Abstract
The Drosophila αPS2βPS integrin is required for diverse development events, including muscle attachment. We characterized six unusual mutations in the αPS2 gene that cause a subset of the null phenotype. One mutation changes a residue in αPS2 that is equivalent to the residue in αV that contacts the arginine of RGD. This change severely reduced αPS2βPS affinity for soluble ligand, abolished the ability of the integrin to recruit laminin to muscle attachment sites in the embryo and caused detachment of integrins and talin from the ECM. Three mutations that alter different parts of the αPS2 β-propeller, plus a fourth that eliminated a late phase of αPS2 expression, all led to a strong decrease in αPS2βPS at muscle ends, but, surprisingly, normal levels of talin were recruited. Thus, although talin recruitment requires αPS2βPS, talin levels are not simply specified by the amount of integrin at the adhesive junction. These mutations caused detachment of talin and actin from integrins, suggesting that the integrin-talin link is weaker than the ECM-integrin link.
Keywords: ECM, integrins, muscle development, ligand-binding site
Introduction
The integrin family of adhesion receptors are essential for development and homeostasis in multicellular animals. Their adhesive roles are exceedingly diverse: from providing simple, stable adhesion between tissues to mediating dynamic cycles of attachment and release between a migrating cell and its substrate (Bokel and Brown, 2002). This diversity in function is thought to be achieved, at least in part, by the expression of different, specialized integrins in different tissues (Hynes, 2002). Identifying the structural elements that are responsible for the different functional properties amongst the integrin family is therefore an important goal.
Integrins are divalent-cation dependent, transmembrane heterodimers that mediate adhesion by binding directly to ligands in the extracellular matrix (ECM) and simultaneously linking to the cytoskeleton through adaptor molecules (Hynes, 2002). In recent years, much advancement has been made towards the understanding of the structural basis of integrin activation and function. The determination of the 3D structure of αVβ3 integrin confirmed predictions: the αβ heterodimer is assembled into a globular, ovoid head that rests on two legs (Xiong et al., 2001). Ligand-bound crystals of αVβ3 and αllbβ3 identified residues in both subunits that directly contact the ligand (Xiong et al., 2002; Xiao et al., 2004). These findings plus extensive mutational analysis and function-blocking antibody studies have led to a detailed map of sites important for integrin function (Humphries et al., 2003). Despite these advances, many questions remain about integrin function within the intact organism. For example, a single integrin heterodimer can bind multiple extracellular ligands, so it is important to understand which receptor-ligand interactions are relevant in a particular tissue at a particular time. Similarly, each tissue expresses a unique pool of cytoplasmic factors, the combination of which may affect integrin activity. For these reasons it is difficult to predict how a mutation characterized in cell culture will affect integrin function in the intact animal.
Our interest is to understand how an integrin heterodimer achieves its numerous functions in different tissues during morphogenesis. In Drosophila, the αPS2βPS integrin (also referred to as PS2 integrin) is required for a number of developmental processes including muscle attachment, midgut morphogenesis and adhesion between wing epithelia in the adult. The αPS2 subunit is most similar to mammalian α5, αV, and αIIb, and forms a heterodimer with the βPS subunit, the orthologue of β1 (Bokel and Brown, 2002). Like its mammalian homologues, αPS2βPS binds Arg-Gly-Asp (RGD) peptides and RGD-containing extracellular ligands when expressed in cell culture (e.g. Graner et al., 1998).
In a previous study we performed a number of genetic screens for mutations in the gene encoding the αPS2 subunit (called the inflated (if) gene) (Bloor and Brown, 1998). Out of the 35 mutant alleles examined, only 6 affected just a subset of αPS2βPS-dependent functions, and these fell into three phenotypic classes based on developmental phenotype and genetic behavior (Table 1). Three alleles (if17, if21, and if35) behaved as typical genetic hypomorphs: they displayed the full range of inflated null embryonic phenotypes but each less severely. The ifSEF allele specifically affected the muscles, while ifC2B and if2B1 particularly affected midgut morphogenesis (Bloor and Brown, 1998). Intriguingly, some inflated mutations were able to complement each other genetically. Such interallelic complementation can occur if two mutations in the same gene affect two different subfunctions; an individual that is transheterozygous for the two alleles appears wild type because it retains normal activity for each subfunction. Transheterozygous flies carrying the ifSEF allele in combination with either ifC2B or if2B1 are fully viable, suggesting that these mutations affect distinct subfunctions.
Table 1. Partial loss of function inflated alleles.
| allele | mutation | location | muscle phenotype | midgut phenotype | wing phenotype | interalleleic complementation |
|---|---|---|---|---|---|---|
| ifC2B | D244 to N | ligand binding pocket | detachment from the ECM | strong | blister | ifC2B / ifSEF are viable |
| if17 | D309 to N | cation coordination | weak cytoskeletal detachment | weak | none | if17 / ifC2B gives escapers |
| if21 | A322 to T | GAP motif | weak cytoskeletal detachment | weak | none | if21 / ifC2B gives escapers |
| if35 | G101 to S | cup tetrapeptide | weak cytoskeletal detachment | weak | none | none |
| ifSEF | Not found | - | strong cytoskeletal detachment | none | none | ifC2B / ifSEF and if2B1 / ifSEF are viable |
| if2B1 | Not found | - | none | strong | none | if2B1 / ifSEF are viable |
In the current study, we utilized these unusual inflated mutants to explore how αPS2 structure relates to its function during different developmental events. A mutant in a residue predicted to directly contact the extracellular ligand led to the identification of a function for integrins in recruiting ECM proteins to muscle attachment sites (MAS). Other alleles led to the discovery that the amount of intracellular integrin-associated proteins, such as talin, that are recruited to integrin adhesive contacts is not simply regulated by the amount of integrin at the adhesive site.
Materials and Methods
Fly stocks and Genetics
All inflated alleles were generated in various mutagenesis screens described in Bloor and Brown (1998). All were generated by mutagenesis with ethyl methanesulfonate except ifC2B, which was generated with N-ethyl-N-nitrosourea, and the null allele ifB4, was generated by γ-rays. We used the X chromosome balancers FM7 eve::lacZ, FM7 Kruppel::Gal4, UAS::GFP, and FM6 P{y+} with w y mutant chromosomes to unambiguously identify hemizygous mutant embryos.
Mapping of inflated mutations
Hemizygous inflated male embryos were identified by the absence of a y+ balancer chromosome. Aliquots of 30 dechorionated y mutant embryos were prepared for PCR and sequencing. They were homogenized in 100 μl 0.5% NP-40 and 0.5% Tween-20 in 1X PCR buffer (Roche), treated with proteinase K (40μg/ml) 1 hour at 50°C, followed 94°C for 10 minutes. PCR fragments spanning each coding exon and their splice junctions were generated from the mutant DNA and sequenced and compared to the published sequence (AE003503) and to other alleles isolated in the same screen, derived from an isogenic stock. Amplification products from two independent embryos collections were sequenced to verify each nucleotide substitution found. In the case of ifSEF and if2B1, a third round of sequencing was performed to confirm that there were no alterations.
Immunofluorescence detection of proteins and microscopy
Stage 15-16 embryos were prepared and labelled using standard antibody staining methods. LamininW was visualized using a polyclonal rabbit antibody, kindly provided by Stephan Baumgartner at 1:50 dilution (Martin et al., 1999). To visualize sarcomeric actin in somatic and visceral muscle, we altered the fixation method, substituting methanol with 80% ethanol and stained with rhodamine-conjugated phalloidin (Molecular Probes). To visualise the muscle cell membrane, UAS::SrcGFP (myristylated GFP) was expressed in the muscles with Mef2::Gal4 (Zervas et al., 2001). For late stage 17 embryos, when standard fixation methods cannot penetrate the cuticle for antibody stainings, we employed a heat fixation protocol described in (Tepass, 1996). Briefly, embryos were dechorionated in 50% bleach for one minute, rinsed in water, immersed in boiling 1X E-wash buffer (100mM NaCl, 0.1% Tween-20) for a few seconds, then immediately cooled by adding 3X volume of ice cold E-wash, and placed on ice. Embryos were then devitellenized in methanol/heptane. Monoclonal antibodies against fasiclin3, myosin, αPS2, βPS were used at 1:5 and polyclonal anti-talin at 1:200 (Brown et al., 2002). Fluorescently labelled secondary antibodies (Molecular Probes) were used at a 1:200 dilution. Images were collected with a Biorad Radiance confocal microscope using 40x/1.30 and 60x/1.40 objectives, and an Olympus Fluoview 1000 confocal microscope using 60x/1.35 objective. All images were assembled in Photoshop 7.0 and labelled with FreeHand MX.
Quantification of immunofluorescence intensities
The intensities of αPS2 and talin immunofluorescence in confocal sections of MAS were quantified using Volocity (Improvision, Inc). Laser and gain settings were fixed when obtaining confocal sections of wild type and mutant embryos at comparable stages of development. Using Volocity, MAS were outlined and the average pixel intensity in the given area was calculated for each channel. The ratios of talin to αPS2 immunofluorescence were calculated and graphed using Microsoft Excel.
Cell Culture, Spreading, TWOW-1 binding, and Flow Cytometry
Drosophila S2 cells were maintained and transformed as described (Jannuzi et al., 2002). Cells were transformed with integrin subunit-expressing plasmids pHSβPS, and pHSαPS2 and the selectable marker-bearing plasmid pH8CO. The pHSαPS2 plasmids express either the m8 or C forms of αPS2 (Brown et al., 1989). Some pHSαPS2 plasmids also had the activating mutation GFFNR>GFANA in the cytoplasmic domain (Jannuzi et al., 2002). Mutations if17, if21 and ifC2B were cloned into these constructs using mutagenic PCR primers and standard techniques and confirmed by sequencing. For transient expression experiments, S2 cells were cotransfected with the three plasmids and grown for 4-5 days in selection medium containing methotrexate. PS2 integrin expression levels were determined by flow cytometry following staining with the αPS2-specific monoclonal antibody CF.2C7 as described (Bunch et al., 2004). Cell spreading on RBB-Tigg (Jannuzi et al., 2002) and DLAM-RGD (Graner et al., 1998) was done as described (Bunch et al., 2004) with the following exceptions. In these transient expression cell spreading experiments the cells were heat shocked at 37°C for 30 minutes and then plated on RBB-Tigg or DLAM-RGD for 4 hours without dispase/collagenase digestion; approximately 90% of the cells expressed the transfected integrin. Subsequent experiments have shown that the heat shock has no effect on integrin expression in these transient expression assays; high levels of integrin are seen with or without heat shock. Spreading experiments were done in M3 medium containing 2 mg/ml BSA (bovine serum albumin). Results are expressed as averages of three experiments with standard errors. We did a full dose response curve (8-2000 ng/ml RBB-Tigg and 0.15-10 μg/ml DLAM-RGD) for wild type and the ifC2B mutant. Concentration curves were also done on if17 and if21, though not in triplicate. 500 ng/ml RBB-Tigg and 5 μg/ml DLAM-RGD, the concentrations used for Fig. 7, give maximal cell spreading for cells expressing wild type PS2 integrins.
Figure 7. Mutations in αPS2 cause similar defects when expressed in S2 cells.
(A) S2 cells expressing βPS plus the m8 or C form of αPS2 from wild type or containing the ifC2B, if17, and if21 mutations were stained with an αPS2 specific monoclonal antibody, and immunofluorescence was quantitated by flow cytometry. Cells expressing the same integrins containing the activating mutation GFFNR>GFANA in the cytoplasmic domain of the αPS2 subunits were similarly stained. Shown are the average mean fluorescent intensities (MFI) and standard errors for three experiments. Each experiment analyzed 10,000 cells. The MFI of untransfected S2 cells was 10.6 +/− 0.6 (not shown). (B) The wild type and mutant cells were spread on 500 ng/ml RBB-Tigg, a fragment of tiggrin, or 5 μg/ml DLAM-RGD, a fragment of the lamininW α chain, and the percentage of the total cells that spread was visually determined. (C) Binding of the soluble monovalent αPS2βPS integrin ligand, TWOW-1, to wild type and mutant receptors. Binding is expressed as a ratio of specific TWOW-1 immunofluorscence over total integrin (as detected with the antibody used in A). Results are the average and standard error for three experiments.
TWOW-1 binding assays were performed as described in detail in (Bunch et al., 2006). Stably transformed cells expressing wild type or mutant integrins were treated with dispase/collegenase at 36°C for 25 minutes to remove extracellular matrix proteins and preexisting integrins. The treatment also induces expression of integrins which are under the control of the heat shock 70 promotor. Following 4 hours recovery at 23°C to allow for expression of new integrins, the cells were stained for αPS2 integrin levels (using the CF2C7 monoclonal antibody) in one tube and incubated with TWOW-1 at 100 μg/ml in another. Following fixation of the bound TWOW-1 and washing, TWOW-1 was detected using a fluorescent anti-mouse secondary antibody. Specific TWOW-1 binding was measured as TWOW-1 Mean Fluorescence Intensity (MFI) minus the TWOW-1 MFI of cells in the presence of EDTA (divalent cations are required for integrin-ligand interactions). For our analyses, this specific binding was expressed relative to surface integrin expression (TWOW-1 MFI/anti-αPS2 MFI).
In situ hybridization
In situ hybridization of whole mount embryos was performed with digioxinogen-labelled antisense RNA probes from an inflated cDNA, or a 1 kb exon fragment of the wing blister gene and and GFP, using standard methods. Embryos mutant for mysXG43 were identified by the absence of FM7Kruppel::Gal4, UAS::GFP labelling with antisense GFP probes. Images were obtained on a Leica DMR microscope with a spot digital camera (Diagnostic Instruments).
Results
Sequencing inflated partial loss of function mutations
In a previous study 35 mutations in the gene encoding the integrin αPS2 subunit (inflated (if)) were characterized (Bloor and Brown, 1998). Twentynine are complete loss of function (amorphic) mutations and six are partial loss of function (hypomorphic). The variable phenotypes displayed by the 6 partial loss of function alleles (Table 1) suggested that they disrupt αPS2 subfunctions, and so identifying the molecular lesions of each should prove informative. We therefore sequenced each mutant allele. The inflated gene contains large introns, so that the 5.7 kb mRNAs are derived from a 31.2 kb primary transcript (Brown et al., 1989). Two alternatively spliced mRNAs are produced, differing by the inclusion (C form) or omission (m8 form) of exon 8, which encodes 25 amino acids. This variation occurs within the ligand binding region, and the two forms of αPS2βPS have different affinities for Drosophila ECM ligands (Fogerty et al., 1994; Graner et al., 1998). Because of the large size of the gene, we restricted our sequencing to the coding regions and splice junctions. Unique sequence alterations were discovered in four of the six if alleles (Fig. 1, Table 1). We did not find any sequence alterations for the ifSEF or if2B1 alleles, suggesting that these mutations lie in the promotor/enhancer elements flanking the gene or within introns.
Figure 1. Sequence alterations caused by inflated mutations.
(A and B) Residues that are altered in inflated mutations are indicated by showing the side chains in red of the equivalent residues of αV, within the structure αVβ3 (αV in blue, β3 in grey) bound to the cyclo-RGD ligand (orange) (Xiong et al., 2002). Green dots are divalent cations. In (B) the β subunit has been removed and the α subunit β-propeller turned to show the face that contacts the β subunit. (C) Portions of a ClustalW alignment between αPS2, αV, and closely related α subunits from C. elegans and human showing conservation of the residues that are mutated. The if35 mutation changes a cup tetrapeptide repeat within the core of the β-propeller. The ifC2B mutation changes the ligand binding pocket. The if17 allele changes the second of four cation binding sites. The divalent cation coordination consensus is outlined on the alignment. The if21 allele changes a GAP repeat located in the core of the β-propeller.
By aligning αPS2 to αV, we were able to examine the position of the mutant residues within the αVβ3 structure (Fig. 1) (Xiong et al., 2002). The extracellular portion of the αV subunit is comprised of a seven bladed β-propeller that rests on an elongated stalk containing a thigh domain and two calf domains. At the central core of the β-propeller, where both if21 and if35 mutations map, extensive contacts with the β3 subunit are made. Four divalent cation-binding sites are found in hairpin loops of blades 4-7, opposite the α/β interface, and the if17 mutation maps to the second of these sites. The ligand-binding pocket resides at the top of the heterodimer in a crevice between the α and β subunits. It is in this site that the ifC2B mutation lies.
To address how each mutation affects the developmental functions of the PS2 integrin, we used new tools to re-examine their phenotypes. Using molecular markers we tested how each mutation affected the expression of αPS2, the attachment of muscles to the ECM, the recruitment of the integrin-associated protein talin, and the linkage of PS2 integrin to the cytoskeleton. Null inflated mutations cause the embryonic somatic muscles to lose their attachment via the tendon matrix to the epidermis, with detachment of the muscle cell membrane from the matrix and a lack of talin recruitment to muscle ends (Bloor and Brown, 1998; Prokop et al., 1998; Brown et al., 2002) (e.g. Fig. 2B). We found that five of the mutations affect muscle attachment, each giving a phenotype distinct from the null phenotype. The muscle defects fell into two distinct categories: membrane detachment from the ECM with retention of talin, and detachment of talin and actin from integrins in the membrane.
Figure 2. The ifC2B mutation causes αPS2βPS to detach from the ECM.
Embryos were triple labelled with antibodies against αPS2 (green), talin (red), and myosin (blue). (A) In wild type embryos, talin and αPS2 colocalize at MAS. (B) Talin localization is dependent on integrins. Talin fails to be recruited to muscle ends in inflated null mutant (ifB4) embryos. (C) In ifC2B mutant embryos, talin colocalizes with αPS2 at MAS, and even at the end of detached muscles (arrowheads). Scale bar 50μm.
Mutation of a residue predicted to contact the extracellular ligand causes detachment from the ECM
The ifC2B mutation alters the presumptive ligand-binding pocket of αPS2, and is equivalent to a change in residue D218 of αV to an N (Fig. 1). This residue forms the major contacts with the arginine side chain of the RGD ligand (Xiong et al., 2002), and so a change to asparagine would eliminate one of the two salt bridges between αV and its ligand. If the equivalent residue in αPS2 is used similarly, this mutation should impair its interaction with RGD-containing ligands.
Embryos homozygous for the ifC2B mutation were previously reported to display a strong midgut phenotype similar to if null mutant embryos (Bloor and Brown, 1998), and this remains the case. However, using a new method to prepare samples for antibody staining we also found that, in contrast to what we reported previously, ifC2B mutant embryos displayed a muscle phenotype similar to, but weaker than, inflated null mutants. Normal levels of αPS2 were found at MAS in ifC2B embryos (Fig. 2C’), and wild type levels of talin were recruited, suggesting that the intracellular function of the integrin was normal (Fig. 2C”). However, αPS2βPS was not able to mediate effective adhesion because the muscles detached, retaining integrin and talin at the ends (Fig. 2C). This differs from the null allele, where the muscle ends lacked both integrin and talin (Fig. 2B). Thus, the ifC2B mutant phenotype is consistent with detachment between integrins and the ECM, as suggested by the molecular change. We also re-examined the phenotype of the if2B1 allele, which shares with ifC2B the ability to complement ifSEF. It still only affected midgut development, and we did not detect any changes in αPS2 subunit expression (data not shown). Since the mutation does not lie in the coding region and the expression was not changed, the defect in this mutant remains unresolved.
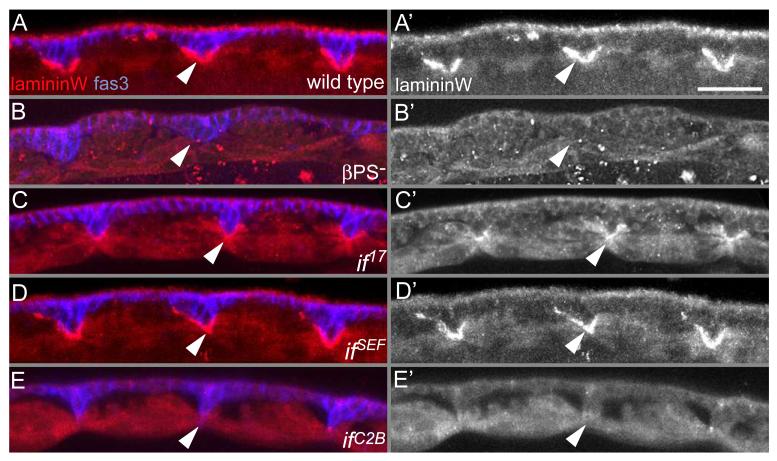
At least two RGD-containing extracellular proteins are present in the tendon matrix of Drosophila MAS: tiggrin and lamininW, the laminin trimer containing the α1,2 subunit encoded by wing blister (Fogerty et al., 1994; Martin et al., 1999). Expressed fragments of each support spreading and attachment of cells expressing αPS2βPS (Fogerty et al., 1994; Graner et al., 1998). To gain further evidence for the ifC2B mutation causing reduced binding to the ECM, we examined the integrin dependence of lamininW recruitment, since tiggrin does not require integrins for its recruitment (Fogerty et al., 1994).
The αPS2 RGD binding site is required for lamininW assembly at muscle attachment sites
In addition to using the ECM as a substrate for adhesion, integrins localize and organize extracellular ligands (Wu et al., 1995; Li et al., 2002). To determine first whether lamininW localization requires integrin, we examined lamininW distribution in embryos lacking βPS (thus lacking all βPS-containing heterodimers), and found that lamininW failed to accumulate at MAS (Fig. 3B vs. A). The absence of localized lamininW appeared to be due to a failure to recruit the secreted protein rather than a failure to express it as lamininW mRNA levels were normal in βPS mutant embryos (Fig. 4). Thus, lamininW recruitment requires integrins and therefore provides an assay in the embryo for integrin binding to an ECM ligand. In ifC2B mutant embryos, lamininW was absent at MAS (Fig. 3E) and between the two cell layers of the midgut (not shown). To test whether loss of lamininW is a general consequence of reduced αPS2 function, we examined if17, if21, and ifSEF mutant embryos and found that lamininW localized normally (Fig. 3C, D and if21 not shown). Therefore, only the ifC2B mutation altered recruitment of a ligand within the embryo, suggesting that it is crucial for ligand binding and the other partial loss of function inflated mutants are not impaired in ligand binding. The ifC2B mutant phenotype is likely to be a consequence of the loss of binding multiple ECM ligands, not just lamininW, because the absence of lamininW causes a weaker phenotype than the ifC2B mutation (Martin et al., 1999).
Figure 3. LamininW localization to basement membranes is dependent on the αPS2 RGD binding site.
(A-E) Embryos were labelled with antibodies against the α chain of lamininW (red, white) and the epidermal septate junction marker, fasiclin III (blue), scale bar 50μm. (A). In wild type, lamininW localized to the ECM between adjacent muscles in a V-shaped pattern. (B) Dependence of lamininW localization on integrins. Embryos that lacked βPS (mysXG43), and therefore lacked all βPS-containing heterodimers, failed to localize lamininW. LamininW localization was not altered in if17 (C) and ifSEF (D) mutant embryos. (E) LamininW was absent from muscle attachment sites in ifC2B mutant embryos.
Figure 4. Integrin is not required for the expression of the lamininW α chain.
(A and B) In situ hybridization against wing blister mRNA shows that the laminin α chain was expressed normally in embryos lacking βPS.
Mutations that reduce αPS2 transport to muscle ends lead to cytoskeletal detachment
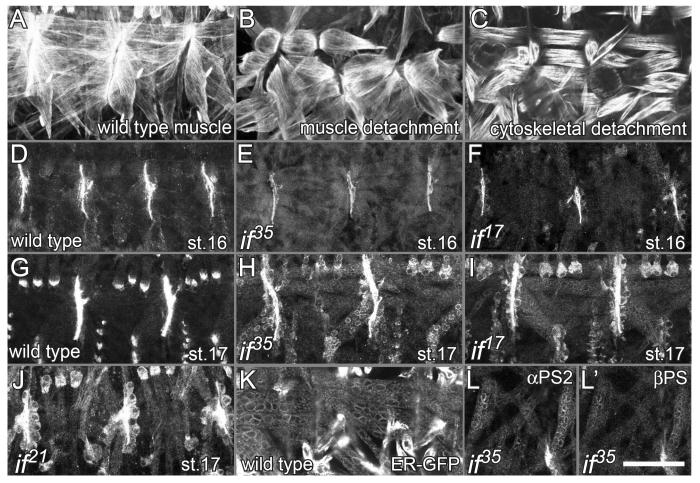
The second class of muscle phenotypes we observed for inflated partial loss of function mutants is the detachment of the cytoskeleton from the muscle cell membrane. This is distinctive from that caused by inflated null or ligand binding mutations because the muscles remained attached to one another, but the cytoskeleton retracted from the muscle ends (Fig. 5A-C, Fig. 6). Similar phenotypes were previously reported for mutations in the integrin-associated proteins integrin-linked kinase and PINCH (Zervas et al., 2001; Clark et al., 2003). The three hypomorphic inflated alleles, if17, if21 and if35, displayed this weak cytoskeletal detachment phenotype. The first muscle defects were observed at the end of embryogenesis (stage 17), when the actin within the muscle retracted, visible as an increase in the gaps between the actin in adjacent muscles (Fig. 5C, Fig. 6D, and data not shown). This defect appears to be caused by a reduction in the amount of αPS2 at MAS.
Figure 5. αPS2 transport to the plasma membrane is defective in inflated mutants that display cytoskeletal detachment.
(A-C) Phalloidin staining of F-actin highlights the muscles. (A) Wild type embryo, (B) muscle detachment in ifC2B mutant embryos at stage 16, (C) actin detachment in stage 17 if17 mutant embryos. (D-J, L) Embryos were labelled with antibodies against αPS2. (D and G) In wild type embryos the αPS2 subunit is localized exclusively to MAS and the levels of αPS2 increase between stage 16 and stage 17. (E and F) At stage 16 the levels of αPS2 localized to muscle ends in if35 and if17mutant embryos were reduced compared to wild type. (H, I, and J) The αPS2 subunit accumulates in perinuclear regions inside the muscle and tendon cells in if17, if35 and if21mutants, as does the (K) endoplasmic reticulum component protein disulfide isomerase, tagged with green fluorescent protein (ER-GFP; (Bobinnec et al., 2003). (L and L’) if35 mutant embryos were double labelled with αPS2 and βPS antibodies. The βPS subunit also fills the inside of muscle and tendon cells. Scale bar 50μm.
Figure 6. Muscle detachment versus cytoskeletal detachment phenotypes observed in different inflated mutant alleles.
Each panel shows muscles with the membrane labelled by expression of green fluorescent protein fused to the myristylation signal from Src (Src-GFP, green), and F-actin by phalloidin staining (red). (A, B) wild type embryos at stage 16 and 17, respectively. (C) In ifC2B embryos the muscles start detaching at stage 16, resulting in rounded up muscles with the membrane (white arrow) in close proximity to actin (red arrow). (D) In if21 mutant embryos (and if17, if35 and ifSEF, not shown), the muscle actin (red arrows) contracted away from the attached muscle ends labelled with Src-GFP (white arrows). Scale bar 50μm.
In if17 if21, and if35 mutant embryos reduced αPS2 at muscle ends was first detected 4 hours earlier, at stage 16 (Fig. 5E,F and not shown). By stage 17, much of αPS2 was found inside the muscles, and also within the cells that the muscles attach to, the tendon cells (Fig. 5H,I,J). The intracellular retention of αPS2 provided a conclusive demonstration that αPS2 is expressed in the tendon cells, as suggested by the inflated null phenotype (Prokop et al., 1998). The endoplasmic reticulum (ER) within the muscles has a similar perinuclear distribution as intracellular αPS2 (Fig. 5K). This suggests that the folding of the mutant proteins was less efficient and/or the ability to form heterodimers was reduced, resulting in their retention in the ER. The formation of heterodimers is required for the transport of integrins from the ER to the plasma membrane (e.g. Rosa and McEver, 1989), and consistent with this, the βPS subunit also accumulated around nuclei in if17 if21, and if35 mutants embryos (Fig. 5L’ and data not shown).
The if17 mutation maps to D309, which is within the second of four divalent cation coordination sites in the αPS2 subunit (Fig. 1) (Xiong et al., 2001). The side chain oxygen atoms from this aspartic acid contribute directly to the coordination of divalent cations (Xiong et al., 2001), and so the change to asparagine eliminates one of the coordinating oxygens. The intracellular accumulation of αPS2 suggests that cation coordination is important for either the folding of the αPS2 protein or its ability to form heterodimers.
The if21 and if35 mutations reside in the central core of the α subunit β-propeller (Fig. 1). The if35 mutation maps to an invariant glycine residue in the second of seven repeated motifs referred to as ‘cup tetrapeptides’ (consensus ϕϕGϕ where ϕ is aromatic) (Xiong et al., 2001). The if21 mutation maps to a conserved alanine within the fifth GAP motif, another repeated structure that contributes to the core of the β-propeller. Together, the seven cup tetrapeptides and six GAP motifs contribute to form a ‘cage motif’ (consensus x17-33-ϕϕGϕx-30-Px2-15Gx5-8) that lies at the center of the contact region between the α and β subunits. In the αVβ3 crystal, the cage motif holds a conserved arginine (R261) from β3 in place (Xiong et al., 2001). Thus, the if21 and if35 mutations could affect folding, but their position suggests that they impair heterodimer formation; either defect would reduce transit from the ER to the plasma membrane. As discussed further below, what is surprising about all three mutations is that the small amount of protein that does reach the plasma membrane has considerable function.
Confirmation of mutant phenotypes in cell culture
To confirm that the sequence alterations we identified do cause the defects observed, we introduced the changes in ifC2B, if17 and if21 into the αPS2 coding sequence and co-expressed each with the βPS subunit in S2 cells. Consistent with the distribution of the mutant proteins in embryos, ifC2B was expressed as well as wild type, while only half the level of if17 and if21 reached the cell surface (Fig. 7A). A series of mutations in the βPS I-like domain also caused a reduction in surface expression of αPS2βPS, especially in the presence of an αPS2 activating mutation in the cytoplasmic domain (GFFNR>GFANA), which is thought to reduce the association between α and β subunits (Vinogradova et al., 2000; Jannuzi et al., 2004). When we added this activating mutation to our mutant alleles, the levels of if17 and if21 were further reduced to approximately 30 and 5 percent, respectively, of the values seen for the GFANA mutation alone or in combination with ifC2B (Fig. 7A). Thus, further destabilisation of the interactions between α and β subunits enhances the failure to reach the cell surface, suggesting that the primary defect may be in the formation of the heterodimer.
We then examined the effect of the mutations on adhesion to ECM ligands. First, we performed cell-spreading assays on plates coated with fragments of tiggrin or lamininW that contain their RGD sites. We previously obtained comparable results assaying cell spreading and cell adhesion (Jannuzi et al., 2004), and have found that spreading is the more robust assay. Untransfected cells lack the αPS2 integrin subunit and do not spread on either substrate (Graner et al., 1998), so the spreading is mediated solely by the transfected αPS2 subunit in combination with βPS. On tiggrin, the D244 to N mutation of ifC2B significantly impaired spreading (Fig. 7B), particularly when present within the m8 form. The other two mutations had a modest effect on spreading, which is likely to be explained by the reduced levels on the cell surface; despite having less surface integrin, they spread better than ifC2B. While the ifC2B mutation had a dramatic affect on lamininW recruitment in vivo, it did not cause a significant reduction in cell spreading on lamininW (Fig. 7B). Recruitment of lamininW may involve capture of soluble protein, so we next tested the affinity of the mutant integrins for soluble ligand with the ligand-mimetic antibody TWOW-1 (Bunch et al., 2006). We measured TWOW-1 binding relative to total cell surface integrin in untreated cells and cells treated with Mn2+, which activates the PS2 integrin. The ifC2B mutation essentially abolished TWOW-1 binding under both conditions (Fig. 7C), demonstrating that this mutation strongly reduced ligand affinity. Both if17 and if21 mutant integrins retained ligand-binding ability, with apparently greater affinity for ligand than wild type in the presence of Mn2+ (Fig. 7C). The increase may be due to reduced association between the two subunits in the mutant heterodimers, comparable to the increase in ligand binding of Mn2+ treated PS2 integrin caused by the GFFNR>GFANA activating mutation (Bunch et al., 2006). Combining this mutation with ifC2B was not able to restore TWOW-1 binding (data not shown).
Normal levels of talin localize to attachment sites despite the reduction of integrins
Talin is the most crucial of the integrin-associated proteins that have been characterized in Drosophila, and removing talin causes an almost identical phenotype to removing integrins (Brown et al., 2002). The recruitment of talin to the plasma membrane is thought to occur by the binding of talin directly to the β cytoplasmic tail (Pfaff et al., 1998; Calderwood et al., 1999; Tanentzapf and Brown, 2006; Tanentzapf et al., 2006). Consistent with this, talin fails to localize to MAS in the absence of αPS2βPS (Brown et al., 2002) (Fig. 2B). We were therefore surprised to find that the levels of talin appeared wild type in if17, if21 and if35 embryos despite the low levels of integrin at muscle ends (Fig. 8B” versus A”; if35 mutants are shown as an example). Even though most αPS2 and βPS was in the ER by late stage 17, talin only colocalized with αPS2βPS at muscle ends (Fig. 8C). This indicates that the βPS subunit in the ER was not able to recruit talin.
Figure 8. Reduced levels of PS2 integrin recruit wild type levels of talin.
Embryos were labelled with antibodies against αPS2 (green) and talin (red). (A and B) The amount of αPS2 transported to muscle ends was reduced in if35 mutants (compare A’ to B’), however talin at MAS was not changed (A” and B”). Scale bar 50μm (C) Magnification of if35 MAS at stage 17, scale bar 25μm. Talin preferentially localized to muscle ends and not with the αPS2 and βPS that is trapped inside the cell. (D) Quantification of average ratios of talin to αPS2 immunofluorescence at direct and indirect MAS from wild type and if35 mutant embryos. An example of a direct MAS is indicated by an arrowhead in B’and B”. The arrows in B’ and B” point to an indirect MAS.
To quantify the ratio of talin to αPS2 that is localized to MAS, we measured the intensity of immunofluorescence of the two proteins. We adjusted the confocal microscope so that the signal from anti-talin antibodies was equivalent to that from anti-αPS2 in wild type embryos and this ratio proved approximately consistent from embryo to embryo, in both indirect and direct MAS (formed by muscles that attach to both tendon cells and other muscles versus those that only attach to tendon cells) and at different developmental stages. Using if35 mutant embryos as an example, we then measured the talin/αPS2 immunofluorescence ratio under identical settings. The ratio at muscle ends was increased to approximately 2 to 1, measuring as high as 3.7 to 1 in some direct MAS (Fig. 8D). This shows that despite the apparent direct interaction between the two proteins, talin and integrin do not have a fixed stoichiometry within integrin adhesive sites (the experiment does not reveal the relative number of molecules, but does show that amount of talin per integrin was increased in the mutants relative to wild type). The imbalance between the levels of integrin and talin in if17, if21, and if35 mutants is coincident with the onset of cytoskeletal detachment. Examination of these embryos closely shows that in the muscles, talin is pulled away from αPS2βPS (Fig. 9B, C). The enlargement in Figure B”” shows free integrin (green only, green arrow) not associated with talin (red arrows, except in the tendon cell toward the top of the figure), showing that the majority of talin is separated from integrin.
Figure 9. Talin separates from integrins when PS2 integrin levels are reduced at muscle attachment sites.
To determine the point at which the link between integrins and the cytoskeleton was failing in if17, if21, and if35 muscles, stage 17 mutant embryos were labelled with antibodies against αPS2, talin, myosin and βPS as indicated. (A) In wild type embryos, talin and αPS2 colocalized at muscle ends. (B) In if17 mutant embryos, talin and myosin detached from the muscle ends, and separated from αPS2 that remains at attachment sites (arrowheads). (C) In if35 mutant embryos talin separated from both integrin subunits and followed the detaching cytoskeleton (arrowheads). A”” to C”” show enlarged segments of panels A to C, respectively. (B””) highlights the separation between integrin (green arrow) and talin at the muscle ends (red arrows). Scale bars 50μm.
αPS2βPS is depleted from muscle ends in ifSEF mutant embryos
The ifSEF allele also caused a cytoskeletal detachment phenotype in late embryonic muscles, but in contrast to if17, if21and if35 mutants, we found that αPS2 was expressed normally in ifSEF embryos at stage 16 (Fig. 10E vs. B). However, 4 hours later at stage 17, levels of αPS2 at MAS were dramatically reduced and this reduction coincided with the onset of cytoskeletal detachment (Fig. 10F vs. C). The level of βPS at MAS was also reduced (Fig. 11), showing that it is the αPS2βPS heterodimer that becomes reduced, not just the αPS2 antibody epitope. In the midgut, αPS2 levels were normal, and consistently, ifSEF embryos did not display any midgut defects (Fig. 10G vs. D) (Bloor and Brown, 1998). Based on this specific loss of αPS2 in the muscle at late stages and the lack of any changes in the coding sequence, we hypothesized that the ifSEF allele affects promoter/enhancer elements in the inflated gene required for a second phase of muscle-specific expression. This was tested by examining the levels of inflated mRNA by in situ hybridisation, which revealed a specific reduction in the somatic muscles versus the visceral muscles (Fig. 10H-K). In addition, this experiment provided further confirmation that αPS2 is expressed in tendon cells, as inflated mRNA was clearly detectable in tendon cells (Fig. 10H,I black arrowhead), which was also reduced in ifSEF mutant embryos (Fig. 10J,K). This mutant therefore provides an opportunity to distinguish between an initial function of αPS2βPS in establishing the muscle attachment junction from a later role in maintaining it.
Figure 10. Cytoskeletal detachment in ifSEF embryos is caused by a late loss of αPS2 expression in muscles.
(A) Stage 17 ifSEF embryonic muscles displayed a cytoskeletal detachment phenotype. Embryos were stained with the indicated antibodies. (B-G) Embryos labelled with αPS2 antibodies. (B-C) The levels of αPS2 increased from stage 16 to stage 17 in wild type muscles. (E) The levels of αPS2 in stage 16 ifSEF muscles were similar to wild type, but at stage 17 (F) αPS2 levels have dropped dramatically in ifSEF muscles. (D and G) αPS2 levels are similar in the visceral muscle layer that surrounds the midgut (arrowheads) in wild type and ifSEF embryos, but reduced at MAS (arrows) in the ifSEF embryo. (H-K) The level of inflated mRNA (encoding αPS2), as revealed by in situ hybridisation, was reduced in the muscles (arrow) and tendon cells (black arrowhead) relative to the visceral mesoderm (white arrowhead) in early stage 16 ifSEF embryos (J,K) relative to wild type (H,I). Scale bars 50μm.
Figure 11. Muscle expression of both αPS2 and βPS are reduced in ifSEF mutants.
The levels of αPS2 (green) in stage 17 ifSEF embryonic muscles (B and B’) is severely reduced compared to wild type (A and A’). βPS, which partners with αPS2 in muscle and αPS1 in the tendon cells, is also reduced from the muscles but remains in the tendon cells, paired with αPS1 (A” vs. B”). This shows that the ifSEF allele cause a loss of the αPS2βPS heteodimer rather than a loss of the epitope recognised by the αPS2 antibody. Scale bar 50μm.
We tested whether talin recruitment was affected by the disappearance of αPS2, and surprisingly talin levels were nearly wild type in ifSEF mutant embryos at the stage when αPS2 was greatly reduced (Fig. 12B). Not only that, talin levels dramatically increased at MAS during the two hour period between mid-stage 16 to stage 17, as the αPS2 levels were dropping. As before, we quantified the ratio of talin and αPS2 immunofluorescence after setting levels in wild type to 1:1 and found that by stage 17 the average ratio in ifSEF mutant embryo MAS increased to approximately 3 to 1. In direct MAS, the average ratio was closer to 4 to 1 (Fig. 12C). These ratios are underestimates, as we have not subtracted the background levels of nonspecific immunofluorescence from the αPS2 antibody in ifSEF embryos. This result provides even stronger evidence that the stoichiometry of integrin and talin is not fixed in the integrin junctions.
Figure 12. Talin remained localized to muscle attachment sites lacking αPS2 in ifSEF mutants.
Embryos were labelled with αPS2, talin and myosin antibodies. (A) In wild type stage 17 embryos, the confocal settings were adjusted so that the levels of talin were approximately equal to the levels of αPS2 at MAS. (B) Using the same confocal settings, αPS2 was barely detectable at stage 17 ifSEF MAS, while the levels of talin remained approximately equivalent to wild type. (C) Quantification of αPS2 and talin immunofluorescence. The average ratio of talin to αPS2 immunofluorescence is represented. Scale bar 50μm.
Discussion
New in vivo functions established for individual residues of αPS2 integrin
In this study, partial loss-of-function alleles in the inflated gene were sequenced and examined for their effects on PS2 integrin function in the Drosophila embryo and in cell culture. By inspection of αPS2 expression, localization, ability to spread on extracellular ligands and recruit the cytoskeletal linker talin, we have assigned roles for individual αPS2 residues during morphogenesis.
Mutation in if17 of D309, an invariant residue in the second of four cation-binding sites, caused intracellular accumulation of αPS2. Divalent cations are critical for integrin function and both α and β subunits harbor multiple cation-binding sites (Hynes, 2002; Xiong et al., 2002); those within α subunits have been previously implicated in integrin biosynthesis. Mutations causing the blood clotting disorder Glanzmann’s thrombasthenia (Basani et al., 1996; Mitchell et al., 2003) that mapped in or near αIIb Ca2+ binding sites caused a reduction in αIIbβ3 surface expression, even though heterodimers could form. Thus, results from these diverse systems solidify the case that in vivo, the structural integrity of cation coordination sites is critical for integrin biogenesis. Mutations in the central core of the β-propeller also disrupted transport of αPS2βPS heterodimers to the plasma membrane. Both if35 and if21 alleles are changes in highly conserved residues contributing to the structure of the cage motif (Xiong et al., 2001). Because it extensively contacts the β3 subunit, this domain was suggested to be important for the formation or stability of heterodimers. Our results support this hypothesis as two different mutations in the αPS2 cage motif caused intracellular accumulation.
It is important to note that while the residues identified by these three alleles are important for αPS2βPS biosynthesis, they are not absolutely necessary for transport to the plasma membrane, because we did observe mutant integrin at MAS, and the integrin that is transported was functional both in the embryo and in cell spreading. These findings offer a possible explanation as to why these alleles do not affect wing adhesion: if the amount of integrin needed for adhesion is only a fraction of the cell surface integrin in the wing cells, then a reduction of this amount will not result in a defect. The muscle may be exceptional in needing all of the available integrin to mediate adhesion.
The mutation of residue D244 in the ifC2B allele demonstrated the importance of this residue for binding to RGD-containing ligands in vivo. Even the conservative change of the aspartic acid to asparagine caused αPS2βPS to detach from the ECM in muscles. D244 is equivalent to D218, which forms the key contact with the arginine of the RGD ligand (Xiong et al., 2002), and we found it astonishing that a random mutagenesis resulted in a mutation of this key residue. We found that the ifC2B mutation significantly impaired the ability of αPS2βPS integrin to mediate cell spreading on tiggrin, bind the soluble ligand TWOW-1, and recruit lamininW within the developing embryo.
The molecular nature of the unusual inflated alleles has not suggested a simple model to explain the interallelic complementation. For example, ifC2B/ifSEF transheterozygotes are viable and fertile, yet we now know that when they are late embryos, the αPS2 subunit derived from the ifSEF allele will have dissappeared from the muscles, leaving only the ifC2B mutant form of αPS2, which is impaired in binding to the ECM. Further work is required to discover why these animals have sufficient integrin function to survive.
Partial loss of function mutations in αPS2 provide new insights into the assembly of the integrin-cytoskeletal link
The distribution of talin in embryos homozygous for the unusual inflated alleles force a revision of our previous working model of the integrins-cytoskeletal link, built up from a number of findings. Mutations in the single talin gene in Drosophila caused an almost identical phenotype to elimination of βPS integrins (Brown et al., 2002), while genetic removal of other linker proteins such as integrin linked kinase (ILK) and PINCH caused substantially weaker phenotypes (Zervas et al., 2001; Clark et al., 2003). This suggested that integrins and talin form a “core” complex, which recruits other proteins with a more specialised function. This genetic model fit with increasing evidence of a direct interaction between talin and the β integrin subunit cytoplasmic tail, culminating with a crystal structure documenting one such interaction (Garcia-Alvarez et al., 2003). Thus, our previous model was that each ligand-bound integrin heterodimer would be bound to talin, which would serve as a scaffold for the assembly of an integrin adhesive junction. This concept was strengthened by the contribution of talin to the first step in integrin adhesion, as indicated by establishment of initial force resistance (Jiang et al., 2003). However, a prediction of this model was that the amount of talin recruited should depend on the amount of integrin, and we show here that this is not the case. Even though integrin is required to recruit talin, a reduction of integrin did not reduce the recruitment of talin, and in addition, integrin could be removed and talin remained at the junction in normal levels.
We can think of a variety of explanations for how talin recruitment could be integrin-dependent, yet not specified by the amount of integrin present. A possible, in our view unlikely, explanation is that normally only a fraction of the integrins at the adhesive sites are bound to talin, so that reducing the number of integrins still leaves enough to bind talin (our experiments did not reveal the relative number of molecules of integrin to talin). The subset of integrins associated with talin could be limited by the amount of active integrin (implying that not all the integrin at MAS is active) or talin, or by competition with other integrin-associated proteins. On the other hand, if there is normally one talin bound to each integrin, then our results would indicate that a single integrin molecule is recruiting additional talin molecules in these mutant conditions. It could be that the initial talin-integrin complex recruits other proteins, such as ILK, PINCH and others, and these proteins recruit more talin. Having talin both in direct contact with integrins and more peripheral in the complex of integrin-associated proteins fits with other recent findings. In addition to the talin head-β subunit cytoplasmic tail interaction, the rod domain contains sites interaction sites for both direct binding to integrins and indirect recruitment (Pfaff et al., 1998; Calderwood et al., 1999; Garcia-Alvarez et al., 2003; Tremuth et al., 2004; Franco et al., 2006; Tanentzapf and Brown, 2006; Tanentzapf et al., 2006). In particular, at MAS the head domain appears to be recruited by direct interaction with integrin and in competition with endogenous talin, while the C-terminal region requires endogenous talin to be recruited, suggesting it is more peripheral (Tanentzapf et al., 2006). In addition, in talin is observed to move with actin flow at the periphery of vertebrate cells, albeit at a reduced rate, rather than staying with the relatively immobile integrins (Brown et al., 2006; Hu et al., 2007), suggesting it provides a slippage clutch and functions in a peripheral position. A third possibility is that talin may be only recruited by binding directly to the integrin cytoplasmic tail, but then it forms additional protein interactions that enable it to dissociate from integrins and remain localised. This fits with the results from the ifSEF allele, which showed that integrins can be removed from muscle ends, yet talin remains.
Integrins at stable junctions are dynamic
We have characterized four mutations that each affect the levels of PS2 integrin at MAS, but in very different ways. Together these mutations reveal the dynamic nature of muscle attachment assembly and growth. The accumulation of αPS2 in the cytoplasm of if17, if21 and if35 mutants, particularly at late stages in embryogenesis, demonstrates that integrins are continuously being synthesized and transported. The rapid disappearance of αPS2 from the membrane in ifSEF embryos suggests that surface receptors are internalized and degraded at MAS. This exchange occurs at a time when an adhesion complex has already formed at MAS containing talin, ILK, PINCH, and other cytoplasmic factors (Zervas et al., 2001; Brown et al., 2002; Clark et al., 2003) (Brown et al., 2006; Hu et al., 2007) (Franco et al., 2006) (Tremuth et al., 2004), yet our results indicate that integrins are not locked into place. Thus muscle attachments are not static and rigid complexes, but are highly dynamic even in the context of stable adhesion.
The exchange of integrins within adhesive complexes has been measured in mammalian cell culture, with an exchange of 50% of β3 integrin within focal adhesions in 5 minutes (Ballestrem et al., 2001). However, turnover may not be a general property of all adhesion components. For example, in β4-containing hemidesmosomes, β4 integrin exchanged in 30 minutes, whereas BP180 (another transmembrane component of hemidesmosomes) failed to exchange (Tsuruta et al., 2003). It will be of interest to measure the relative exchange rates of integrin and talin in the Drosophila embryo.
Stabilizing the integrin cytoplasmic linkage
The subcellular position of the structural failure that occurs in the different if alleles is likely to reflect the relative strengths of interaction inside and outside the cell. Integrins are connecting two multiprotein complexes, the ECM and cytoskeleton. When integrins are completely removed, the muscle membrane detaches from the ECM, indicating that the remaining integrin-independent interactions between the cytoskeleton and the plasma membrane are stronger than those linking the plasma membrane to the ECM. However, when the integrins are reduced, the break happens between the membrane and the cytoskeleton, indicating that small amounts of integrin can mediate a stronger attachment to the ECM than the cytoskeleton. This fits very well with the force of interactions measured by optical tweezers, which have measured integrin-ECM binding at approximately 100pN (Litvinov et al., 2002), while integrin-talin interactions require only 2pN to break (Jiang et al., 2003). The intracellular connection may well be strengthened by the formation of the more elaborate complex of intracellular proteins, but our results suggest that the strength of the intracellular connection does not match the extracellular bond. Thus, even though a small number of integrins can mediate strong adhesion of the muscle membrane to the ECM, additional integrins must be recruited to build up the intracellular link to the cytoskeleton so that it becomes equally strong.
Interallelic complementation between if alleles: developmental changes in integrin function
In our previous study we showed that ifC2B/ifSEF transheterozygous flies are viable and fertile (Bloor and Brown, 1998). We now know that the αPS2 subunit derived from the ifSEF allele dissappears from the muscles, so we infer that the muscle ends of ifC2B/ifSEF late embryos only contain the ifC2B mutant form of αPS2, which is impaired in binding to the ECM. There are a variety of ways to explain how the ifC2B mutant integrin functions relatively normally late in development even though it is non-functional earlier. One explanation is that it does not bind ligand with sufficient affinity to recruit soluble lamininW, but is able to bind to lamininW recruited earlier by the ifSEF mutant αPS2, consistent with our adhesion assays in culture. However, the muscles continue to grow during larval life, suggesting that the ability to recruit lamininW would continue to be important. A second hypothesis is that new ECM proteins are synthesised late in development that can be bound by the ifC2B mutant integrin with sufficiently high affinity. We know there must be additional ligands involved, since for example the loss of lamininW alone does not give an embryonic phenotype equivalent to the ifC2B mutant phenotype (Martin et al., 1999). This contrasts with the adhesion between wing epithelia. Viable mutations in the wing blister locus, which reduce the amount of the lamininW, cause, as its name implies, blisters in adult wings (Martin et al., 1999), similar to those produced by the absence of integrins. Consistent with this, ifC2B is the only hypomorphic mutation that causes wing blisters (in adult mitotic clones) (Bloor and Brown, 1998). Therefore the ifC2B adult phenotype can be explained by the loss of lamininW binding and recruitment in the wing.
Acknowledgements
We are grateful to I. Delon, M. Narasimha, K. Röper, and G. Tanentzapf for critically reading the manuscript. This work was supported by grants from the Wellcome Trust to D.D. and N.H.B. (62169, 31315), and the National Institute of Health to T.A.B. and D.L.B. (R01 GM042474).
References
- Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319–32. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basani RB, Vilaire G, Shattil SJ, Kolodziej MA, Bennett JS, Poncz M. Glanzmann thrombasthenia due to a two amino acid deletion in the fourth calcium-binding domain of alpha IIb: demonstration of the importance of calcium-binding domains in the conformation of alpha IIb beta 3. Blood. 1996;88:167–73. [PubMed] [Google Scholar]
- Bloor JW, Brown NH. Genetic analysis of the Drosophila alphaPS2 integrin subunit reveals discrete adhesive, morphogenetic and sarcomeric functions. Genetics. 1998;148:1127–42. doi: 10.1093/genetics/148.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinnec Y, Marcaillou C, Morin X, Debec A. Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil Cytoskeleton. 2003;54:217–25. doi: 10.1002/cm.10094. [DOI] [PubMed] [Google Scholar]
- Bokel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3:311–21. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci. 2006;119:5204–14. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- Brown NH, Gregory SL, Rickoll WL, Fessler LI, Prout M, White RA, Fristrom JW. Talin is essential for integrin function in Drosophila. Dev Cell. 2002;3:569–79. doi: 10.1016/s1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Brown NH, King DL, Wilcox M, Kafatos FC. Developmentally regulated alternative splicing of Drosophila integrin PS2 alpha transcripts. Cell. 1989;59:185–95. doi: 10.1016/0092-8674(89)90880-5. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Helsten TL, Kendall TL, Shirahatti N, Mahadevan D, Shattil SJ, Brower DL. Amino acid changes in Drosophila alphaPS2betaPS integrins that affect ligand affinity. J Biol Chem. 2006;281:5050–7. doi: 10.1074/jbc.M508550200. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Miller SW, Brower DL. Analysis of the Drosophila betaPS subunit indicates that regulation of integrin activity is a primal function of the C8-C9 loop. Exp Cell Res. 2004;294:118–29. doi: 10.1016/j.yexcr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–4. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- Clark KA, McGrail M, Beckerle MC. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development. 2003;130:2611–21. doi: 10.1242/dev.00492. [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development. 1994;120:1747–58. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Senetar MA, Simonson WT, Huttenlocher A, McCann RO. The conserved C-terminal I/LWEQ module targets Talin1 to focal adhesions. Cell Motil Cytoskeleton. 2006;63:563–81. doi: 10.1002/cm.20145. [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC. Structural determinants of integrin recognition by talin. Mol Cell. 2003;11:49–58. doi: 10.1016/s1097-2765(02)00823-7. [DOI] [PubMed] [Google Scholar]
- Graner MW, Bunch TA, Baumgartner S, Kerschen A, Brower DL. Splice variants of the Drosophila PS2 integrins differentially interact with RGD-containing fragments of the extracellular proteins tiggrin, ten-m, and D-laminin 2. J Biol Chem. 1998;273:18235–41. doi: 10.1074/jbc.273.29.18235. [DOI] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–5. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Symonds EJ, Mould AP. Mapping functional residues onto integrin crystal structures. Curr Opin Struct Biol. 2003;13:236–43. doi: 10.1016/s0959-440x(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jannuzi AL, Bunch TA, Brabant MC, Miller SW, Mukai L, Zavortink M, Brower DL. Disruption of C-terminal cytoplasmic domain of betaPS integrin subunit has dominant negative properties in developing Drosophila. Mol Biol Cell. 2002;13:1352–65. doi: 10.1091/mbc.01-08-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannuzi AL, Bunch TA, West RF, Brower DL. Identification of integrin beta subunit mutations that alter heterodimer function in situ. Mol Biol Cell. 2004;15:3829–40. doi: 10.1091/mbc.E04-02-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–7. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–90. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov RI, Shuman H, Bennett JS, Weisel JW. Binding strength and activation state of single fibrinogen-integrin pairs on living cells. Proc Natl Acad Sci U S A. 2002;99:7426–31. doi: 10.1073/pnas.112194999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Zusman S, Li X, Williams EL, Khare N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S. wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J Cell Biol. 1999;145:191–201. doi: 10.1083/jcb.145.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WB, Li JH, Singh F, Michelson AD, Bussel J, Coller BS, French DL. Two novel mutations in the alpha IIb calcium-binding domains identify hydrophobic regions essential for alpha IIbbeta 3 biogenesis. Blood. 2003;101:2268–76. doi: 10.1182/blood-2002-07-2266. [DOI] [PubMed] [Google Scholar]
- Pfaff M, Liu S, Erle DJ, Ginsberg MH. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem. 1998;273:6104–9. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- Prokop A, Martin-Bermudo MD, Bate M, Brown NH. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophila embryos. Dev Biol. 1998;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Rosa JP, McEver RP. Processing and assembly of the integrin, glycoprotein IIb-IIIa, in HEL cells. J Biol Chem. 1989;264:12596–603. [PubMed] [Google Scholar]
- Tanentzapf G, Brown NH. An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat Cell Biol. 2006;8:601–6. doi: 10.1038/ncb1411. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Martin-Bermudo MD, Hicks MS, Brown NH. Multiple factors contribute to integrin-talin interactions in vivo. J Cell Sci. 2006;119:1632–44. doi: 10.1242/jcs.02859. [DOI] [PubMed] [Google Scholar]
- Tepass U. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev Biol. 1996;177:217–25. doi: 10.1006/dbio.1996.0157. [DOI] [PubMed] [Google Scholar]
- Tremuth L, Kreis S, Melchior C, Hoebeke J, Ronde P, Plancon S, Takeda K, Kieffer N. A fluorescence cell biology approach to map the second integrin-binding site of talin to a 130-amino acid sequence within the rod domain. J Biol Chem. 2004;279:22258–66. doi: 10.1074/jbc.M400947200. [DOI] [PubMed] [Google Scholar]
- Tsuruta D, Hopkinson SB, Jones JC. Hemidesmosome protein dynamics in live epithelial cells. Cell Motil Cytoskeleton. 2003;54:122–34. doi: 10.1002/cm.10089. [DOI] [PubMed] [Google Scholar]
- Vinogradova O, Haas T, Plow EF, Qin J. A structural basis for integrin activation by the cytoplasmic tail of the alpha IIb-subunit. Proc Natl Acad Sci U S A. 2000;97:1450–5. doi: 10.1073/pnas.040548197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–24. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–45. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–5. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–18. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]