
Other groups have evaluated the role of Vhlh in the β cell (Zehetner et al. 2008; Cantley et al. 2009; Cheng et al. 2010; Choi et al. 2011). However, seemingly contradictory results have been published. Zehetner et al. (2008) reported a hypoglycemic phenotype in animals with Vhlh−/− β cells, while Cantley et al. (2009) observed hyperglycemia in 12-wk-old animals under fed conditions. In animal models of disease, the genetic background, the specific Cre, and the intricacies of gene expression in transgenics can produce conflicting data. Although no clear explanation emerges about the discrepancies, the development of diabetes mellitus in the Vhlh−/− animal in our hands is a robust, consistent phenotype. Interestingly, deletion of HIF also results in a β-cell phenotype (Cheng et al. 2010) with perturbed glucose homeostasis, indicating once again that either activation or attenuation of multiple regulatory pathways function can have similarly deleterious effects on the target tissue. Choi et al. (2011) reported hyperglycemia during random blood glucose measurements in the β-cell-specific knockout of Vhlh but did not attribute this to a loss of β-cell identity.
Measurements in vivo have determined rodent islet oxygen partial pressure to be 31–37 mmHg (equivalent to 4%–5%) (Carlsson et al. 1998). At 2% oxygen, which mimics physiological hypoxia for the islet, β-cell-specific genes are down-regulated in β-cell lines and isolated mouse islets. A down-regulation in insulin expression has been previously reported when β-cell lines are incubated under 1% oxygen (Vasir et al. 1998). Significantly, our data show that human islets respond to hypoxia by up-regulating canonical HIF targets, with simultaneous down-regulation of PDX-1, MAFA, and NKX6-1. These data encourage us to explore the effects of hypoxia in islets during transplantation, where oxygenation impacts islet function (Carlsson et al. 1998, 2000; Sakata et al. 2010). Interestingly, both low oxygenation and loss of β-cell differentiation have been reported in islet grafts in animals (Laybutt et al. 2007). HIF stabilization in transplanted islets (Miao et al. 2006), presumably due to delayed vascularization of the transplanted cells, could induce changes in the β-cell transcriptome that negatively impact successful transplantation and graft function. Identifying small molecules that block such dedifferentiation would be a useful step toward therapy.
T2D constitutes a heterogeneous collection of hyperglycemic phenotypes that manifest due to some combination of insulin resistance and a failing β-cell cohort. Rodent models, such as the ZDF rat, have revealed that β-cell hyperplasia occurs due to increased metabolic demand, perhaps mimicking the human condition (Paulsen et al. 2010). An adaptive expansion of β-cell mass may generate regions of hypoxia, as islets from ZDF rats display elevated levels of HIF target genes such as Ldha (Li et al. 2006). Islets isolated from T2D patients have dysregulation of HIF components as well as the progenitor markers SOX9 and HNF6, among others (Gunton et al. 2005; Weir et al. 2009), indicating that in a subset of patients, hypoxia may impact the β-cell transcriptome.
In summary, sustained activation of the hypoxia pathway triggers dedifferentiation of the β cell in part by activating transcriptional regulators such as Sox9 and Hnf6, which results in a progressive defect in glucose regulation resembling T2D. Loss of β-cell identity appears in a model of Fox01 depletion when β cells are stressed due to pregnancy or aging (Puri and Hebrok 2012; Talchai et al. 2012). Compromised expression of β-cell genes has been associated with β-cell dysfunction in several animal models (Laybutt et al. 2003; Kjorholt et al. 2005; Tweedie et al. 2006). Loss of β-cell differentiation has been proposed as an end result of β-cell decompensation in T2D (Weir and Bonner-Weir 2004; Weir et al. 2009). Although T2D is a diverse set of diseases, β-cell dedifferentiation due to either genetic or environmental insults (for instance, hypoxia) in a subset of patients may be a potential mechanism (Guo et al. 2013).
Materials and methods
Ethics statement
Mice were maintained in the barrier facility according to protocols approved by the Committee on Animal Research at the University of California at San Francisco.
Transgenic mice
Ins-Cre mice were obtained from Dr. Pedro Herrera's laboratory (Herrera 2000). The Pdx-1-CreER, Vhlh−/− (VhlhloxP/loxP), and Sox9 mice have been described previously (Haase et al. 2001; Gu et al. 2002; Kim et al. 2011).
Tissue histology, immunofluorescence analysis, and islet morphometry
Pancreata isolated from adult mice were fixed in Z-Fix (Anatech) for 12–16 h at 4°C and processed for paraffin embedding. Hematoxylin/eosin staining, immunohistochemistry, and immunofluorescence analyses were performed as described previously (Kawahira et al. 2005). The following primary antibodies were used: guinea pig anti-insulin (1:300; Millipore), mouse anti-insulin (1:300; Sigma), guinea pig anti-insulin (1:200; Dako North America, Inc.), rabbit anti-glucagon (1:300), guinea pig anti-glucagon (1:300; Millipore), mouse anti-glucagon (1:500; Sigma), rabbit anti-Glut2 (1:500), rabbit anti-Sox9 (1:200; Chemicon), rat anti-somatostatin (1:200; Millipore), mouse anti-Nkx6.1 (1:200; Developmental Studies Hybridoma Bank), rabbit anti-MafA (1:200; Bethyl Laboratories, Inc.), guinea pig anti-Pdx1 (1:200; gift from Mike German, University of California at San Francisco), mouse anti-Pdx1 (1:200; Developmental Studies Hybridoma Bank), rabbit anti-Pdx-1 (1:200; Chemicon), goat anti-Pdx-1 (1:200; R&D Systems), rabbit anti-cleaved caspase-3 (1:200; Cell Signaling), rat anti-HA (1:200; Roche Applied Science), mouse anti-synaptophysin (1:200; Biogenex), goat anti-NeuroD (1:200; Santa Cruz Biotechnology), and mouse anti-β-catenin (1:200; BD Biosciences). Primary antibodies were detected with Alexa-488-, Alexa-546-, or Alexa-555- and Alexa-633-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). For immunohistochemistry, biotinylated anti-rabbit, anti-mouse, anti-guinea pig (Vector Laboratories), and anti-rat (Jackson ImmunoResearch Laboratories, Inc.) antibodies were used as secondary antibodies at a 1:200 dilution. For islet mass quantification, sections 100 μm apart were stained with anti-insulin antibody, and the total islet area was quantified as a percent of total pancreatic area. Paraffin-embedded pancreata from human donors (nondiabetic and T2D) were obtained from the Juvenile Diabetes Research Foundation (JDRF) Network for Pancreatic Organ Donors with Diabetes (nPOD).
Bright-field images were acquired using a Zeiss Axio Imager D1 microscope. Zeiss Axioscope2 wide-field and Zeiss ApoTome microscopes were used to visualize and photograph fluorescence. Confocal microscopy was carried out using the Leica SL confocal microscope. Unless otherwise noted, all photomicrographs shown are representative of at least three independent samples of the indicated genotype.
Quantitative PCR and gene expression array
RNA isolation, cDNA preparation, and quantitative PCR were performed as described previously (Cano et al. 2006). RNA expression of target genes was normalized to cyclophilin expression for mouse samples and to TATA-box-binding protein for human samples. One control was set to 1, and all other controls and test samples were normalized to that. The Ct values for the control samples were between 15 (for insulin in islets) and 33 (for genes expressed at lower levels, such as Hnf6). Primer sequences are available on request. For microarray analysis, three biological replicates were collected for control and transgenic islets, and RNA was extracted using the MinElute columns (Qiagen) and hybridized to Affymetrix chips (GeneChip Mouse Gene 1.0 ST array).
Intraperitoneal glucose tolerance test, insulin tolerance test, and hormone measurement in vivo
All physiological analyses were carried out on male mice. After a 16- to 18-h fast, adult mice were weighed, and blood glucose level was measured using the Contour glucometer. Mice were injected intraperitoneally with 1 M glucose solution at 10 μL per gram of body weight. Blood glucose was measured every 30 min for 2 h after injection. For the insulin tolerance test, mice were fasted for 16–18 h, and body weight and blood glucose were measured. Insulin was injected intraperitoneally with a dose of 1 U per kilogram of body weight. Blood glucose was measured every 30 min for 2 h after injection. For in vivo hormone measurement, blood was collected either before and after a glucose challenge or from mice in the resting fed state from the tail vein and spun down to collect serum, which was stored at −80°C with protease inhibitors (Roche). Insulin concentration was calculated using the Insulin EIA kit (ALPCO) per the manufacturer's instructions.
Cell line and islet culture
MIN6 cells were cultured in DMEM supplemented with penicillin and streptomycin, 71.5 μM 2-mercaptoethanol, and 15% fetal bovine serum. Ins-1 cells were cultured as described previously (Hohmeier et al. 2000). The Islet Production Facility Core at the Diabetes Center at University of California at San Francisco isolated islets from adult mice. Human islets were purchased from Prodo Laboratories and cultured in proprietary medium according to the company's instructions. Cells and islets were incubated at either 20% or 2% oxygen for the specified times before collection and processed for gene expression analysis as described above.
Western blotting
Ins-1 cells were homogenized in RIPA buffer, electrophoresed on SDS-PAGE gels, and transferred to PVDF. Blocking was carried out using 5% milk in TBST for 1 h at room temperature. Subsequently, blots were incubated with primary antibodies overnight at 4°C and detected using HRP-conjugated secondary antibodies (1:5000 dilution; Santa Cruz Biotechnology, Inc.) and ECL (Amersham Biosciences). Primary antibodies used for Western blotting were rabbit anti-Sox9 (1:1000; Chemicon), rabbit anti-Pdx-1 (1:1000; Chemicon), and mouse anti-β-tubulin (1:2000; Sigma).
Statistical analyses
Data are presented as mean ± SD and were subjected to two-tailed t-test. P-value < 0.05 was considered to be significant. Array qualities were analyzed using the affyPLM package in R/Bioconductor and RMA Express software (http://rmaexpress.bmbolstad.com). The data were normalized by robust multichip averaging method. Control and low-performing probe sets (those with intensity values below a threshold across all samples; the threshold was taken to be the global lowest 25th percentile of intensity values) were excluded from analysis. Out of 35,556 probe sets, 23,911 remained after filtering. Moderated t-statistics (as implemented in the limma package in R/Bioconductor) were used to test for differentially expressed probe sets. The false discovery rate (FDR) was controlled by estimating the Q-values of the P-values. A change in gene expression was identified as significant if the Q-value was <0.05. GOstat was used to search for significant GO terms for the significant genes. Significant GO terms were identified as those having an FDR-adjusted P-value (Benjamini) <0.05.
Acknowledgments
We thank Dr. Mike German for providing antibody reagents. We also thank Dr. John P. Morris IV, Dr. David Cano, Dr. Alexandra Folias, and Dr. Holger Russ for critical reading of the manuscript, and Cecilia Austin and Debbie Ngow for expert help with mouse islet isolation and tissue processing. Work in M.H.'s laboratory was supported by a National Institutes of Health grant (DK60533) and a Juvenile Diabetes Research Foundation grant. Image acquisition was supported by the University of California at San Francisco Diabetes and Endocrinology Research Center (DERC) microscopy core P30 DK63720. Islet isolation was performed by the University of California at San Francisco DERC islet core P30 DK63720.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.227785.113.
References
- Bar Y, Russ HA, Knoller S, Ouziel-Yahalom L, Efrat S 2008. HES-1 is involved in adaptation of adult human β-cells to proliferation in vitro. Diabetes 57: 2413–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano DA, Sekine S, Hebrok M 2006. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology 131: 1856–1869 [DOI] [PubMed] [Google Scholar]
- Cantley J, Selman C, Shukla D, Abramov AY, Forstreuter F, Esteban MA, Claret M, Lingard SJ, Clements M, Harten SK, et al. 2009. Deletion of the von Hippel-Lindau gene in pancreatic β cells impairs glucose homeostasis in mice. J Clin Invest 119: 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Silva HS, Conboy IM 2008. Aging of signal transduction pathways, and pathology. Exp Cell Res 314: 1951–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson PO, Liss P, Andersson A, Jansson L 1998. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 47: 1027–1032 [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Andersson A, Carlsson C, Hellerstrom C, Hoglund E, King A, Kallskog O, Liss P, Mattsson G, Olsson R, et al. 2000. Engraftment and growth of transplanted pancreatic islets. Ups J Med Sci 105: 107–123 [DOI] [PubMed] [Google Scholar]
- Chen H, Houshmand G, Mishra S, Fong GH, Gittes GK, Esni F 2010. Impaired pancreatic development in Hif2-α deficient mice. Biochem Biophys Res Commun 399: 440–445 [DOI] [PubMed] [Google Scholar]
- Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O'Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, et al. 2010. Hypoxia-inducible factor-1α regulates β cell function in mouse and human islets. J Clin Invest 120: 2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Cai EP, Schroer SA, Wang L, Woo M 2011. Vhl is required for normal pancreatic β cell function and the maintenance of β cell mass with age in mice. Lab Invest 91: 527–538 [DOI] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A 2009. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 138: 449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville MI, Eizirik DL 2006. Notch signaling: A mediator of β-cell de-differentiation in diabetes? Biochem Biophys Res Commun 339: 1063–1068 [DOI] [PubMed] [Google Scholar]
- Dotsch J, Knerr I, Rascher W, Meissner U 2006. Does defective hypoxia signalling cause type 2 diabetes? Eur J Endocrinol 154: 781–782 [DOI] [PubMed] [Google Scholar]
- Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T 2009. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 16: 379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457 [DOI] [PubMed] [Google Scholar]
- Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, et al. 2005. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122: 337–349 [DOI] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R 2013. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 123: 3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R 2001. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci 98: 1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J 2003. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol 260: 426–437 [DOI] [PubMed] [Google Scholar]
- Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M 2006. Stabilization of β-catenin impacts pancreas growth. Development 133: 2023–2032 [DOI] [PubMed] [Google Scholar]
- Herrera PL 2000. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127: 2317–2322 [DOI] [PubMed] [Google Scholar]
- Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB 2000. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49: 424–430 [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292: 464–468 [DOI] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC 1999. Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J Biol Chem 274: 14112–14121 [DOI] [PubMed] [Google Scholar]
- Kaelin WG Jr 2005. The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res Commun 338: 627–638 [DOI] [PubMed] [Google Scholar]
- Kaelin WG Jr 2008. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8: 865–873 [DOI] [PubMed] [Google Scholar]
- Kahn SE 2003. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46: 3–19 [DOI] [PubMed] [Google Scholar]
- Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M 2005. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol 280: 111–121 [DOI] [PubMed] [Google Scholar]
- Kim Y, Murao H, Yamamoto K, Deng JM, Behringer RR, Nakamura T, Akiyama H 2011. Generation of transgenic mice for conditional overexpression of Sox9. J Bone Miner Metab 29: 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR 2005. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of β-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 54: 2755–2763 [DOI] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M 2011. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138: 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacraz G, Giroix MH, Kassis N, Coulaud J, Galinier A, Noll C, Cornut M, Schmidlin F, Paul JL, Janel N, et al. 2009. Islet endothelial activation and oxidative stress gene expression is reduced by IL-1Ra treatment in the type 2 diabetic GK rat. PLoS ONE 4: e6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Parent A, Hebrok M 2011. Elevated Hedgehog/Gli signaling causes β-cell dedifferentiation in mice. Proc Natl Acad Sci 108: 17010–17015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Hebrok M 2010. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult β-cell function. Diabetes 59: 1211–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC 2003. Critical reduction in β-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem 278: 2997–3005 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Hawkins YC, Lock J, Lebet J, Sharma A, Bonner-Weir S, Weir GC 2007. Influence of diabetes on the loss of β cell differentiation after islet transplantation in rats. Diabetologia 50: 2117–2125 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF 2006. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes 55: 2965–2973 [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS 2007. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci 104: 10500–10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R 2003. Members of the large Maf transcription family regulate insulin gene transcription in islet β cells. Mol Cell Biol 23: 6049–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275 [DOI] [PubMed] [Google Scholar]
- Miao G, Ostrowski RP, Mace J, Hough J, Hopper A, Peverini R, Chinnock R, Zhang J, Hathout E 2006. Dynamic production of hypoxia-inducible factor-1α in early transplanted islets. Am J Transplant 6: 2636–2643 [DOI] [PubMed] [Google Scholar]
- Morris JP IV, Cano DA, Sekine S, Wang SC, Hebrok M 2010. β-Catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest 120: 508–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA 2003. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci 100: 14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H, Kai M, Odate S, Iwasaki H, Morifuji Y, Ogino T, Morisaki T, Nakashima Y, Katano M 2011. Hypoxia activates the hedgehog signaling pathway in a ligand-independent manner by upregulation of Smo transcription in pancreatic cancer. Cancer Sci 102: 1144–1150 [DOI] [PubMed] [Google Scholar]
- Paulsen SJ, Vrang N, Larsen LK, Larsen PJ, Jelsing J 2010. Stereological assessment of pancreatic β-cell mass development in male Zucker diabetic fatty (ZDF) rats: Correlation with pancreatic β-cell function. J Anat 217: 624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ 2006. Islet β cell failure in type 2 diabetes. J Clin Invest 116: 1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Hebrok M 2010. Cellular plasticity within the pancreas–lessons learned from development. Dev Cell 18: 342–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Hebrok M 2012. Diabetic β cells: To be or not to be? Cell 150: 1103–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Cano DA, Hebrok M 2009. A role for von Hippel-Lindau protein in pancreatic β-cell function. Diabetes 58: 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Garcia-Nunez A, Hebrok M, Cano DA 2013. Elimination of von hippel-lindau function perturbs pancreas endocrine homeostasis in mice. PLoS ONE 8: e72213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausa F, Samadani U, Ye H, Lim L, Fletcher CF, Jenkins NA, Copeland NG, Costa RH 1997. The cut-homeodomain transcriptional activator HNF-6 is coexpressed with its target gene HNF-3 β in the developing murine liver and pancreas. Dev Biol 192: 228–246 [DOI] [PubMed] [Google Scholar]
- Sakata N, Chan NK, Ostrowski RP, Chrisler J, Hayes P, Kim S, Obenaus A, Zhang JH, Hathout E 2010. Hyperbaric oxygen therapy improves early posttransplant islet function. Pediatr Diabetes 11: 471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL 2013. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123: 3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M 2007. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci 104: 1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HP, Kopp JL, Sandhu M, Dubois CL, Seymour PA, Grapin-Botton A, Sander M 2012. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 139: 2488–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D 2012. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150: 1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MK, Rastalsky N, Lee JH, Habener JF 2000. Hedgehog signaling regulation of insulin production by pancreatic β-cells. Diabetes 49: 2039–2047 [DOI] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL 2010. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464: 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie E, Artner I, Crawford L, Poffenberger G, Thorens B, Stein R, Powers AC, Gannon M 2006. Maintenance of hepatic nuclear factor 6 in postnatal islets impairs terminal differentiation and function of β-cells. Diabetes 55: 3264–3270 [DOI] [PubMed] [Google Scholar]
- Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC 1998. Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes 47: 1894–1903 [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S 2004. Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53: S16–S21 [DOI] [PubMed] [Google Scholar]
- Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S 2009. Towards better understanding of the contributions of overwork and glucotoxicity to the β-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 11: 82–90 [DOI] [PubMed] [Google Scholar]
- Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV 2011. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev 25: 1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehetner J, Danzer C, Collins S, Eckhardt K, Gerber PA, Ballschmieter P, Galvanovskis J, Shimomura K, Ashcroft FM, Thorens B, et al. 2008. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic β cells. Genes Dev 22: 3135–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yang F, Cornelia R, Tang W, Swisher S, Kim H 2011. Hypoxia-inducible factor-1 is a positive regulator of Sox9 activity in femoral head osteonecrosis. Bone 48: 507–513 [DOI] [PubMed] [Google Scholar]