Abstract
The development of novel therapies against neurodegenerative disorders requires the ability to detect their early, presymptomatic manifestations in order to enable treatment before irreversible cellular damage occurs. Precocious signs indicative of neurodegeneration include characteristic changes in certain protein levels, which can be used as diagnostic biomarkers when they can be detected in fluids such as blood plasma or cerebrospinal fluid. In the case of synucleinopathies, cerebrospinal alpha-synuclein (α-syn) has attracted great interest as a potential biomarker; however, there is ongoing debate regarding the association between cerebrospinal α-syn levels and neurodegeneration in Parkinson disease and synucleinopathies. Post-translational modifications (PTMs) have emerged as important determinants of α-syn's physiological and pathological functions. Several PTMs are enriched within Lewy bodies and exist at higher levels in α-synucleinopathy brains, suggesting that certain modified forms of α-syn might be more relevant biomarkers than the total α-syn levels. However, the quantification of PTMs in bodily fluids poses several challenges. This review describes the limitations of current immunoassay-based α-syn quantification methods and highlights how these limitations can be overcome using novel mass-spectrometry-based assays. In addition, we describe how advances in chemical synthesis, which have enabled the preparation of α-syn proteins that are site-specifically modified at single or multiple residues, can facilitate the development of more accurate assays for detecting and quantifying α-syn PTMs in health and disease.
Strong genetic and neuropathological evidence suggests that α-synuclein (α-syn)1 has a central role in the development of several neurodegenerative disorders, collectively known as synucleinopathies, of which the most common is Parkinson disease (PD). PD is a movement disorder that is characterized by the loss of dopamine-producing neurons and the presence of intracellular protein inclusions (known as Lewy bodies (LBs)) in the brain stem of affected patients. Primary diagnosis of PD relies on motor symptoms, which appear only when more than 75% of the dopaminergic neurons in the substantia nigra have degenerated (1, 2), and current therapies offer only transient and symptomatic treatment. Despite the lack of effective therapies, there is consensus that early intervention with lifestyle changes and disease-modifying strategies could dramatically change the course of the disease. Therefore, the identification and validation of biomarkers of PD is crucial for early diagnosis, monitoring the progression of the disease, designing clinical trials, and assessing the effectiveness of therapeutic strategies.
The presence of fibrillar and aggregated forms of α-syn within LBs combined with the findings that genetic mutations (3–7) or gene duplication or triplication (8, 9) promote α-syn aggregation and fibrillization and cause early-onset forms of PD suggest that the process of LB formation plays a central role in neurodegeneration and the pathogenesis of PD. The molecular factors that contribute to triggering α-syn aggregation and LB formation remain unknown. Studies using animal and cell culture models of synucleinopathies, as well as from human PD cases, suggest that an increase in the level of α-syn is sufficient to trigger its aggregation and neurodegeneration (10–13). In addition, post-translational modifications such as phosphorylation and ubiquitination have emerged as consistent markers of α-syn pathology. For example, α-syn within LBs has been shown to be phosphorylated (at S87, S129, or Y125) (14–16), ubiquitinated (K12, K21, or K23) (17, 18), truncated (at its C terminus) (17, 19), and oxidized (by tyrosine nitration (20, 21)). Monomeric, oligomeric, and post-translationally modified α-syn can be detected in the cerebrospinal fluid (CSF) and plasma (22, 23), making α-syn an ideal target for biomarker discovery. Some disease-associated α-syn modifications—namely, pS129 and ubiquitination (at multiple sites)—have also been detected in α-syn CSF and plasma from control cases and from PD, multiple system atrophy, and LB dementia cases (24). The extent to which α-syn levels and post-translational modifications in the CSF and blood plasma reflect the protein's condition in the CNS or correlate with disease progression or severity remains unknown.
This review article focuses on presenting an overview of the progress that has been made toward developing sensitive methods to detect and quantify α-syn levels. In addition to discussing the major challenges and bottlenecks in developing such methods, we also highlight recent advances in the chemical synthesis of α-syn and mass spectrometry techniques that will help researchers overcome these challenges and provide unique opportunities to screen for novel biomarkers of PD and related synucleinopathies.
Full-length α-syn
The overwhelming majority of methods employed to quantify α-syn in biological fluids rely on classical sandwich ELISA assays. Early studies seeking to establish a correlation between PD and α-syn levels in human CSF using ELISA-based methods suggested that PD patients could be characterized by a lower total α-syn CSF level (25–31); similar findings were recently reported for blood plasma (32). Other groups have quantified CSF α-syn using “quasi-solution,” bead-based Luminex® xMAP immunoassays, which are reportedly more sensitive than conventional ELISAs (33) and also reported lower total α-syn in PD patients than in healthy individuals (34, 35). Bidinosti and colleagues developed a novel, capture-independent time-resolved FRET immunoassay that allowed the quantification of α-syn in a single step, thus greatly improving assay throughput. They also measured lower total α-syn in the CSF of PD patients than in control subjects (36), suggesting that lower CSF α-syn levels may be of diagnostic value for identifying PD patients.
There are, however, conflicting reports about the nature of the change in α-syn levels between PD patients and controls; for example, Ohrfelt and colleagues observed no significant difference in total CSF α-syn between PD patients and age-matched healthy individuals (37). Furthermore, it has not been possible to use total CSF α-syn levels measured via immunoassay-based methods to distinguish between different synucleinopathies and other neurodegenerative disorders. Kasuga and colleagues reported significantly lower α-syn levels in the CSF of diffuse Lewy body disease patients than in patients with AD and other dementias (38), whereas others have found no significant differences between CSF α-syn levels when comparing diffuse Lewy body disease to other disorders (39, 40).
α-syn Oligomers
Several studies have suggested that oligomeric prefibrillar species of α-syn, rather than fibrils or LBs, are the primary toxic species in PD and related synucleinopathies (41, 42). Therefore, significant efforts and resources have been devoted to developing oligomer-specific antibodies and assays to detect and quantify oligomers in CSF, plasma, and brain tissues (35, 43). However, given the complexity and structural and morphological heterogeneity of α-syn oligomers, it is unlikely that a single antibody will be able to detect all forms of α-syn oligomers equally. To address this limitation, several groups have sought to develop general ELISA assays to detect all types of α-syn oligomers, irrespective of their structure or size. This is achieved by using the same monoclonal antibody for both the capture and the detection steps. Thus, recognition by the detection antibody can occur only if the epitope used by the capture antibody is also displayed (i.e. in an oligomer-specific situation) (35, 43). In these assays, fibrils would also be detected, but given their large size and reduced solubility, the fibrils could be removed via filtration or centrifugation protocols prior to sample analysis. Using such assays, elevated levels of α-syn oligomers have been detected in the CSF of PD patients (28, 43). However, such a difference was not observed when analyzing α-syn oligomers in blood plasma (32).
α-syn Post-translational Modifications
Phosphorylation at S129 (pS129) is one of the main disease-associated α-syn post-translational modifications (PTMs). Studies from human postmortem samples (14, 17) and animal models have shown that this PTM is largely up-regulated under pathological conditions: in human brain samples, pS129 α-syn represents more than 90% of the total α-syn found in LBs, compared with ∼4% under physiological conditions (14). In transgenic mice overexpressing α-syn (44), rat models overexpressing human α-syn using adeno-associated viral infection (45), and Drosophila models of PD (46), α-syn-induced pathology results in an increased detection of pS129 α-syn, the levels of which are positively correlated with the development of pathology.
Thus, several groups have explored the relationship between pS129 levels and PD to determine whether this modification is a viable biomarker candidate. Detecting and quantifying modified forms of α-syn in the CSF or blood plasma is inherently more challenging than detecting unmodified α-syn because of the much lower concentrations of modified forms of α-syn in biological fluids (estimated to be 4 to 10 times lower than that of total α-syn in the case of pS129 (34)) and the reversible nature of some of these modifications. Foulds et al. first found that pS129 α-syn could be detected in human plasma (24) by using a sandwich ELISA assay and a pS129-specific detection antibody, and they reported slightly higher pS129 α-syn levels in PD patients than in control subjects, thus suggesting a potential diagnostic value for this PTM. Analysis of oligomeric pS129 α-syn (by using the same phospho-specific antibody for both capture and detection as reported before (43)) did not reveal significant differences between PD patients and healthy subjects (24). Interestingly, in the same study it was demonstrated via Western blot analysis that phosphorylated α-syn in plasma is also mono- as well as polyubiquitinated (24), although the ubiquitination sites and chain lengths were not determined. Using a bead-based immunoassay employing a pS129-specific capture antibody, Wang and colleagues confirmed the results obtained by Foulds et al. by demonstrating a significantly higher pS129/total α-syn ratio in the CSF of PD and multiple system atrophy patients relative to healthy controls, but they observed no difference between controls and patients suffering from progressive supranuclear palsy or Alzheimer disease (34). They also found a weak but significant correlation between CSF pS129 α-syn levels and PD symptom severity as determined based on the Unified Parkinson's Disease Rating Scale rating scale (47).
Limitations of Immunoassay-based Techniques
Several factors influence the sensitivity and reproducibility of the different assays described above, among which are nonstandardized CSF and blood plasma collection and handling protocols (for example, Barbour and others have demonstrated that such samples can easily be contaminated by α-syn originating from lysed red blood cells and platelets (48)), the small number of samples in some studies (37), and, most important, the great diversity of capture and detection antibodies and variability in the quality and purity of the molecular standards used by different groups.
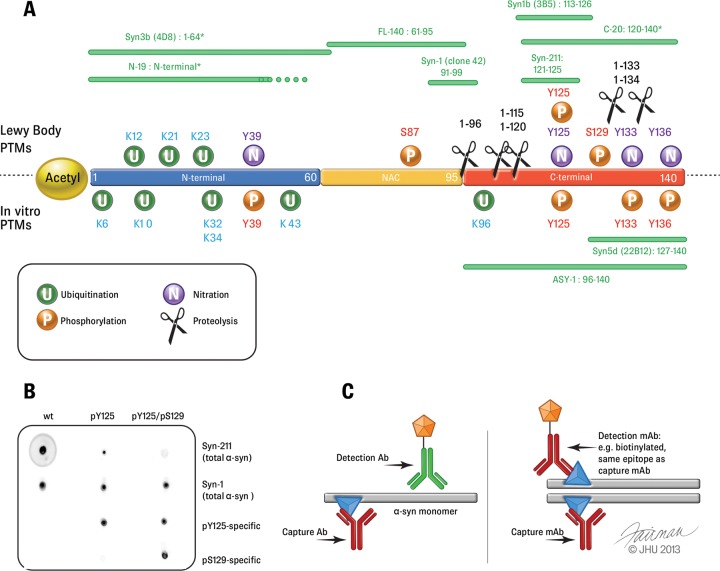
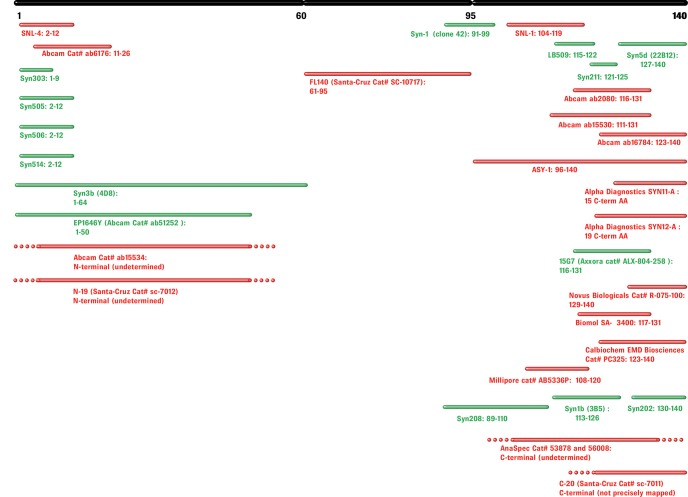
The majority of the antibodies used in such immunoassays have epitopes within the C-terminal region of α-syn encompassing residues 120–140 (See Figs. 1 and 2), which includes most of the protein's PTMs, including phosphorylation and truncations (49). Fig. 1 shows a map of the most commonly used antibodies for α-syn quantification in biological fluids, as well as the most commonly observed PTMs, splicing isoforms, and proteolytic fragments of α-syn. Therefore, antibodies such as Syn-211, which recognizes residues 121–125 and is one of the most commonly used antibodies to capture α-syn, will not capture C-terminally truncated variants of α-syn. Interestingly, the majority of disease-associated PTMs (phosphorylation, nitration, and truncations) cluster within residues 120–137. Furthermore, several metal binding sites have been shown to occur within this region of α-syn and alter the conformation of the C terminus (16, 50, 51). Because ELISA-based immunoassays are performed under nondenaturing conditions, it is likely that PTM or metal-dependent conformational changes with the C terminus may also affect the detection of modified forms of α-syn in the CSF and/or blood plasma. Indeed, unpublished results from our laboratory show that phosphorylation of C-terminal tyrosines decreases the sensitivity of C-terminal antibodies such as Syn-211, and a full-length modified α-syn phosphorylated at both Y125 and S129 was not detectable by this antibody (Fig. 1B).2 The more comprehensive list of antibodies shown in Fig. 2 (including antibodies for applications other than ELISAs) illustrates the predominance of C-terminally directed antibodies and highlights how the majority of available antibodies may show altered detection sensitivities toward C-terminally modified α-syn. Similarly, α-syn self-association and the formation of oligomers are likely to mask some epitopes within specific populations of oligomeric species, thereby precluding the possibility of accurate assessment of the levels of total α-syn oligomers. Other groups have developed their own oligomer-specific antibodies by using oligomeric α-syn as an immunogen (52, 53). Several protocols have been elaborated to induce α-syn oligomerization and isolate purified or enriched preparations of α-syn oligomers; for example, several groups have isolated α-syn oligomers immediately after dissolving lyophilized recombinant α-syn in saline solution (52, 54) or via incubation in the presence of metal ions (55), dopamine, or 4-oxo-2-nonenal (53). The size and structural properties of the oligomers vary greatly depending on the method used. Therefore, it is very likely that any oligomer-specific antibodies obtained by using a specific oligomer preparation may detect only a subset of α-syn oligomers in vivo. Furthermore, it is not known whether these antibodies will detect post-translationally modified (e.g. phosphorylated or ubiquitinated) α-syn oligomers with the same sensitivity as in the detection of unmodified α-syn.
Fig. 1.
Post-translational modifications of α-syn. A, the locations of the main α-syn PTMs (phosphorylation, ubiquitination, nitration, and truncation) are shown. Disease-associated PTMs identified in Lewy bodies are shown in the upper part of the scheme, and those identified from in vitro studies are shown below. Green bars above and below the sequence of α-syn show epitope maps of the most frequently used non-PTM and non-oligomer-specific antibodies for immunoassay-based α-syn quantification in the CSF and blood plasma. Stars indicate putative or incomplete epitope mapping. B, dot blot showing evidence that PTMs can interfere with the detection of modified α-syn by antibodies. Using the Syn-211 clone, the sensitivity for pY125 α-syn was reduced, and double-phosphorylated α-syn (pY125/pS129) was nearly undetected. The antibodies used for detection are shown on the right-hand side of the blots. C, schemes depicting the ordinary, α-syn monomer-directed ELISA assays (left) and the principle of the oligomer-specific ELISA system (right). Adapted from Ref. 23.
Fig. 2.
Epitope mapping of α-syn antibodies. Monoclonal and polyclonal antibodies are shown in green and red, respectively. Residue numbers corresponding to the epitopes are shown in bold (when available).
Integrity and Stability of Protein Standards
The accuracy and reliability of α-syn quantification is also dependent on the quality of the α-syn standards used to produce the calibration curves. Full-length recombinant α-syn can be obtained from different commercial vendors; Bidinosti et al. assessed and compared the use of recombinant human α-syn from different commercial sources as standards and found differences between the supplier-provided and actual protein concentrations, but these variations were much smaller than the values of α-syn concentrations in human CSF and therefore were not a major source of error (36). Nevertheless, one must still ensure of the purity of the final product, ideally by employing SDS-PAGE, analytical HPLC, and mass spectrometric characterization.
Regarding phosphorylated α-syn, two studies have been published to date (24, 34). In these studies, the phosphorylated protein standards were prepared via in vitro phosphorylation of recombinant α-syn using CK2 kinase (56). However, studies have shown that the extent of CK2 phosphorylation of α-syn does not exceed 5% (51). In the studies mentioned above, large amounts of kinase were added to compensate for its inefficiency (24, 34), which might have led to additional artifactual phosphorylation events. Therefore, in the case of post-translationally modified forms of α-syn, it is important to use conditions that lead to efficient and quantitative conversion of the protein as determined by mass spectrometry and/or to purify the modified protein of interest using RP-HPLC or other chromatographic methods.
Until recently, standards for the majority of post-translationally modified forms of full-length α-syn standards have been inaccessible because of the lack of enzymatic or chemical methods permitting site-specific modification of α-syn. For example, it is not possible to enzymatically prepare α-syn that is site-specifically and homogeneously phosphorylated at Y125 or S87 because the kinases that phosphorylate at these residues also phosphorylate at other sites. For example, Syk is an efficient tyrosine kinase, but it phosphorylates all C-terminal tyrosine residues (57), and the kinases that are specific (such as Lyn, Fyn, and Src) have been found to be inefficient (57, 58). Similarly, kinases that efficiently phosphorylate α-syn only at S87 remain to be identified; CKI phosphorylates α-syn at both S87 and S129 (16, 56). Moreover, the chemical conditions used to induce nitration of α-syn (e.g. treatment with tetranitromethane or peroxynitrite) result in nitration of all four tyrosine residues (59–61), and side reactions resulting in the formation of o,o′-dityrosine crosslinks have been reported (59). Because of the immense diversity of possible ubiquitination patterns (localization and combinations of modified sites, interubiquitin linkages, ubiquitin chain lengths), it has not been possible to generate well-defined ubiquitinated α-syn using in vitro methods and E3 ligases (62). To address these limitations, our laboratory has recently developed chemical and semisynthetic approaches that address these limitations and enable the introduction of site-specific PTMs at single or multiple residues in α-syn (see below).
To determine the limit of detection of an immunoassay, known amounts of the desired standards are usually spiked into a biological fluid (CSF, plasma, etc.) that was previously immunodepleted of the target antigen. Because some α-syn PTMs, particularly phosphorylation, are reversible, it is important to consider that spiked modified α-syn standards as well as the analytes of interest could be degraded or undergo dephosphorylation by phosphatases, affecting the accuracy and reproducibility of the measurements. The reversibility of some α-syn PTMs calls for the development of sample-processing protocols aimed at stabilizing the desired modification. As an example, the use of pervanadate to inhibit tyrosine phosphatases should be considered with caution: although pervanadate is very useful for stabilizing tyrosine-phosphorylated proteins for qualitative immunoblot and immunocytochemical analyses, the hydrogen peroxide used in its preparation is likely to induce heterogeneous methionine oxidations in the protein.
Mass Spectrometry for the Identification and Quantification of α-syn PTMs
Initial analysis of the chemical integrity of α-syn within LBs mainly involved the use of traditional immunohistochemistry techniques, which required the development of highly specific antibodies targeting specific regions of α-syn and key PTMs in the protein, a tedious and slow process. New methods based primarily on mass spectrometry are rapidly changing the analysis of α-syn. In recent years, the mass spectrometry field has witnessed steady technological evolution that has led to the introduction of new instruments with improved sensitivity, resolution, and mass accuracy (63, 64), often combined with attractive alternative fragmentation techniques such as electron-transfer dissociation (65) and higher-energy C-trap dissociation (66). New methods combining improved sample preparation, selective fractionation of specific peptide and protein subsets, high-performance mass spectrometry, and innovative bioinformatic tools have already delivered impressive results in the PTM analysis field for phosphorylation (67, 68), ubiquitination (69, 70), and acetylation (71). Mass spectrometry is rapidly becoming established not only as the method of choice for protein identification and PTM characterization, but also as an attractive alternative for quantifying changes in the levels of modified proteins. Parallel to this evolution, there has been growing interest in the clinical field in gaining access to specific quantitative assays that are easier to develop and capable of quantifying proteins and PTMs relevant to a disease state in large cohorts of samples. Although α-syn and its diverse proteoforms (72) constitute one of the major components of LBs, the use of mass spectrometry to characterize and quantify the various forms of α-syn in LBs, CSF, and blood has been limited. Early mass-spectrometry-based discovery studies identified more than 500 proteins associated with LBs (73, 74). In addition to the ubiquitous Nα-terminal acetylation and some C-terminally truncated forms, phosphorylation at residue S129 emerged as one of the major and most commonly detected PTMs (14, 17). Two additional phosphorylation sites involving residue S87 in the NAC region and Y125 in the C-terminal region of α-syn were identified within LBs and in cell culture studies using more conventional biochemical techniques (16, 75).
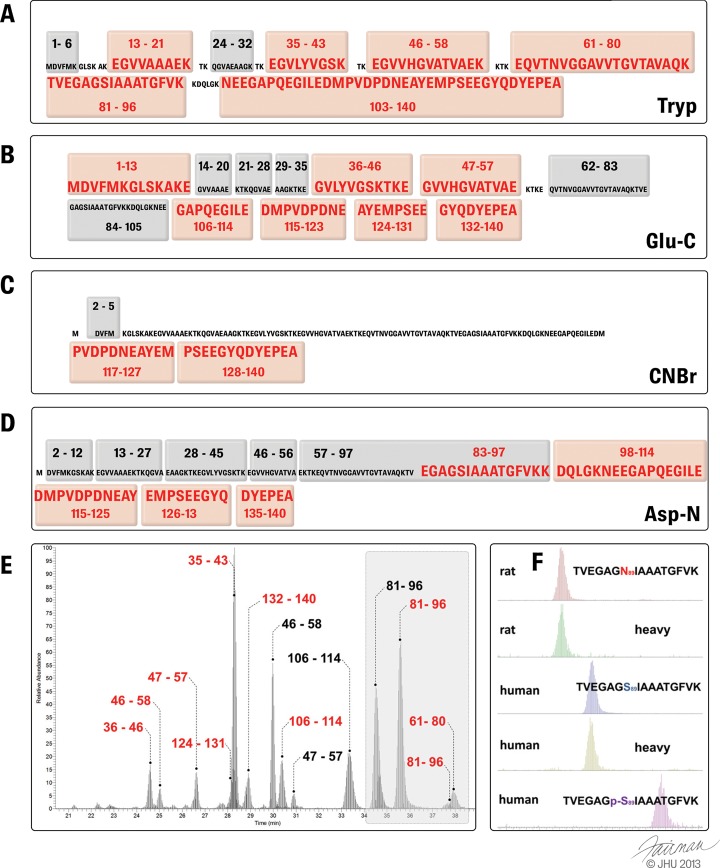
Monitoring PTM abundance is only possible if peptides carrying the modification of interest can be detected via MS. Therefore, enzymatic digestion constitutes a fundamental step in achieving sufficient sequence coverage. A major bottleneck associated with enzymatic proteolysis is the risk of generating proteolytic peptides of poor or reduced solubility or MS ionization properties, particularly when working with aggregation-prone proteins and amyloid-forming proteins such as α-syn. This in turn results in a compromise of the overall achievable protein sequence coverage, as observed in the case of trypsin proteolysis of α-syn. Trypsin digestion generates a large, hydrophilic, acidic C-terminal fragment of residues 103–140 carrying 11 glutamate or aspartate residues and 2 methionines.
This large C-terminal tryptic fragment is generally difficult to analyze via MS due not only to its size (4286.73 Da) but also, and more importantly, to the complexity and heterogeneity of the PTMs that can occur within this region (Fig. 1). This situation is further complicated by the acidic nature of this peptide (due to the number of E/D residues). Although the peptide is highly soluble under buffered conditions, neutralization of the its negative charges in the acidified aqueous solution used to resolubilize the sample prior to MS analysis increases its propensity to precipitate. Therefore, the use of alternative enzymes or combined proteolytic approaches (Figs. 5A–5D) to generate smaller peptide fragments containing the various PTMs of interest should improve the characterization and quantification of C-terminal single and multiple PTMs within this region via mass spectrometry (76). For example, the overall sequence coverage achieved by Glu-C proteolysis enables the unambiguous identification of all pathologically relevant PTMs (namely, pY125 or pS129, nY133, and other nitrated C-terminal Tyr residues) typically found within the C-terminal stretch of α-syn. In the following sections, we provide an overview of how mass spectrometry techniques have been applied to identify and map disease-relevant α-syn PTMs, namely, phosphorylation, truncation, and tyrosine nitration.
Fig. 5.
Proteolytic peptides typically observed via mass spectrometry following trypsin (A), Glu-C (B), cyanogen bromide (C), and Asp-N (D) digestion. (MS preferential peptides are highlighted in red.) E, liquid chromatography MS spectrum of a typical LC-SRM analysis of α-syn tryptic and Glu-C peptides derived from rat nigral tissue. Endogenous rat syn (black) and viral induced overexpressed human α-syn (red) can be quantified during a single LC-SRM run. F, zoom of the highlighted area in E showing the total ion extraction chromatogram of tryptic fragment 81–96 with its heavy-labeled α-syn isoform (15N) below. A shift in retention time can be observed between rat and human isoforms accounting for the difference in sequence at residue 87 or for the phosphorylation occurring at p-S87, whereas the retention time of surrogate peptides (15N heavy isoform) corresponds to the endogenous form.
Identification and Quantification of Phosphorylated α-syn Species
Phosphorylation is one of the best-characterized α-syn PTMs but remains challenging to analyze via mass spectrometry. This reversible modification results in the introduction of a negative charge, is highly dynamic, and often leads to substoichiometric occupancy of the regulated phosphorylation sites, all of which can lead to signal suppression in the presence of other nonphosphorylated peptides. The introduction of efficient phosphopeptide enrichment strategies (reviewed in Ref. 77), such as immobilized metal ion chromatography (78, 79), titanium dioxide (TiO2) chromatography (80, 81), or phosphotyrosine immunoprecipitation (82), with or without fractionation, has facilitated an increasing number of comparative studies encompassing different tissues (68) and cell lines (83). Collision-induced dissociation is traditionally used to fragment and identify phosphorylated peptides, although when available electron-transfer dissociation and higher-energy C-trap dissociation are preferred fragmentation techniques. These two fragmentation methods allow for more efficient fragmentation of the peptide backbone while minimizing the intense neutral loss of phosphate usually observed in collision-induced dissociation, thereby facilitating the localization of the phosphorylation site. Several thousand phosphorylation sites can be detected, mapped back onto thousands of proteins, and even quantified in a single state-of-the-art MS experiment (84). Some of these phosphorylation sites are highly conserved and tightly regulated, whereas others seem to lack any biological function or regulation. Monitoring of their regulation is increasingly performed by measuring their abundances relative to their nonphosphorylated counterparts using targeted MS methods (85). The use of heavy isotope-labeled surrogate peptides as in the AQUA approach (85) is a more reliable way to detect and locate the phosphorylation site of a phosphopeptide, because identical peptide fragmentation patterns and HPLC retention times offer unambiguous identification and quantitation of the endogenous peptide forms (86). Moreover, the use of heavy isotope-labeled surrogate phosphopeptides enables the in situ characterization of peptide charge states and fragmentation behavior, because in silico predictions using software-embedded algorithms might not necessarily accurately represent the properties of endogenous peptides. However, a disadvantage of spiked-in surrogate peptides is that the solubilization or digestibility properties of some heavy-labeled reference peptides might not represent those of the in situ–generated peptides following enzymatic proteolysis. This problem can be overcome by using full-length internal standard proteins labeled with stable isotopes, a strategy known as protein standard absolute quantification (87, 88). To this end, we have developed a 15N-labeled α-syn protein standard that can be spiked into biological tissue extracts at an early stage of analytical processing. Early spiking of the accurately quantified (using amino acid analysis) 15N-labeled α-syn protein helps to compensate for any methodology-associated losses or artifactually introduced PTMs associated with tissue extraction and SDS-PAGE analysis.
Identification of Truncated α-syn in Brain Extracts
C-terminal truncated forms of α-syn have been detected in LBs and are thought to play a role in the initiation of α-syn aggregation and LB formation due to the increased aggregation propensity of α-syn variants lacking parts of or the entire C terminus (19, 89, 90). Although several important C-terminal truncations have been identified using specific antibodies against different segments of the α-syn C terminus, direct mass spectrometric evidence of their presence in tissues was confirmed only recently. C-terminal peptides produced by means of trypsinolysis in standard bottom-up/shotgun approaches are notoriously difficult to detect via mass spectrometry, particularly in the absence of specific enrichment methods such as the C-TAILS subtractive enrichment method (91). An attractive alternative to these rather demanding methods is the use of highly purified full-length proteins carrying the truncations of interest as multipurpose standards to build MS methods optimized for the detection of specific peptides in the protein. This approach has already proven useful in determining the level of truncation of troponin 1 during the development of a selected reaction monitoring (SRM) assay (92). Using a full-length protein instead of the corresponding synthetic peptides more accurately reflects the local environment around the cleavage sites, because the kinetic effects induced by the secondary structure of the truncated forms may affect the digestion efficiency (93), and because the freshly generated peptides do not necessarily behave in the same manner as the corresponding synthetic peptides, as is the case for α-syn and many other proteins (94). Differential peptide decay (95) and resolubilization issues can lead to significant underestimation in quantitative experiments based on stable isotope standard dilution (87). Correct resolubilization and stability need to be carefully controlled when using AQUA peptides (96). This approach was implemented by our group in a recent study aimed at detecting the presence of truncated forms of α-syn and their phosphorylated counterparts in brain extracts of a mouse model overexpressing human A30P, which shows accumulation of pS129 α-syn similar to that observed in human synucleinopathy patients.3 In this study, recombinant truncated forms of α-syn at Y133 and D135 were used (i) to optimize the conditions for detecting the corresponding C-terminal peptides in mouse model brain extract samples, (ii) to test the specificity of newly developed antibodies targeting the alternative C termini, and (iii) to compare the propensity of the truncated forms to be phosphorylated at S129 by PLK-3 relative to the full-length protein. In addition to the C-terminal peptide of the full-length α-syn, only the D135 form could be unambiguously detected, demonstrating the preferred accumulation of the D135 truncation over the Y133 truncation in symptomatic mice.
Nitrated α-syn
Oxidative stress has been reported as a possible key regulatory mechanism associated with the pathogenesis of different neurodegenerative diseases. Immunohistochemical studies of LBs have indicated that tyrosine nitration is a common feature of synucleinopathies (97). The first strong evidence of α-syn-specific nitration in pathological inclusions was provided via the use of monoclonal antibodies (21), which demonstrated that nitration affects α-syn solubility and therefore may promote fibrillogenesis. Nitration of tyrosine residues is often characterized by means of mass spectrometry. This PTM significantly affects the physicochemical properties of proteins and is often present at very low levels. Nitration enrichment methodologies prior to MS analysis include antibody-based enrichment, chemical conversion to 3-aminotyrosine followed by selective derivatization of the primary amine, and extensive fractionation of 3-nitrotyrosine (3NT)-containing peptides (reviewed in Refs. 98 and 99). However, in discovery experiments, the detection of tyrosine nitration by mass spectrometry is often associated with high false discovery rates and erroneous localization of the modification (100). In several instances, ambiguities in 3NT detection and localization could be clarified with the use of synthetic peptide standards bearing the modification; high-resolution, high-mass-accuracy MS instruments; and more stringent tolerances on parent ion selection (101). Another approach using SRM combined with the use of protein standards carrying the modifications of interest has also proven to be suitable for the simultaneous identification and quantitation of nitrated tyrosine residues in α-syn (102). Fully nitrated 3NT-containing peptides were selectively detected by monitoring the diagnostic immonium ion at m/z 181.1 associated with tyrosine nitration. Danielson et al. introduced combined proteolysis with trypsin and Asp-N to increase the sequence coverage (Figs. 4 and 5D), which enabled the successful identification of all four 3NT residues, of which one is found in the N-terminal region (Y39) and three (Y125, Y133, and Y136) are located within the C-terminal region of α-syn (102).
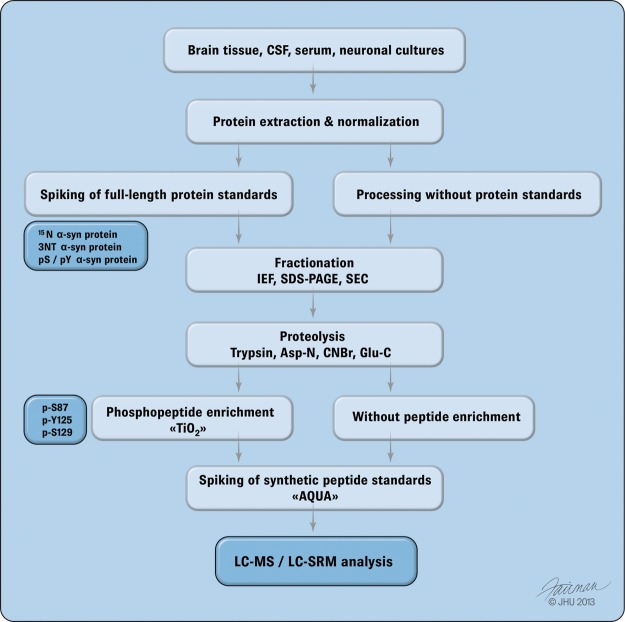
Fig. 4.
Overview of the main workflow from sample preparation to MS analysis. The diagram outlines the required workflow for sample preparation prior to MS analysis and quantification via selected reaction monitoring (SRM). Total protein extraction is typically carried out by means of SDS followed by protein equalization using a BCA assay. Prior to processing, protein extracts are spiked with a full-length heavy isoform 15N α-syn protein carrying the PTM of interest such as nitration (3NT) or phosphorylation at tyrosine or serine residues. Samples are typically fractionated via isoelectric focusing (IEF), SDS-PAGE, or size-exclusion chromatography (SEC). Following fractionation, samples are digested by trypsin (Tryp), Asp-N, cyanogen bromide (CNBr), or Glu-C using consecutive or combined digestions. Gel bands are extracted at the migration height of α-syn protein and subjected to individual proteolysis. For combined proteolytic analysis, such as in the case of Tryp and Glu-C digestions, samples are run in duplicate and digested separately or sequentially as in the case of Tryp and Asp-N proteolysis. Phosphopeptide enrichment (i.e. using titanium dioxide (TiO2)) chromatography allows for the enrichment of pathologically relevant phosphorylated peptides such as at residues p-S87, p-Y125, and p-S129. If full-length protein standards are not available, then the use of synthetic heavy-labeled surrogate peptides (AQUA) allows for individual spiking of reference peptides bearing PTMs of particular interest. Following proteolysis and reference peptide spiking, samples are analyzed via liquid chromatography (LC) coupled with SRM.
α-syn Splicing Isoforms
Transcription of the α-syn gene has been shown to give rise to at least four splicing variants (103, 104). The expressed isoforms other than the 140-residue protein are, in decreasing expression order, α-syn-112 (104, 105), which lacks exon 5 (residues 103–130), and two other minor isoforms: α-syn-126 (105), which lacks exon 3 (residues 41–54), and α-syn-98 (106), which lacks exons 3 and 5. Several groups have reported significant differences in the expression levels of these transcripts between patients suffering from synucleinopathies and control cases. For example, Alzheimer disease and LB dementia brains seem to be characterized by higher α-syn-98 mRNA levels (106) and lower α-syn-126 expression (107), whereas an increased level of α-syn-112 was reported to be specific for LB dementia patients (108). A recent study suggested a global trend for increased expression of the four α-syn splicing variant mRNAs between control and PD patients, but the expression levels were found to vary between different brain regions (109). Surprisingly, clinical studies of α-syn isoforms have so far relied only on mRNA analysis, mainly because the levels of expression of the corresponding proteins are very low. It has been suggested that an imbalance between the principal (α-syn-140) isoform and splicing variants may play a role in α-syn aggregation (110), and oxidative stress may be among the factors inducing this disequilibrium (111). For example, overexpression of α-syn-112 has been shown to sensitize PC12 cells to respiratory chain dysfunction (112, 113). More research is required in order to determine whether the splicing isoform protein levels follow the same patterns as the mRNA transcripts and whether they could be used as potential biomarkers. We anticipate that mass-spectrometry-based proteomics is particularly well suited to this task.
Mass-spectrometry-based Techniques as Novel Tools for Quantifying α-syn and Its Modifications in Biological Fluids
In this section, we highlight recent advances in mass spectrometry techniques and the chemical synthesis of α-syn that we believe will help researchers overcome many of the challenges mentioned above and provide unique opportunities to screen and identify novel biomarkers of PD and related synucleinopathies. We present specific protocols that our groups and others have optimized to enable reliable and multiplexed quantification of α-syn and its PTMs. In addition, we discuss the advantages of using full-length, site-specifically modified protein standards instead of peptides for absolute quantification of α-syn and how such protein standards could be produced using synthetic and semisynthetic approaches pioneered by our group (58, 62, 114, 115).
Absolute Quantitation of α-syn Protein and PTMs Using SRM-MS
Early diagnosis of PD requires the development of accurate analytical tools for the differential diagnosis of other synucleinopathies. The assessment of both p-S129 and total α-syn levels has been suggested to improve diagnostic classification; however, CSF levels of α-syn do not necessarily correlate with disease severity (34). Because phosphorylation at S129 is strongly associated with α-syn pathology (14, 17) in PD and diffuse Lewy body disease (44–46), recent developments in clinical validation have focused on developing quantitative assays specifically tailored for monitoring this modification and its combination with others. In addition to the immunological methods described above, SRM-MS has emerged as an indispensable analytical tool enabling accurate protein quantification and unambiguous identification and validation of various PTMs found in proteins. Important efforts to improve diagnostic specificity and interlaboratory reproducibility have been invested in developing robust analytical assays to complement immunological methods for biomarker validation in clinical diagnostics (116). The selectivity of the SRM method is achieved through the specific selection of the parent ions of interest in the first quadrupole (Q1) over a narrow m/z range, followed by a fragmentation step in Q2 combined with the fast sequential scanning of preselected fragment ions in Q3 (117). This selective mode of data acquisition permits the sensitive detection and identification of peptide abundance over a wide range of protein abundance within complex sample mixtures (118, 119). SRM-MS has already been employed for the quantification of nitrated α-syn and proteins in biological or clinical samples and for targeting PTMs for which adequate antibodies often do not exist (102). SRM-MS could serve as a valuable analytical tool for the validation of α-syn as a biomarker. SRM-MS assays enable the detection of low-abundant proteins in blood or CSF in the picogram to nanogram per milliliter range, a level of sensitivity similar to that of a Luminex assay showing a decrease of α-syn concentration in CSF below 0.5 ng/ml, which was recently suggested to be indicative of PD pathology (34). The decrease in α-syn concentration in CSF associated with the pathology appears to be in line with other studies using ELISA assays, which, however, show a disparity in absolute levels of α-syn at 1.6 pg/μl (29). The discrepancies in absolute α-syn levels found between different studies are suggested to be the result of differences in antibody specificity and the different protocols used in these studies (35).
Synthetic and Semisynthetic α-syn: Novel Tools for Mass-spectrometry-based Quantification of Modified α-syn in Biological Samples
Until recently, the generation of site-specifically modified forms of α-syn has not been possible. To address this limitation, our laboratory developed total chemical synthesis and semisynthetic strategies that enabled, for the first time, site-specific modification of α-syn and the generation of full-length α-syn containing single or multiple PTMs, which had not been accessible via chemical or enzymatic modification methods.
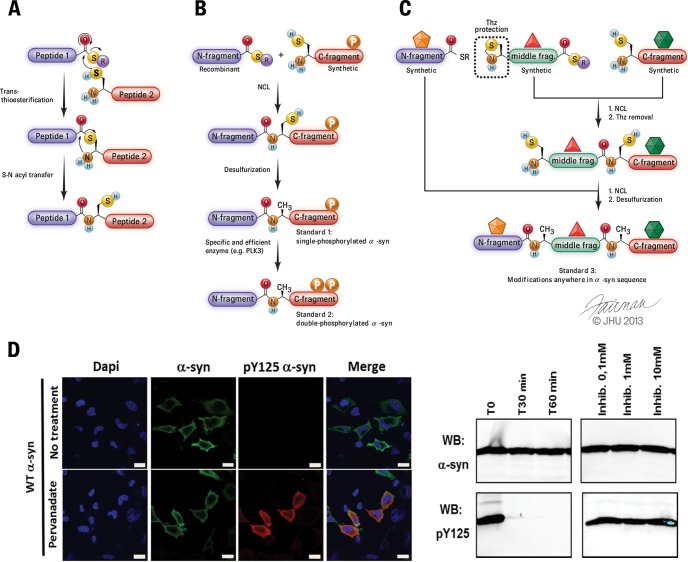
Protein semisynthesis, also known as expressed protein ligation (120), involves the ligation of two fragments: one fragment of the protein of interest that does not contain PTMs is produced by expression in E. coli, and the remaining fragment containing the desired modified residues is chemically synthesized via solid-phase peptide synthesis. The two purified, unprotected fragments are then covalently linked together by a regioselective reaction known as native chemical ligation (121), which generates a native peptide bond at the ligation site (Fig. 3A–3C). This method requires one of the fragments to bear an N-terminal cysteine, a residue not present within the sequence of α-syn. However, the ligation site can be positioned at an alanine residue that is temporarily mutated to cysteine. Following ligation, a cysteine-specific desulfurization step (122) affords the full-length protein with the native alanine residue at the ligation site (Fig. 3).
Fig. 3.
Chemical protein synthesis as a tool to generate well-defined full-length protein standards for absolute quantification. A, mechanism of the native chemical ligation reaction, a core component of chemical protein synthesis approaches. B, expressed protein ligation can be combined with enzymatic modifications using specific enzymes, such as PLK3, thus allowing the preparation of α-syn modified at multiple residues to enable investigation of how single or multiple PTMs influence α-syn detection by different antibodies and comparison of the performance of mass-spectrometry-based quantification to the immunoassays currently in use. Adapted from Ref. 58. C, total chemical synthesis of α-syn using three fragments, all produced via chemical peptide synthesis, allowing the introduction of PTMs and/or various labels (shown as colored stars) anywhere within the α-syn′ sequence (whereas semisynthesis, shown in B, is mostly restricted to PTMs within the C or N terminus of α-syn). The central fragment (green) initially bears a temporary thiazolidine (Thz) protection to prevent the peptide from polymerizing and/or undergoing cyclization. Adapted from Ref. 131. D, immunocytochemistry (left) and Western blot (right) show that pY125 is a very labile modification, highlighting that the semisynthetic pY125 α-syn standard can be very useful for developing protocols to stabilize this modification in biological samples.
Most, but not all, naturally modified amino acids (e.g. phospho-serine/threonine/tyrosine, nitro-tyrosine, acetylated lysine) are commercially available in forms that are ready for use in solid-phase peptide synthesis, thus allowing greater flexibility for introducing multiple modifications within the same domain of the protein, which is relevant for many proteins (e.g. α-syn (49), exon 1 of the Huntingtin protein (123), clustered tau phosphorylation sites (124), histones (125), etc.) in which several PTMs cluster within the N- or C-terminal regions of the protein. When there are additional modifications outside these regions, the introduction of multiple PTMs can still be achieved using total chemical synthesis. In total chemical synthesis, proteins are produced by means of the chemical ligation of multiple (usually three to four) synthetic peptides, which provides greater flexibility for introducing multiple modifications at any position with the protein of interest. Our group has successfully developed such an approach (Fig. 3C) that can be used to place one or several PTMs anywhere within α-syn's sequence.
Despite the major advances made in the chemical synthesis of proteins and the fact that these methods have been used to produce proteins as large as a few hundred residues, such as the signaling protein Smad2 (126) or a fully synthetic 304-residue tetra-ubiquitin protein (127), as complex as ion channels (128) and other membrane proteins (129), or as challenging as prion proteins (130), post-translationally modified synthetic proteins have not been used in the development of assays to quantify PTMs and/or optimize assays in which such modifications are likely to influence the accuracy and sensitivity of the methods used.
Using this approach, we have been able to produce highly pure and homogeneous preparations of several modified forms of α-syn, including mono-ubiquitination at lysine 6 (62) and 12 (114), phosphorylation at Y125 (58), and N-terminal acetylation of α-syn (115). The availability of these reagents allowed us to gain critical insight about the role of these modifications in regulating the structure, aggregation, membrane interactions, subcellular localization, and degradation of α-syn. Many PTMs are reversible, and phosphorylation/dephosphorylation of proteins can occur on a very rapid timescale, which precludes the accumulation of such modifications to a level at which the PTMs can be detected. This has led many to conclude that some PTMs do not occur in vivo. The availability of site-specifically modified synthetic proteins provides unique opportunities to investigate the turnover of PTMs and the development of optimal conditions under which PTMs would be stable and/or protected against removal during sample collection and processing of brain, CSF, and blood samples to ensure detection and accurate quantification of the modified protein as discussed above. For example, using semisynthetic α-syn phosphorylated at Y125 (pY125), we were able to demonstrate that phosphorylation at this residue is tightly regulated and that pY125 is highly susceptible to dephosphorylation by phosphatases; dephosphorylation occurs within 30 min upon addition to cell lysates or after microinjection into primary neurons (Fig. 3D). These observations led to the development of conditions that enabled the stabilization and detection of pY125 α-syn in cell cultures and primary neurons (58).
The availability of modified α-syn standards prepared via chemical synthesis methods is instrumental for the development of more accurate assays to quantify α-syn levels. These assays could then be used to investigate the relationships connecting modified α-syn levels, α-syn pathology, and disease progression. As discussed above, most disease-linked α-syn modifications occur within the protein's C terminus, which is also the site of binding for many of the antibodies used in α-syn immunoassays (see Fig. 1A and Fig. 2), and single and multiple C-terminal PTMs thus affect the recognition of the protein by C-terminal antibodies (see Fig. 1B). Modified α-syn standards produced via chemical protein synthesis will thus facilitate screens for antibodies and/or combinations of antibodies whose binding to α-syn is not affected by PTMs and achieve quantitative capture of total α-syn within biological samples such as CSF and blood plasma. As emphasized above, protein chemical synthesis enables the preparation of chemically homogeneous protein standards, which is in turn crucial for mass-spectrometry-based quantification methods, because heterogeneity in MS samples (for example, due to methionine oxidation) results in signal spread over several species and consequently lower sensitivity. Homogeneous protein standards will facilitate the development of MS sample processing protocols that minimize these problems.
CONCLUDING REMARKS
The central involvement of α-syn in Parkinson disease and related synucleinopathies has made this protein a significant target for biomarker discovery and early diagnosis schemes. The presence of numerous PTMs within α-syn under pathological conditions complicates the search for α-syn-derived biomarkers because of the need for further research to characterize the role of these PTMs in α-syn pathogenesis, and because they may complicate α-syn quantification. This situation has heightened the need for site-specific studies of α-syn PTMs. The development of synthetic approaches that have enabled the generation of pure standards with predefined and well-characterized modifications represents a major advance toward understanding the role of PTMs in health and disease. The availability of these reagents holds great promise for the development of accurate and sensitive assays capable of quantifying changes in protein levels or PTMs and the correlation of these changes with disease pathology or severity. The use of semisynthetic or recombinant protein standards with targeted mass spectrometry offers the attractive possibility of building hypothesis-driven experiments in which mass spectrometry, instead of antibodies, is used as a specific readout (119). The presence of the specific modifications on the full-length protein standards also permits consideration of the effect of the protease kinetics, which may directly influence the quantification precision, and will help minimize the false discovery rate and clarify ambiguous PTM localization within modified peptides.
Our group successfully developed a total chemical synthesis scheme for α-syn (131) that will allow us to introduce modifications at any site within the protein, enabling greater flexibility in studying combinations of different PTMs, such as phosphorylation at S87 together with C-terminal phosphorylation or N-terminal ubiquitination. Moreover, this strategy allows a greater level of control of the labeling schemes that are required for mass-spectrometry-based quantitation in order to distinguish between signals from the standards and from analytes of interest (132). We foresee semisynthetic protein synthesis as a useful way to generate more accurate standards for multiple PTM monitoring, particularly in the case of cross-talk between disease-relevant PTMs (75, 133).
Using a recombinant 15N-labeled α-syn protein standard, we have recently developed an SRM assay for accurately quantifying α-syn levels in a rat model of PD (Figs. 5E and 5F). The assay builds on the availability of an accurately quantified fully 15N-labeled α-syn protein standard and enables monitoring and accurate quantification of abundance changes in endogenous rat and human α-syn protein in vitro and in vivo. The development of semisynthetic α-syn protein standards with PTMs is therefore of tremendous interest, because they could serve as ultimate standards for the MS-based discovery, identification, and quantitation of endogenous α-syn protein in postmortem brain extracts, blood samples, or CSF of clinically relevant cases. The combination of SRM and full-length heavy isotope-labeled α-syn standards ultimately serves as a foundation for future clinical developments aimed at accurately monitoring not only the protein abundance but also relevant PTMs associated with disease progression and severity.
Footnotes
2 Lashuel group, unpublished data.
3 Fournier et al., submitted for publication.
1 The abbreviations used are:
- CSF
- cerebrospinal fluid
- LB
- Lewy body
- PD
- Parkinson disease
- PTM
- post-translational modification
- SRM
- selected reagent monitoring
- α-syn
- α-synuclein.
REFERENCES
- 1. Lang A. E., Lozano A. M. (1998) Parkinson's disease. First of two parts. N. Engl. J. Med. 339, 1044–1053 [DOI] [PubMed] [Google Scholar]
- 2. Lang A. E., Lozano A. M. (1998) Parkinson's disease. Second of two parts. N. Engl. J. Med. 339, 1130–1143 [DOI] [PubMed] [Google Scholar]
- 3. Lesage S., Anheim M., Letournel F., Bousset L., Honore A., Rozas N., Pieri L., Madiona K., Durr A., Melki R., Verny C., Brice A. (2013) G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 73, 459–471 [DOI] [PubMed] [Google Scholar]
- 4. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 5. Proukakis C., Dudzik C. G., Brier T., MacKay D. S., Cooper J. M., Millhauser G. L., Houlden H., Schapira A. H. (2013) A novel alpha-synuclein missense mutation in Parkinson disease. Neurology 80, 1062–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spira P. J., Sharpe D. M., Halliday G., Cavanagh J., Nicholson G. A. (2001) Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann. Neurol. 49, 313–319 [PubMed] [Google Scholar]
- 7. Zarranz J. J., Alegre J., Gomez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atares B., Llorens V., Gomez Tortosa E., del Ser T., Munoz D. G., de Yebenes J. G. (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 8. Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., Destee A. (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 [DOI] [PubMed] [Google Scholar]
- 9. Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) Alpha-synuclein locus triplication causes Parkinson's disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 10. Kirik D., Rosenblad C., Burger C., Lundberg C., Johansen T. E., Muzyczka N., Mandel R. J., Bjorklund A. (2002) Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J. Neurosci. 22, 2780–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 [DOI] [PubMed] [Google Scholar]
- 12. van der Putten H., Wiederhold K. H., Probst A., Barbieri S., Mistl C., Danner S., Kauffmann S., Hofele K., Spooren W. P., Ruegg M. A., Lin S., Caroni P., Sommer B., Tolnay M., Bilbe G. (2000) Neuropathology in mice expressing human alpha-synuclein. J. Neurosci. 20, 6021–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luk K. C., Song C., O'Brien P., Stieber A., Branch J. R., Brunden K. R., Trojanowski J. Q., Lee V. M. (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 106, 20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M. S., Shen J., Takio K., Iwatsubo T. (2002) Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 [DOI] [PubMed] [Google Scholar]
- 15. Kiely A. P., Asi Y. T., Kara E., Limousin P., Ling H., Lewis P., Proukakis C., Quinn N., Lees A. J., Hardy J., Revesz T., Houlden H., Holton J. L. (2013) Alpha-synucleinopathy associated with G51D SNCA mutation: a link between Parkinson's disease and multiple system atrophy? Acta Neuropathol. 125, 753–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paleologou K. E., Oueslati A., Shakked G., Rospigliosi C. C., Kim H. Y., Lamberto G. R., Fernandez C. O., Schmid A., Chegini F., Gai W. P., Chiappe D., Moniatte M., Schneider B. L., Aebischer P., Eliezer D., Zweckstetter M., Masliah E., Lashuel H. A. (2010) Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J. Neurosci. 30, 3184–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., Barbour R., Huang J., Kling K., Lee M., Diep L., Keim P. S., Shen X., Chataway T., Schlossmacher M. G., Seubert P., Schenk D., Sinha S., Gai W. P., Chilcote T. J. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281, 29739–29752 [DOI] [PubMed] [Google Scholar]
- 18. Hasegawa M., Fujiwara H., Nonaka T., Wakabayashi K., Takahashi H., Lee V. M., Trojanowski J. Q., Mann D., Iwatsubo T. (2002) Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J. Biol. Chem. 277, 49071–49076 [DOI] [PubMed] [Google Scholar]
- 19. Li W., West N., Colla E., Pletnikova O., Troncoso J. C., Marsh L., Dawson T. M., Jakala P., Hartmann T., Price D. L., Lee M. K. (2005) Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc. Natl. Acad. Sci. U.S.A. 102, 2162–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duda J. E., Giasson B. I., Chen Q., Gur T. L., Hurtig H. I., Stern M. B., Gollomp S. M., Ischiropoulos H., Lee V. M., Trojanowski J. Q. (2000) Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am. J. Pathol. 157, 1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giasson B. I., Duda J. E., Murray I. V., Chen Q., Souza J. M., Hurtig H. I., Ischiropoulos H., Trojanowski J. Q., Lee V. M. (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 [DOI] [PubMed] [Google Scholar]
- 22. Borghi R., Marchese R., Negro A., Marinelli L., Forloni G., Zaccheo D., Abbruzzese G., Tabaton M. (2000) Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci. Lett. 287, 65–67 [DOI] [PubMed] [Google Scholar]
- 23. El-Agnaf O. M., Salem S. A., Paleologou K. E., Cooper L. J., Fullwood N. J., Gibson M. J., Curran M. D., Court J. A., Mann D. M., Ikeda S., Cookson M. R., Hardy J., Allsop D. (2003) Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 17, 1945–1947 [DOI] [PubMed] [Google Scholar]
- 24. Foulds P. G., Mitchell J. D., Parker A., Turner R., Green G., Diggle P., Hasegawa M., Taylor M., Mann D., Allsop D. (2011) Phosphorylated alpha-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson's disease. FASEB J. 25, 4127–4137 [DOI] [PubMed] [Google Scholar]
- 25. Wennstrom M., Surova Y., Hall S., Nilsson C., Minthon L., Bostrom F., Hansson O., Nielsen H. M. (2013) Low CSF levels of both alpha-synuclein and the alpha-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PloS One 8, e53250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wennstrom M., Londos E., Minthon L., Nielsen H. M. (2012) Altered CSF orexin and alpha-synuclein levels in dementia patients. J. Alzheimers Dis. 29, 125–132 [DOI] [PubMed] [Google Scholar]
- 27. Tokuda T., Salem S. A., Allsop D., Mizuno T., Nakagawa M., Qureshi M. M., Locascio J. J., Schlossmacher M. G., El-Agnaf O. M. (2006) Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem. Biophys. Res. Commun. 349, 162–166 [DOI] [PubMed] [Google Scholar]
- 28. Tokuda T., Qureshi M. M., Ardah M. T., Varghese S., Shehab S. A., Kasai T., Ishigami N., Tamaoka A., Nakagawa M., El-Agnaf O. M. (2010) Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75, 1766–1772 [DOI] [PubMed] [Google Scholar]
- 29. Mollenhauer B., Locascio J. J., Schulz-Schaeffer W., Sixel-Doring F., Trenkwalder C., Schlossmacher M. G. (2011) Alpha-synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 10, 230–240 [DOI] [PubMed] [Google Scholar]
- 30. Mollenhauer B., Cullen V., Kahn I., Krastins B., Outeiro T. F., Pepivani I., Ng J., Schulz-Schaeffer W., Kretzschmar H. A., McLean P. J., Trenkwalder C., Sarracino D. A., Vonsattel J. P., Locascio J. J., El-Agnaf O. M., Schlossmacher M. G. (2008) Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 213, 315–325 [DOI] [PubMed] [Google Scholar]
- 31. Mollenhauer B., Trautmann E., Taylor P., Manninger P., Sixel-Doring F., Ebentheuer J., Trenkwalder C., Schlossmacher M. G. (2013) Total CSF alpha-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci. Lett. 532, 44–48 [DOI] [PubMed] [Google Scholar]
- 32. Gorostidi A., Bergareche A., Ruiz-Martinez J., Marti-Masso J. F., Cruz M., Varghese S., Qureshi M. M., Alzahmi F., Al-Hayani A., de Munain A. L., El-Agnaf O. M. A. (2012) Alpha-synuclein levels in blood plasma from LRRK2 mutation carriers. PloS One 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vignali D. A. (2000) Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243, 243–255 [DOI] [PubMed] [Google Scholar]
- 34. Wang Y., Shi M., Chung K. A., Zabetian C. P., Leverenz J. B., Berg D., Srulijes K., Trojanowski J. Q., Lee V. M.-Y., Siderowf A. D., Hurtig H., Litvan I., Schiess M. C., Peskind E. R., Masuda M., Hasegawa M., Lin X., Pan C., Galasko D., Goldstein D. S., Jensen P. H., Yang H., Cain K. C., Zhang J. (2012) Phosphorylated α-synuclein in Parkinson's disease. Sci. Transl. Med. 4, 121ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hong Z., Shi M., Chung K. A., Quinn J. F., Peskind E. R., Galasko D., Jankovic J., Zabetian C. P., Leverenz J. B., Baird G., Montine T. J., Hancock A. M., Hwang H., Pan C., Bradner J., Kang U. J., Jensen P. H., Zhang J. (2010) DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 133, 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bidinosti M., Shimshek D. R., Mollenhauer B., Marcellin D., Schweizer T., Lotz G. P., Schlossmacher M. G., Weiss A. (2012) Novel one-step immunoassays to quantify alpha-synuclein: applications for biomarker development and high-throughput screening. J. Biol. Chem. 287, 33691–33705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohrfelt A., Grognet P., Andreasen N., Wallin A., Vanmechelen E., Blennow K., Zetterberg H. (2009) Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders—a marker of synapse loss? Neurosci. Lett. 450, 332–335 [DOI] [PubMed] [Google Scholar]
- 38. Kasuga K., Tokutake T., Ishikawa A., Uchiyama T., Tokuda T., Onodera O., Nishizawa M., Ikeuchi T. (2010) Differential levels of alpha-synuclein, beta-amyloid42 and tau in CSF between patients with dementia with Lewy bodies and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 81, 608–610 [DOI] [PubMed] [Google Scholar]
- 39. Noguchi-Shinohara M., Tokuda T., Yoshita M., Kasai T., Ono K., Nakagawa M., El-Agnaf O. M., Yamada M. (2009) CSF alpha-synuclein levels in dementia with Lewy bodies and Alzheimer's disease. Brain Res. 1251, 1–6 [DOI] [PubMed] [Google Scholar]
- 40. Spies P. E., Melis R. J., Sjogren M. J., Rikkert M. G., Verbeek M. M. (2009) Cerebrospinal fluid alpha-synuclein does not discriminate between dementia disorders. J. Alzheimers Dis. 16, 363–369 [DOI] [PubMed] [Google Scholar]
- 41. Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 42. Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Williamson R. E., Lansbury P. T., Jr. (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U.S.A. 97, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. El-Agnaf O. M., Salem S. A., Paleologou K. E., Curran M. D., Gibson M. J., Court J. A., Schlossmacher M. G., Allsop D. (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 20, 419–425 [DOI] [PubMed] [Google Scholar]
- 44. Kahle P. J., Neumann M., Ozmen L., Muller V., Jacobsen H., Spooren W., Fuss B., Mallon B., Macklin W. B., Fujiwara H., Hasegawa M., Iwatsubo T., Kretzschmar H. A., Haass C. (2002) Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 3, 583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamada M., Iwatsubo T., Mizuno Y., Mochizuki H. (2004) Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. Journal Neurochem. 91, 451–461 [DOI] [PubMed] [Google Scholar]
- 46. Chen L., Feany M. B. (2005) Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 8, 657–663 [DOI] [PubMed] [Google Scholar]
- 47. Goetz C. G., Tilley B. C., Shaftman S. R., Stebbins G. T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M. B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A. E., Lees A., Leurgans S., LeWitt P. A., Nyenhuis D., Olanow C. W., Rascol O., Schrag A., Teresi J. A., van Hilten J. J., LaPelle N., and Movement Disorder Society, U. R. T. F (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 [DOI] [PubMed] [Google Scholar]
- 48. Barbour R., Kling K., Anderson J. P., Banducci K., Cole T., Diep L., Fox M., Goldstein J. M., Soriano F., Seubert P., Chilcote T. J. (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 5, 55–59 [DOI] [PubMed] [Google Scholar]
- 49. Oueslati A., Fournier M., Lashuel H. A. (2010) Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: implications for Parkinson's disease pathogenesis and therapies. Prog. Brain Res. 183, 115–145 [DOI] [PubMed] [Google Scholar]
- 50. Lu Y., Prudent M., Fauvet B., Lashuel H. A., Girault H. H. (2011) Phosphorylation of alpha-synuclein at Y125 and S129 alters its metal binding properties: implications for understanding the role of alpha-synuclein in the pathogenesis of Parkinson's disease and related disorders. ACS Chem. Neurosci. 2, 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paleologou K. E., Schmid A. W., Rospigliosi C. C., Kim H. Y., Lamberto G. R., Fredenburg R. A., Lansbury P. T., Jr., Fernandez C. O., Eliezer D., Zweckstetter M., Lashuel H. A. (2008) Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J. Biol. Chem. 283, 16895–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Emadi S., Barkhordarian H., Wang M. S., Schulz P., Sierks M. R. (2007) Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J. Mol. Biol. 368, 1132–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fagerqvist T., Lindstrom V., Nordstrom E., Lord A., Tucker S. M., Su X., Sahlin C., Kasrayan A., Andersson J., Welander H., Nasstrom T., Holmquist M., Schell H., Kahle P. J., Kalimo H., Moller C., Gellerfors P., Lannfelt L., Bergstrom J., Ingelsson M. (2013) Monoclonal antibodies selective for alpha-synuclein oligomers/protofibrils recognize brain pathology in Lewy body disorders and alpha-synuclein transgenic mice with the disease-causing A30P mutation. J. Neurochem. 126, 131–144 [DOI] [PubMed] [Google Scholar]
- 54. Lashuel H. A., Petre B. M., Wall J., Simon M., Nowak R. J., Walz T., Lansbury P. T. (2002) α-Synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J. Mol. Biol. 322, 1089–1102 [DOI] [PubMed] [Google Scholar]
- 55. Lowe R., Pountney D. L., Jensen P. H., Gai W. P., Voelcker N. H. (2004) Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 13, 3245–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okochi M., Walter J., Koyama A., Nakajo S., Baba M., Iwatsubo T., Meijer L., Kahle P. J., Haass C. (2000) Constitutive phosphorylation of the Parkinson's disease associated alpha-synuclein. J. Biol. Chem. 275, 390–397 [DOI] [PubMed] [Google Scholar]
- 57. Negro A., Brunati A. M., Donella-Deana A., Massimino M. L., Pinna L. A. (2002) Multiple phosphorylation of alpha-synuclein by protein tyrosine kinase Syk prevents eosin-induced aggregation. FASEB J. 16, 210–212 [DOI] [PubMed] [Google Scholar]
- 58. Hejjaoui M., Butterfield S., Fauvet B., Vercruysse F., Cui J., Dikiy I., Prudent M., Olschewski D., Zhang Y., Eliezer D., Lashuel H. A. (2012) Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: alpha-synuclein phosphorylation at tyrosine 125. J. Am. Chem. Soc. 134, 5196–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Souza J. M., Giasson B. I., Chen Q., Lee V. M., Ischiropoulos H. (2000) Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 275, 18344–18349 [DOI] [PubMed] [Google Scholar]
- 60. Yamin G., Uversky V. N., Fink A. L. (2003) Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 542, 147–152 [DOI] [PubMed] [Google Scholar]
- 61. Uversky V. N., Yamin G., Munishkina L. A., Karymov M. A., Millett I. S., Doniach S., Lyubchenko Y. L., Fink A. L. (2005) Effects of nitration on the structure and aggregation of alpha-synuclein. Brain Res. Mol. Brain Res. 134, 84–102 [DOI] [PubMed] [Google Scholar]
- 62. Hejjaoui M., Haj-Yahya M., Kumar K. S., Brik A., Lashuel H. A. (2011) Towards elucidation of the role of ubiquitination in the pathogenesis of Parkinson's disease with semisynthetic ubiquitinated alpha-synuclein. Angew. Chem. 50, 405–409 [DOI] [PubMed] [Google Scholar]
- 63. Makarov A., Denisov E., Lange O., Horning S. (2006) Dynamic range of mass accuracy in LTQ Orbitrap hybrid mass spectrometer. J. Am. Soc. Mass Spectrom. 17, 977–982 [DOI] [PubMed] [Google Scholar]
- 64. Michalski A., Damoc E., Hauschild J. P., Lange O., Wieghaus A., Makarov A., Nagaraj N., Cox J., Mann M., Horning S. (2011) Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol. Cell. Proteomics 10, M111.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Syka J. E., Coon J. J., Schroeder M. J., Shabanowitz J., Hunt D. F. (2004) Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 101, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Olsen J. V., Macek B., Lange O., Makarov A., Horning S., Mann M. (2007) Higher-energy C-trap dissociation for peptide modification analysis. Nat. Methods 4, 709–712 [DOI] [PubMed] [Google Scholar]
- 67. Jedrychowski M. P., Huttlin E. L., Haas W., Sowa M. E., Rad R., Gygi S. P. (2011) Evaluation of HCD- and CID-type fragmentation within their respective detection platforms for murine phosphoproteomics. Mol. Cell. Proteomics 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lundby A., Secher A., Lage K., Nordsborg N. B., Dmytriyev A., Lundby C., Olsen J. V. (2012) Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat. Commun. 3, 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim W., Bennett E. J., Huttlin E. L., Guo A., Li J., Possemato A., Sowa M. E., Rad R., Rush J., Comb M. J., Harper J. W., Gygi S. P. (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Udeshi N. D., Svinkina T., Mertins P., Kuhn E., Mani D. R., Qiao J. W., Carr S. A. (2012) Refined preparation and use of anti-K-ε-GG antibody enables routine quantification of 10,000s of ubiquitination sites in single proteomics experiments. Mol. Cell. Proteomics 12, 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 72. Smith L. M., Kelleher N. L., and Consortium for Top Down P, (2013) Proteoform: a single term describing protein complexity. Nat. Methods 10, 186–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leverenz J. B., Umar I., Wang Q., Montine T. J., McMillan P. J., Tsuang D. W., Jin J., Pan C., Shin J., Zhu D., Zhang J. (2007) Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol. 17, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xia Q., Liao L., Cheng D., Duong D. M., Gearing M., Lah J. J., Levey A. I., Peng J. (2008) Proteomic identification of novel proteins associated with Lewy bodies. Front. Biosci. 13, 3850–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen L., Periquet M., Wang X., Negro A., McLean P. J., Hyman B. T., Feany M. B. (2009) Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J. Clin. Invest. 119, 3257–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Switzar L., Giera M., Niessen W. M. (2013) Protein digestion: an overview of the available techniques and recent developments. J. Proteome Res. 12, 1067–1077 [DOI] [PubMed] [Google Scholar]
- 77. Fila J., Honys D. (2012) Enrichment techniques employed in phosphoproteomics. Amino Acids 43, 1025–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Porath J. (1988) High-performance immobilized-metal-ion affinity chromatography of peptides and proteins. J. Chromatogr. 443, 3–11 [DOI] [PubMed] [Google Scholar]
- 79. Posewitz M. C., Tempst P. (1999) Immobilized gallium(III) affinity chromatography of phosphopeptides. Anal. Chem. 71, 2883–2892 [DOI] [PubMed] [Google Scholar]
- 80. Larsen M. R., Thingholm T. E., Jensen O. N., Roepstorff P., Jorgensen T. J. (2005) Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol. Cell. Proteomics 4, 873–886 [DOI] [PubMed] [Google Scholar]
- 81. Pinkse M. W., Uitto P. M., Hilhorst M. J., Ooms B., Heck A. J. (2004) Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal. Chem. 76, 3935–3943 [DOI] [PubMed] [Google Scholar]
- 82. Rush J., Moritz A., Lee K. A., Guo A., Goss V. L., Spek E. J., Zhang H., Zha X. M., Polakiewicz R. D., Comb M. J. (2005) Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23, 94–101 [DOI] [PubMed] [Google Scholar]
- 83. Rigbolt K. T. G., Prokhorova T. A., Akimov V., Henningsen J., Johansen P. T., Kratchmarova I., Kassem M., Mann M., Olsen J. V., Blagoev B. (2011) System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Sci. Signal. 4, rs3. [DOI] [PubMed] [Google Scholar]
- 84. Choudhary C., Mann M. (2010) Decoding signalling networks by mass spectrometry-based proteomics. Nat. Rev. Mol. Cell Biol. 11, 427–439 [DOI] [PubMed] [Google Scholar]
- 85. Gerber S. A., Rush J., Stemman O., Kirschner M. W., Gygi S. P. (2003) Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl. Acad. Sci. U.S.A. 100, 6940–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mayya V., Rezual K., Wu L., Fong M. B., Han D. K. (2006) Absolute quantification of multisite phosphorylation by selective reaction monitoring mass spectrometry: determination of inhibitory phosphorylation status of cyclin-dependent kinases. Mol. Cell. Proteomics 5, 1146–1157 [DOI] [PubMed] [Google Scholar]
- 87. Brun V., Dupuis A., Adrait A., Marcellin M., Thomas D., Court M., Vandenesch F., Garin J. (2007) Isotope-labeled protein standards: toward absolute quantitative proteomics. Mol. Cell. Proteomics 6, 2139–2149 [DOI] [PubMed] [Google Scholar]
- 88. Dupuis A., Hennekinne J. A., Garin J., Brun V. (2008) Protein Standard Absolute Quantification (PSAQ) for improved investigation of staphylococcal food poisoning outbreaks. Proteomics 8, 4633–4636 [DOI] [PubMed] [Google Scholar]
- 89. Liu C. W., Giasson B. I., Lewis K. A., Lee V. M., Demartino G. N., Thomas P. J. (2005) A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J. Biol. Chem. 280, 22670–22678 [DOI] [PubMed] [Google Scholar]
- 90. Ohrfelt A., Zetterberg H., Andersson K., Persson R., Secic D., Brinkmalm G., Wallin A., Mulugeta E., Francis P. T., Vanmechelen E., Aarsland D., Ballard C., Blennow K., Westman-Brinkmalm A. (2011) Identification of novel alpha-synuclein isoforms in human brain tissue by using an online nanoLC-ESI-FTICR-MS method. Neurochem. Res. 36, 2029–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schilling O., Barre O., Huesgen P. F., Overall C. M. (2010) Proteome-wide analysis of protein carboxy termini: C terminomics. Nat. Methods 7, 508–511 [DOI] [PubMed] [Google Scholar]
- 92. Picard G., Lebert D., Louwagie M., Adrait A., Huillet C., Vandenesch F., Bruley C., Garin J., Jaquinod M., Brun V. (2012) PSAQ standards for accurate MS-based quantification of proteins: from the concept to biomedical applications. J. Mass Spectrom. 47, 1353–1363 [DOI] [PubMed] [Google Scholar]
- 93. Brownridge P., Beynon R. J. (2011) The importance of the digest: proteolysis and absolute quantification in proteomics. Methods 54, 351–360 [DOI] [PubMed] [Google Scholar]
- 94. Simicevic J., Schmid A. W., Gilardoni P. A., Zoller B., Raghav S. K., Krier I., Gubelmann C., Lisacek F., Naef F., Moniatte M., Deplancke B. (2013) Absolute quantification of transcription factors during cellular differentiation using multiplexed targeted proteomics. Nat. Methods 10, 570–576 [DOI] [PubMed] [Google Scholar]
- 95. Shuford C. M., Sederoff R. R., Chiang V. L., Muddiman D. C. (2012) Peptide production and decay rates affect the quantitative accuracy of protein cleavage isotope dilution mass spectrometry (PC-IDMS). Mol. Cell. Proteomics 11, 814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chambers A. G., Percy A. J., Yang J., Camenzind A. G., Borchers C. H. (2013) Multiplexed quantitation of endogenous proteins in dried blood spots by multiple reaction monitoring-mass spectrometry. Mol. Cell. Proteomics 12, 781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Good P. F., Hsu A., Werner P., Perl D. P., Olanow C. W. (1998) Protein nitration in Parkinson's disease. J. Neuropathol. Exp. Neurol. 57, 338–342 [DOI] [PubMed] [Google Scholar]
- 98. Dekker F., Abello N., Wisastra R., Bischoff R. (2012) Enrichment and detection of tyrosine-nitrated proteins. Curr. Protoc. Protein Sci. Ch. 14, Unit 14.13 [DOI] [PubMed] [Google Scholar]
- 99. Feeney M. B., Schoneich C. (2013) Proteomic approaches to analyze protein tyrosine nitration. Antioxid. Redox Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stevens S. M., Jr., Prokai-Tatrai K., Prokai L. (2008) Factors that contribute to the misidentification of tyrosine nitration by shotgun proteomics. Mol. Cell. Proteomics 7, 2442–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ghesquiere B., Helsens K., Vandekerckhove J., Gevaert K. (2011) A stringent approach to improve the quality of nitrotyrosine peptide identifications. Proteomics 11, 1094–1098 [DOI] [PubMed] [Google Scholar]
- 102. Danielson S. R., Held J. M., Schilling B., Oo M., Gibson B. W., Andersen J. K. (2009) Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson's disease. Anal. Chem. 81, 7823–7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ueda K., Xia Y., Masliah E. (2002) Characterization of the human NACP/alpha-synuclein gene: genomic structure, transcription start site, promoter region and polymorphisms. Neurobiol. Aging 23, S411. [DOI] [PubMed] [Google Scholar]
- 104. Ueda K., Saitoh T., Mori H. (1994) Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer's disease amyloid. Biochem. Biophys. Res. Commun. 205, 1366–1372 [DOI] [PubMed] [Google Scholar]
- 105. Campion D., Martin C., Heilig R., Charbonnier F., Moreau V., Flaman J. M., Petit J. L., Hannequin D., Brice A., Frebourg T. (1995) The NACP/synuclein gene: chromosomal assignment and screening for alterations in Alzheimer disease. Genomics 26, 254–257 [DOI] [PubMed] [Google Scholar]
- 106. Beyer K., Domingo-Sabat M., Lao J. I., Carrato C., Ferrer I., Ariza A. (2008) Identification and characterization of a new alpha-synuclein isoform and its role in Lewy body diseases. Neurogenetics 9, 15–23 [DOI] [PubMed] [Google Scholar]
- 107. Beyer K., Humbert J., Ferrer A., Lao J. I., Carrato C., Lopez D., Ferrer I., Ariza A. (2006) Low alpha-synuclein 126 mRNA levels in dementia with Lewy bodies and Alzheimer disease. Neuroreport 17, 1327–1330 [DOI] [PubMed] [Google Scholar]
- 108. Beyer K., Lao J. I., Carrato C., Mate J. L., Lopez D., Ferrer I., Ariza A. (2004) Differential expression of alpha-synuclein isoforms in dementia with Lewy bodies. Neuropathol. Appl. Neurobiol. 30, 601–607 [DOI] [PubMed] [Google Scholar]
- 109. McLean J. R., Hallett P. J., Cooper O., Stanley M., Isacson O. (2012) Transcript expression levels of full-length alpha-synuclein and its three alternatively spliced variants in Parkinson's disease brain regions and in a transgenic mouse model of alpha-synuclein overexpression. Mol. Cell. Neurosci. 49, 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McLean P. J., Hyman B. T. (2002) An alternatively spliced form of rodent alpha-synuclein forms intracellular inclusions in vitro: role of the carboxy-terminus in alpha-synuclein aggregation. Neurosci. Lett. 323, 219–223 [DOI] [PubMed] [Google Scholar]
- 111. Kalivendi S. V., Yedlapudi D., Hillard C. J., Kalyanaraman B. (2010) Oxidants induce alternative splicing of alpha-synuclein: implications for Parkinson's disease. Free Radic. Biol. Med. 48, 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ma K. L., Yuan Y. H., Song L. K., Han N., Chen N. H. (2011) Over-expression of alpha-synuclein 98 triggers intracellular oxidative stress and enhances susceptibility to rotenone. Neurosci. Lett. 491, 148–152 [DOI] [PubMed] [Google Scholar]
- 113. Ma K. L., Song L. K., Long W. A., Yuan Y. H., Zhang Y., Song X. Y., Niu F., Han N., Chen N. H. (2013) Deletion in exon 5 of the SNCA gene and exposure to rotenone leads to oligomerization of alpha-synuclein and toxicity to PC12 cells. Brain Res. Bull. 90, 127–131 [DOI] [PubMed] [Google Scholar]
- 114. Shabek N., Herman-Bachinsky Y., Buchsbaum S., Lewinson O., Haj-Yahya M., Hejjaoui M., Lashuel H. A., Sommer T., Brik A., Ciechanover A. (2012) The size of the proteasomal substrate determines whether its degradation will be mediated by mono- or polyubiquitylation. Mol. Cell 48, 87–97 [DOI] [PubMed] [Google Scholar]
- 115. Fauvet B., Fares M. B., Samuel F., Dikiy I., Tandon A., Eliezer D., Lashuel H. A. (2012) Characterization of semisynthetic and naturally Nalpha-acetylated alpha-synuclein in vitro and in intact cells: implications for aggregation and cellular properties of alpha-synuclein. J. Biol. Chem. 287, 28243–28262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Addona T. A., Abbatiello S. E., Schilling B., Skates S. J., Mani D. R., Bunk D. M., Spiegelman C. H., Zimmerman L. J., Ham A. J., Keshishian H., Hall S. C., Allen S., Blackman R. K., Borchers C. H., Buck C., Cardasis H. L., Cusack M. P., Dodder N. G., Gibson B. W., Held J. M., Hiltke T., Jackson A., Johansen E. B., Kinsinger C. R., Li J., Mesri M., Neubert T. A., Niles R. K., Pulsipher T. C., Ransohoff D., Rodriguez H., Rudnick P. A., Smith D., Tabb D. L., Tegeler T. J., Variyath A. M., Vega-Montoto L. J., Wahlander A., Waldemarson S., Wang M., Whiteaker J. R., Zhao L., Anderson N. L., Fisher S. J., Liebler D. C., Paulovich A. G., Regnier F. E., Tempst P., Carr S. A. (2009) Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat. Biotechnol. 27, 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lange V., Picotti P., Domon B., Aebersold R. (2008) Selected reaction monitoring for quantitative proteomics: a tutorial. Mol. Syst. Biol. 4, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Picotti P., Aebersold R. (2012) Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat. Methods 9, 555–566 [DOI] [PubMed] [Google Scholar]
- 119. Picotti P., Bodenmiller B., Aebersold R. (2013) Proteomics meets the scientific method. Nat. Methods 10, 24–27 [DOI] [PubMed] [Google Scholar]
- 120. Muir T. W., Sondhi D., Cole P. A. (1998) Expressed protein ligation: a general method for protein engineering. Proc. Natl. Acad. Sci. U.S.A. 95, 6705–6710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dawson P. E., Muir T. W., Clark-Lewis I., Kent S. B. (1994) Synthesis of proteins by native chemical ligation. Science 266, 776–779 [DOI] [PubMed] [Google Scholar]
- 122. Wan Q., Danishefsky S. J. (2007) Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew. Chem. 46, 9248–9252 [DOI] [PubMed] [Google Scholar]
- 123. Ehrnhoefer D. E., Sutton L., Hayden M. R. (2011) Small changes, big impact: posttranslational modifications and function of huntingtin in Huntington disease. Neuroscientist 17, 475–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lippens G., Amniai L., Wieruszeski J. M., Sillen A., Leroy A., Landrieu I. (2012) Towards understanding the phosphorylation code of tau. Biochem. Soc. Trans. 40, 698–703 [DOI] [PubMed] [Google Scholar]
- 125. Lennartsson A., Ekwall K. (2009) Histone modification patterns and epigenetic codes. Biochim. Biophys. Acta 1790, 863–868 [DOI] [PubMed] [Google Scholar]
- 126. Ottesen J. J., Huse M., Sekedat M. D., Muir T. W. (2004) Semisynthesis of phosphovariants of Smad2 reveals a substrate preference of the activated T beta RI kinase. Biochemistry 43, 5698–5706 [DOI] [PubMed] [Google Scholar]
- 127. Kumar K. S., Bavikar S. N., Spasser L., Moyal T., Ohayon S., Brik A. (2011) Total chemical synthesis of a 304 amino acid K48-linked tetraubiquitin protein. Angew. Chem. 50, 6137–6141 [DOI] [PubMed] [Google Scholar]
- 128. Valiyaveetil F. I., MacKinnon R., Muir T. W. (2002) Semisynthesis and folding of the potassium channel KcsA. J. Am. Chem. Soc. 124, 9113–9120 [DOI] [PubMed] [Google Scholar]
- 129. Lahiri S., Brehs M., Olschewski D., Becker C. F. (2011) Total chemical synthesis of an integral membrane enzyme: diacylglycerol kinase from Escherichia coli. Angew. Chem. 50, 3988–3992 [DOI] [PubMed] [Google Scholar]
- 130. Olschewski D., Seidel R., Miesbauer M., Rambold A. S., Oesterhelt D., Winklhofer K. F., Tatzelt J., Engelhard M., Becker C. F. (2007) Semisynthetic murine prion protein equipped with a GPI anchor mimic incorporates into cellular membranes. Chem. Biol. 14, 994–1006 [DOI] [PubMed] [Google Scholar]
- 131. Fauvet B., Butterfield S. M., Fuks J., Brik A., Lashuel H. (2013) One-pot total chemical synthesis of human α-synuclein. Chem. Commun. [DOI] [PubMed] [Google Scholar]
- 132. Kaiser S. E., Riley B. E., Shaler T. A., Trevino R. S., Becker C. H., Schulman H., Kopito R. R. (2011) Protein standard absolute quantification (PSAQ) method for the measurement of cellular ubiquitin pools. Nat. Methods 8, 691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang Z., Udeshi N. D., Slawson C., Compton P. D., Sakabe K., Cheung W. D., Shabanowitz J., Hunt D. F., Hart G. W. (2010) Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci. Signal. 3, ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]