Abstract
Malaria, an infectious disease caused by parasites of the Plasmodium genus, is one of the world's major public health concerns causing up to a million deaths annually, mostly because of P. falciparum infections. All of the clinical symptoms are associated with the blood stage of the disease, an obligate part of the parasite life cycle, when a form of the parasite called the merozoite recognizes and invades host erythrocytes. During erythrocyte invasion, merozoites are directly exposed to the host humoral immune system making the blood stage of the parasite a conceptually attractive therapeutic target. Progress in the functional and molecular characterization of P. falciparum merozoite proteins, however, has been hampered by the technical challenges associated with expressing these proteins in a biochemically active recombinant form. This challenge is particularly acute for extracellular proteins, which are the likely targets of host antibody responses, because they contain structurally critical post-translational modifications that are not added by some recombinant expression systems. Here, we report the development of a method that uses a mammalian expression system to compile a protein resource containing the entire ectodomains of 42 P. falciparum merozoite secreted and cell surface proteins, many of which have not previously been characterized. Importantly, we are able to recapitulate known biochemical activities by showing that recombinant MSP1-MSP7 and P12-P41 directly interact, and that both recombinant EBA175 and EBA140 can bind human erythrocytes in a sialic acid-dependent manner. Finally, we use sera from malaria-exposed immune adults to profile the relative immunoreactivity of the proteins and show that the majority of the antigens contain conformational (heat-labile) epitopes. We envisage that this resource of recombinant proteins will make a valuable contribution toward a molecular understanding of the blood stage of P. falciparum infections and facilitate the comparative screening of antigens as blood-stage vaccine candidates.
Parasites of the Plasmodium genus are the etiological agents responsible for malaria, an infectious disease mostly occurring in developing countries with up to 40% of the world's population described as being at risk of the disease. Among the Plasmodium species that can affect humans, Plasmodium falciparum is responsible for the highest mortality, causing around one million deaths annually, mostly in children under the age of five (1). The clinical symptoms of malaria occur during the cyclic asexual blood stage of the parasite lifecycle when merozoites, that have invaded and replicated within host erythrocytes, are released into the bloodstream before invading new red blood cells (2). Despite intensive efforts from the research community there is currently no licensed vaccine for malaria. The leading candidate RTS,S/AS01, which targets the pre-erythrocytic stage of the disease and was tested in phase III trials, conferred 30 to 50% protection from clinical malaria, depending on the age group studied (3, 4). This limited efficacy has led to calls for a more effective vaccine and many have suggested that a combinatorial vaccine that additionally targets the blood stage may increase efficacy.
A vaccine targeting the proteins expressed on the surface of the blood stage of the parasite is conceptually attractive because merozoites are repeatedly and directly exposed to the human humoral immune system and naturally acquired antibodies against these proteins have been shown to confer at least partial immunity (5–8). Despite this, only a few antigens discovered before the completion of the parasite genome sequence have been assessed in detail (9) and clinical vaccine trials using antigens that target the blood stage have so far shown limited efficacy, mostly caused by antigenic diversity (10). The sequencing of the parasite genome (11) has identified all possible targets but the systematic screening of these new candidates to assess their potential as a vaccine is hampered by the inability to systematically express recombinant Plasmodium proteins in their native conformation (12–15). Likely explanations might be the high (∼80%) A:T content of the P. falciparum genome resulting in low codon usage compatibility in heterologous expression systems, the large size (> 50 kDa) of many proteins, the presence of long stretches of highly repetitive amino acids, and the difficulty in identifying clear structural domains within these proteins using standard prediction computer programs (11). Extracellular proteins, in particular, present an additional challenge because they often have signal peptides and transmembrane regions that can negatively impact expression (16–18) and contain structurally important disulfide bonds. However, unlike most other eukaryotic extracellular proteins, Plasmodium cell surface and secreted proteins are not modified by N-linked glycans because of the absence of the necessary enzymes (19).
To express Plasmodium proteins for basic research and vaccine development, a diverse range of expression systems have been tried (12) ranging from bacteria (17, 18), yeast (13), Dictyostelium (20), and plants (21) to mammalian cells (22) and cell-free systems (23–25). To circumvent the problem of codon usage, bacterial (26) and yeast (27) strains with modified tRNA pools have been developed, or sequences of the gene of interest synthesized and codon-optimized to match that of the expression host (28, 29). Although Escherichia coli has been the most popular expression system because of its relative simplicity and cost effectiveness, large-scale production of soluble functional Plasmodium falciparum recombinant proteins remains challenging with success rates ranging from just 6 to 21% (17, 18) and is often hindered by the need for complex refolding procedures. Similarly, attempts have been made to compile large panels of parasite proteins using in vitro translation systems (23, 25, 30, 31). These systems, however, require reducing conditions and are therefore not generally suitable for the systematic expression of extracellular proteins that occupy an oxidizing environment and critically require the formation of disulfide bonds for proper function. As a result, functional analyses of extracellular parasite proteins have often been restricted to smaller subfragments of the proteins that can be expressed in a soluble form rather than the entire extracellular region. Although eukaryotic expression systems are able to add disulfide bonds, they also often inappropriately glycosylate parasite proteins, adding further complication (32). A generic method that would overcome these technical challenges to express, in a systematic way, panels of recombinant Plasmodium proteins that have retained their native function and conformation would therefore be a valuable resource for the molecular investigations of erythrocyte invasion and the development of a blood stage vaccine.
To generate a resource of correctly folded recombinant merozoite proteins, we used a mammalian expression system and established the parameters necessary for high-level expression. Using this method, we compiled a panel of 42 proteins that corresponds to the repertoire of abundant cell surface and secreted merozoite proteins of the 3D7 strain of Plasmodium falciparum. Biochemical activity of these proteins was demonstrated by recapitulating known protein interactions and by showing conformation-sensitive immunoreactivity of the recombinant proteins using immune sera.
EXPERIMENTAL PROCEDURES
Design and Synthesis of P. falciparum Merozoite Cell Surface and Secreted Expression Plasmids
The regions corresponding to the entire extracellular domains of 51 merozoite cell-surface proteins from the P. falciparum 3D7 strain were determined by using transmembrane and GPI-anchor (33, 34), or signal peptide (35) prediction software. Sequences encoding the extracellular domains of these proteins, with the exception of their signal peptide, were made by gene synthesis (GeneartAG) and are presented in Table I. All sequences were codon-optimized for expression in human cells and all potential N-linked glycosylation sites (NXS/T) were modified by substituting the serine or threonine residue with an alanine residue to prevent the inappropriate addition of large glycans that are absent in the native P. falciparum proteins. The coding sequences were flanked by unique NotI and AscI sites and cloned into a derivative of the pTT3 expression vector (36) between the leader sequence of the mouse variable κ light chain 7–33 (37), and a rat Cd4 domains 3 and 4 tag followed by an enzymatic biotinylation sequence as previously described (38). All expression constructs were cotransfected with the BirA biotinylation enzyme into HEK293E cells and are available from Addgene, a non-profit plasmid repository (www.addgene.org). The soluble biotinylated recombinant proteins were collected from the cell culture supernatant 6 days post-transfection, and dialyzed into HBS before analysis. During gene synthesis, constructs encoding the full-length ectodomain of RH1, RH2b and RH4 proved to be toxic in bacteria, and only subfragments could be produced as presented in Table I.
Table I. Details of the P. falciparum merozoite surface protein library. Each protein within the library is grouped according to its predicted subcellular localization (Sub-cell. Locn). The region of the protein (typically the full-length ectodomain) that was selected for expression is identified by the N- and C-terminal residues and their positions. The levels at which each protein was expressed was determined by ELISA against a known purified, quantified standard. All transfections were performed transiently using an unoptimized noncommercial transfection reagent, which routinely achieves 40–60% transfection efficiency. Expression levels are given as a guide only given the significant batch-to-batch variability observed using this approach and grouped into “high” (between 5 μg/ml–50 μg/ml:), “medium” (0.5 μg/ml–5 μg/ml) and “low” (0.005 μg/ml–0.5 μg/ml). The expression of nine proteins (PF3D7_1431400, EBL1, PTRAMP, RAP1, RAP2, RAP3, CLAG3.2, RhopH2, and RON3) could not be detected (n.d.). † Pf34 is also GPI-linked Prod: this is a footnote place after detected (n.d).
| Sub-cell. Locn | Official nomenclature | Synonym/s | Accession number | Region expressed | Len. (aa) | Exp. level |
|---|---|---|---|---|---|---|
| Surface (GPI-Anchored) | MSP1 | PFI1475w | V20-S1701 | 1682 | medium | |
| MSP2 | PFB0300c | I20-N246 | 227 | high | ||
| MSP4 | PFB0310c | Y29-S253 | 225 | high | ||
| MSP5 | PFB0305c | N22-S251 | 230 | high | ||
| MSP10 | PFF0995c | H27-S503 | 477 | medium | ||
| P12 | Pf12 | PFF0615c | H26-S323 | 298 | medium | |
| P12p | PFF0620c | Y21-T349 | 329 | low | ||
| P38 | Pf38 | PFE0395c | Q22-S328 | 307 | medium | |
| Pf92 | PF13_0338 | A26-S770 | 745 | low | ||
| Pf113 | PF14_0201 | Y23-K942 | 920 | medium | ||
| PF3D7_1136200 | PF11_0373 | L19-G656 | 638 | medium | ||
| PF3D7_1431400 | PF14_0293 | N25-S968 | 944 | n.d. | ||
| Peripheral | MSP3 | MSP3.1, SPAM | PF10_0345 | K26-H354 | 328 | high |
| MSP6 | MSP3.2 | PF10_0346 | Y17-N371 | 355 | low | |
| H101 | MSP3.3 | PF10_0347 | Q23-N424 | 402 | low | |
| MSP11 | H103, MSP3.7 | PF10_0352 | K27-Y405 | 379 | medium | |
| MSP7 | PF13_0197 | T28-M351 | 324 | high | ||
| MSRP1 | PF13_0196 | Y22-T379 | 358 | medium | ||
| MSRP2 | MAL13P1.174 | K24-T280 | 257 | low | ||
| MSRP3 | PF13_0193 | Q24-S298 | 275 | medium | ||
| P41 | Pf41 | PFD0240c | K21-S378 | 358 | high | |
| MSP9 | p101, ABRA | PFL1385c | N24-S742 | 719 | low | |
| Micronemal | AMA1 | Pf83, RMA1 | PF11_0344 | Q25-T541 | 517 | high |
| EBA140 | BAEBL | MAL13P1.60 | I26-P1135 | 1110 | low | |
| EBA175 | MAL7P1.176 | A21-P1424 | 1404 | low | ||
| EBA181 | JESEBL | PFA0125c | I27-S1488 | 1462 | low | |
| EBL1 | PF13_0115 | K22-N2584 | 2563 | n.d. | ||
| ASP | PFD0295c | A20-S708 | 689 | low | ||
| MTRAP | PF10_0281 | I23-K432 | 410 | medium | ||
| PTRAMP | TRAMP, TSP-3 | PFL0870w | N25-S306 | 282 | n.d. | |
| GAMA | PSOP9 | PF08_0008 | L22-P710 | 689 | medium | |
| Rhoptry | RH1 | RBP1, NBP1 | PFD0110w | Q24-T666 | 643 | very low |
| RH2b | RBP2b | MAL13P1.176 | H25-S1028 | 1003 | very low | |
| RH4 | RBP4 | PFD1150c | I27-T1148 | 1122 | very low | |
| RH5 | PFD1145c | F25-Q526 | 502 | low | ||
| RAP1 | PF14_0102 | I23-D782 | 760 | n.d. | ||
| RAP2 | PFE0080c | D22-L398 | 387 | n.d. | ||
| RAP3 | PFE0075c | N23-K400 | 378 | n.d. | ||
| CLAG3.2 | RhopH1(3.2) | PFC0110w | K21-H1416 | 1396 | n.d. | |
| RhopH2 | PFI1445w | L20-S1378 | 1359 | n.d. | ||
| RhopH3 | PFI0265c | K25-L897 | 873 | low | ||
| SPATR | PFB0570w | E22-C250 | 229 | high | ||
| AARP | PFD1105w | K18-P191 | 174 | low | ||
| Pf34† | PV2 | PFD0955w | N25-S306 | 282 | high | |
| RON3 | PFL2505c | N22-N249 | 228 | n.d. | ||
| RON6 | PFB0680w | F16-T949 | 934 | low | ||
| RAMA | MAL7P1.208 | Y17-K838 | 821 | low | ||
| Other | TLP | TRAP2 | PFF0800w | E24-P1306 | 1283 | low |
| PTEX150 | Pf112 | PF14_0344 | A20-N993 | 974 | low | |
| ETRAMP10.2 | PfJ323 | PF10_0323 | R25-R52 | 28 | medium | |
| PF3D7_0606800 | PFF0335c | V23-K299 | 277 | high |
Enzyme-linked Immunosorbant Assay (ELISA)
The biotinylated ectodomains of the P. falciparum library were serially diluted 1:2 up to a final dilution of 1:128 and all dilutions were immobilized on streptavidin-coated plates (NUNC) before being incubated for one hour with 1 μg/ml OX68 antibody (AbD Serotec), which binds the Cd4 tag. The plates were washed in PBS/0.1% Tween20 (PBST) before incubation with an anti-mouse immunoglobulin antibody coupled to alkaline phosphatase (Sigma) for one hour at room temperature. After washes in PBST and PBS, wells were incubated with p-nitrophenyl phosphate at 1 mg/ml and optical density measurements taken at 405 nm.
For the immunogenicity study, proteins were either immobilized as above or denatured for 10 min at 80 °C before immobilization on streptavidin-coated plates and incubation with pooled sera from 10 malaria-exposed or malaria-naïve individuals used at a 1:1000 dilution in PBST/2% BSA.
Western Blot
Between 5 and 30 μl of dialyzed transfection medium containing the recombinant proteins was resolved by SDS-PAGE under reducing conditions before blotting onto Hybond-P PVDF membrane (GE Healthcare) overnight at 30 V. Membranes were blocked with 2% BSA in PBST and incubated with 0.02 μg/ml of streptavidin-HRP (Jackson Immunoresearch) diluted in PBST/0.2% BSA and detected with the Supersignal West pico chemiluminescent substrate (Pierce).
AVEXIS Screen
Interactions between the MSP1 and MSP7, and P12 and P41 proteins were identified using the AVEXIS1 method as previously described (38). Briefly, the codon-optimized sequences of the four proteins were subcloned into a prey construct between the leader sequence of the mouse variable κ light chain 7–33 (37), and a rat Cd4 domains 3 and 4 tag followed by the pentamerization domain of rat cartilaginous oligomeric matrix protein and the beta-lactamase coding sequence, as previously described (38). The prey pentamers were screened against the whole biotinylated merozoite library, and positive interactions identified by detecting colorimetric turnover of nitrocefin (Calbiochem, San Diego, CA) at 485 nm.
Flow Cytometry
Biotinylated EBA140-Cd4d3 + 4, EBA175-Cd4d3 + 4 ectodomains or Cd4 domains 3 + 4 alone (negative control) were immobilized around streptavidin-coated Nile Red fluorescent 0.4–0.6 μm microbeads (Spherotech Inc.) by incubation for 45 min at 4 °C and then presented to human erythrocytes. After incubating for 1 h at 4 °C, the cells were analyzed by flow cytometry using an LSR II machine (BD Biosciences). To test for binding specificity, purified human erythrocytes were either treated with 0.1 mU/106 cells of Vibrio cholera neuraminidase (Sigma) for 1 h at 37 °C and washed twice, or preincubated with the anti-GYPA BRIC 256 monoclonal antibody at a concentration of 0.5 μg/106 cells, before incubation with EBA175-coated microbeads.
RESULTS
Optimization of the Parameters for the Expression of Extracellular Merozoite Proteins in Mammalian Cells
To establish which factors are important for the expression of merozoite surface proteins, we selected the Plasmodium falciparum RH5 protein as an example. RH5 is a 526 amino acid protein secreted by the rhoptries upon erythrocyte invasion and has features that typify many merozoite surface and secreted proteins: it exhibits a very high (79.8%) A+T content in its coding region, does not possess any obvious structural domains and contains a predicted N-terminal signal peptide that gives very low scores when queried by eukaryotic signal sequence prediction programs such as SignalP3.0 (0.022 using Hidden Markov model, and 0.543 using the neural network model) (35). The protein sequence also contains four N-linked glycosylation sequons (NXS/T). Using a mammalian expression system, we have recently shown that an RH5 expression construct containing a codon-optimized sequence, mutated N-linked glycosylation sites and an exogenous signal peptide derived from a mouse antibody could produce a biochemically active RH5 protein that is able to bind its receptor, Basigin (39) and to elicit invasion-blocking antibodies (40). In contrast, RH5 expressed in bacterial expression systems required refolding procedures (41).
To determine the relative importance of the factors affecting the expression levels of full-length RH5, we assessed the individual contributions of each modification by comparing four constructs that contained: (1) the native sequence (RH5-N), (2) the codon-optimized coding sequence for expression in human cells in which the endogenous RH5 signal sequence was retained (RH5-E), (3) the codon-optimized coding sequence using an exogenous high-scoring signal peptide (RH5-X), and (4) the codon-optimized coding sequence using an exogenous signal peptide and in which the four threonine residues in the context of potential N-linked glycosylation sites were mutated to alanine (RH5-O) (39). Although these four mutations modify the protein coding sequence, we considered they would probably be less disruptive than the inappropriate addition of large glycan moieties, which are likely to sterically hinder protein binding interfaces and antibody epitopes. All four coding sequences were flanked by unique NotI and AscI restriction enzyme sites and subcloned into a plasmid that contained a rat Cd4 domain 3 and 4 (Cd4d3 + 4) tag followed by an enzymatically biotinylatable sequence. The Cd4d3 + 4 tag has been successfully used to express the ectodomains of cell surface proteins from diverse structural families in a soluble form while retaining the native binding properties of the fused protein (42). Using this approach, we were able to detect the secretion of a recombinant RH5 protein expressed at moderate level from the native mRNA sequence by ELISA. Although codon-optimization of the sequence on its own did not significantly improve expression of the RH5 protein, the addition of an exogenous signal sequence improved the expression level by ∼3.5-fold (Fig. 1A). This was further enhanced by removal of potential N-linked glycosylation sites which increased expression by 5.5-fold. In summary, the addition of an exogenous high-scoring signal peptide and mutation of N-linked glycosylation sites led to improved expression of RH5.
Fig. 1.
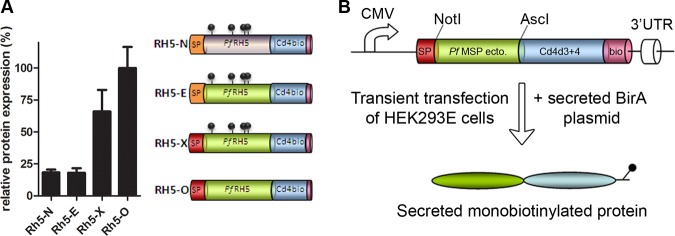
Addition of an exogenous signal peptide and mutation of potential N-linked glycosylation sites contribute to increased expression of recombinant full-length PfRH5 using a mammalian expression system. A, Four different full-length P. falciparum RH5 protein expression constructs were synthesized: RH5-N, Native sequence; RH5-E, codon-optimized sequence (shaded green) with Endogenous signal peptide (orange); RH5-X, codon-optimized sequence with an eXogenous high-scoring mouse antibody signal peptide (red); RH5-O, codon-Optimized sequence with exogenous signal peptide and mutation of potential N-linked glycosylation sites. The approximate positions of potential N-linked glycosylation sites are represented by gray lollipops. The relative levels at which each of these constructs were expressed is shown; bar charts show mean ± S.D.; n = 3. B, Schematic representation of the merozoite protein constructs and procedure to produce soluble recombinant monobiotinylated Cd4d3 + 4-tagged proteins in mammalian cells.
A Resource of Recombinant P. falciparum Merozoite Surface Proteins
Having determined the parameters that improved the expression levels of RH5, we used the same systematic approach to compile a resource of recombinant soluble P. falciparum merozoite surface proteins in a format that could be used in a wide range of biochemical, immunological and functional studies. From the literature, we selected 51 secreted and membrane-tethered merozoite proteins, which likely represent the repertoire of abundant merozoite surface proteins and consisted of 12 GPI-anchored, 10 peripheral, 9 micronemal, 16 rhoptry, and 4 other proteins (Table I). In the case of membrane-tethered proteins, we truncated the protein just before the predicted transmembrane or GPI-anchor to ensure the protein was soluble while preserving the binding functions of the extracellular region. All coding regions were made by gene synthesis and codon-optimized for expression in human cells, any potential N-linked glycosylation sequon was mutated, and the mouse antibody signal sequence was used. For three of the 51 proteins (RH1, RH2b and RH4), cloning of the entire ectodomain concatenated from 1 kbp gene synthesis fragments failed, possibly because of toxicity in bacteria (Geneart AG, personal communication). We therefore used a PCR-based approach to construct smaller subdomains of these proteins from the synthesized fragments (Table I). All proteins were expressed as Cd4d3 + 4-tagged enzymatically monobiotinylated proteins by transiently transfecting HEK293E cells (Fig. 1B).
Using this approach, 39 out of 51 proteins (76%) could be detected directly from the spent cell culture supernatant by ELISA, whereas another three (RH1, RH2b and RH4 subdomains) had to be purified first, suggesting extremely low levels of expression. Detectable expression was not observed for nine proteins (EBL1, PTRAMP, RAP1, RAP2, RAP3, CLAG3.2, RhopH2, RON3, and PF3D7_1431400), mostly belonging to the rhoptry and rhoptry neck category. When quantified against a reference, the expression levels of the recombinant soluble proteins varied widely, covering four orders of magnitude, with 10 of them (25%) expressed above 5 μg of protein per ml of cell culture supernatant (Table I). To determine the integrity of the recombinant proteins, the monomeric biotinylated proteins that could be detected by ELISA were normalized, resolved by SDS-PAGE and detected by anti-biotin blotting (Fig. 2). With the exception of RAMA and TLP, which gave smaller fragments than expected, all 37 remaining merozoite proteins were produced at a size compatible with their calculated expected molecular mass. Twelve of the proteins that could be detected from the cell culture supernatant had a molecular mass above 100 kDa, including the entire ectodomain of MSP1, the largest protein in the library. In addition to their full-length ectodomains, smaller fragments were also observed for some of the proteins, suggesting proteolytic processing.
Fig. 2.
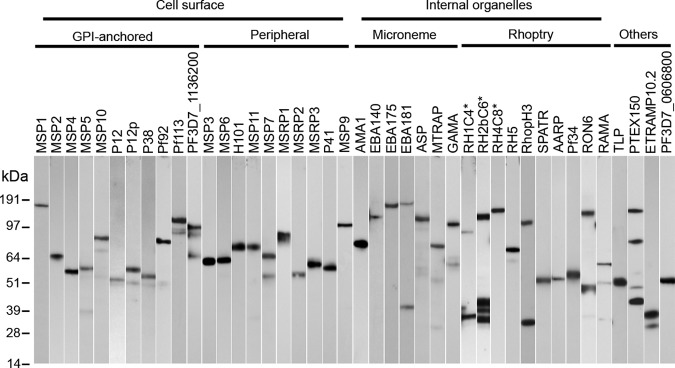
A library of recombinant P. falciparum merozoite surface and secreted proteins. A Western blot of the P. falciparum merozoite proteins that were normalized, resolved by SDS-PAGE under reducing conditions, blotted, and probed with streptavidin-HRP. Proteins labeled with an asterisk were purified before loading. Each protein additionally contains a C-terminal rat Cd4 tag (∼25kDa) and is enzymatically monobiotinylated during expression.
The Recombinant Merozoite Proteins Recapitulate Known Protein–Protein Interactions
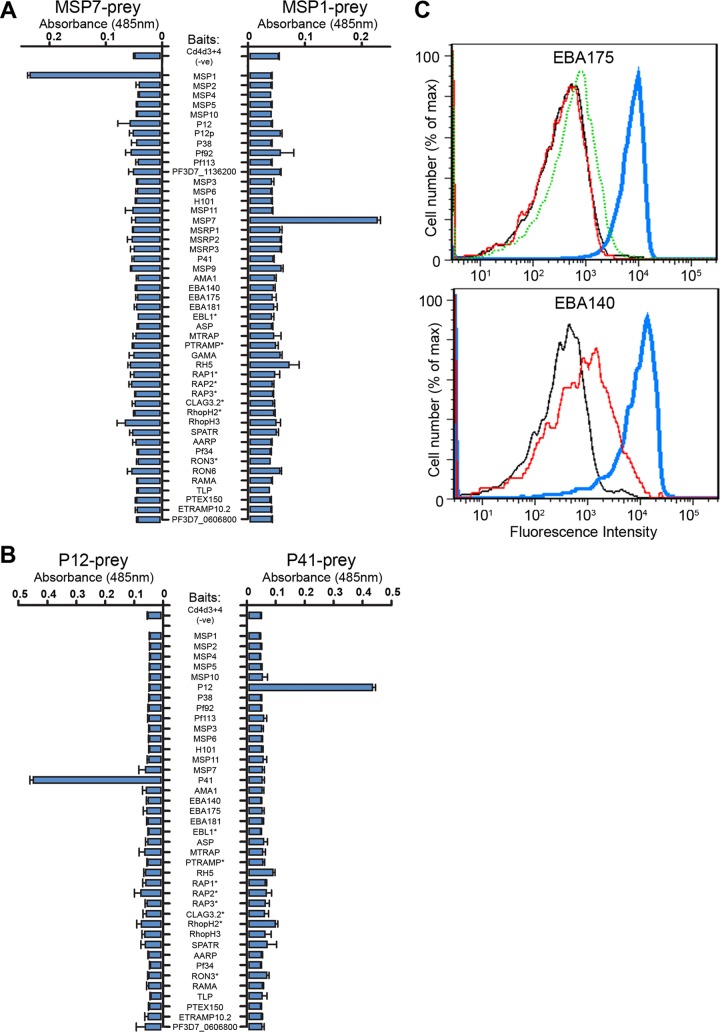
One important factor that must be considered when expressing proteins recombinantly is whether they have retained their native biochemical activity. To demonstrate this, we sought to recapitulate well-characterized known protein interactions that were first identified using native proteins. MSP1, one of the most abundant proteins displayed on the surface of merozoites, has been biochemically well characterized and is known to associate in a complex with MSP7 (43–45). To demonstrate that the recombinant MSP1 and MSP7 expressed using our method interacted directly, we used AVEXIS - an assay designed to detect extracellular protein–protein interactions (38). Both MSP1 and MSP7 were expressed as pentameric enzymatically tagged “preys” that were systematically screened for interactions across the entire library of proteins. We observed that both the MSP1 and MSP7 preys were able to interact with the corresponding MSP7 and MSP1 baits and did not interact with any other proteins in the library, including other members of the MSP7 family (46) (Fig. 3A). As previously observed by others, no interaction was detected between MSP6 and MSP7, nor between MSP6 and the unprocessed form of MSP1 (45). We used a similar approach to detect the direct interaction between two members of the 6-cys family—P12 and P41—which have recently been shown to form a complex at the merozoite surface (47) (Fig. 3B).
Fig. 3.
Recombinant P. falciparum merozoite proteins recapitulate known biochemical activities. The interactions between recombinant MSP1-MSP7 (A), and P12-P41 (B) were detected in both bait-prey orientations by screening the P. falciparum merozoite library with the indicated prey proteins using the AVEXIS assay. Baits labeled with an asterisk were below threshold levels required for the assay. Bar charts show mean ± S.D.; n = 3. C, Recombinant biotinylated EBA175 (top panel) and EBA140 (bottom panel) immobilized on fluorescent streptavidin-coated beads bound to untreated erythrocytes (blue line). Binding was blocked by pre-treating the erythrocytes with neuraminidase (red line) or, for EBA175, pre-incubating erythrocytes with an anti-GYPA monoclonal antibody (dotted green line). Negative controls were Cd4d3 + 4-coated beads (black line).
To extend this validation, we next focused on two micronemal proteins, EBA175 and EBA140, that are known to interact with host proteins on the erythrocyte surface: Glycophorin-A (GYPA) and Glycophorin-C (GYPC), respectively (22, 48). Both interactions are known to be dependent on sialic acid residues so that pre-treatment of erythrocytes with neuraminidase is sufficient to abolish binding (22, 48). To test whether we could recapitulate these interactions, highly avid arrays of the EBA140 or EBA175 ectodomains were immobilized via their biotin tags around streptavidin-coated fluorescent microbeads and tested to see if they bound human erythrocytes using FACS analysis. Clear binding of both recombinant EBA175 and EBA140 to the erythrocyte surface was observed relative to controls (Fig. 3C). Importantly, this binding could be specifically blocked by pre-treatment of erythrocytes with neuraminidase, or, in the case of EBA175-coated microbeads, by pre-incubation of the red blood cells with an anti-GYPA monoclonal antibody. This additionally showed that GYPA is the only major receptor for EBA175 on the erythrocyte surface. Together, these data demonstrate that the recombinant proteins within our library retain their known native extracellular binding properties, suggesting that they are correctly folded and functional.
The Recombinant Merozoite Library Proteins are Immunoreactive and Contain Conformational Epitopes
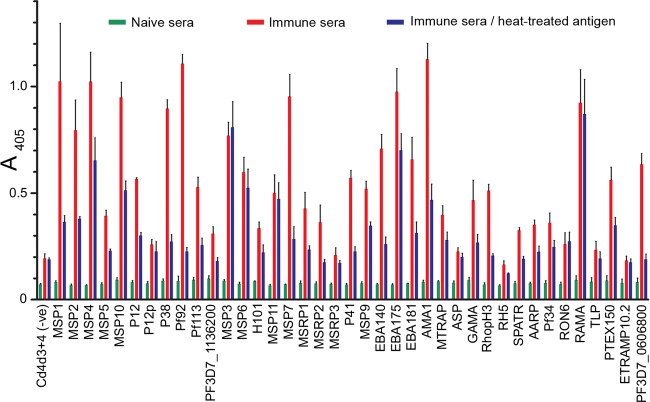
Recapitulating known interactions is one way to demonstrate the biochemical activity of the recombinant proteins, however, P. falciparum merozoite surface and secreted antigens are relatively poorly characterized and the majority of them have no known interacting partner. Therefore, to systematically demonstrate that the proteins within the panel adopt a native conformation, we tested their immunoreactivity using sera from patients living in malaria-endemic regions with the rationale that protective antibody responses will largely recognize the parasite proteins in their native, folded conformation. Thirty-nine proteins from the library were expressed at sufficient levels, captured in an orientated fashion on a streptavidin-coated microtitre plate and their relative immunoreactivity to pooled sera from Kenyan adults compared with that from malaria-naïve UK individuals (Fig. 4). Most proteins were immunoreactive with strong reactivity observed for most of the GPI-anchored surface proteins, MSP3, MSP7, EBA140, EBA175, AMA1, and RAMA. Seven of them (P12p, MSRP3, ASP, RH5, RON6, TLP, and the very small ETRAMP10.2) showed little serological response when compared with the negative control. Although these proteins may not contain epitopes present in the native protein, we cannot rule out that they are poorly immunogenic. For example, the recombinant RH5 protein, which is biochemically active and can bind the Basigin receptor, is known to be poorly immunogenic in natural infections (49). To show that the antibodies were recognizing conformational epitopes within the protein library, all proteins were denatured by heat-treatment before being captured via their biotin tag. The immunoreactivity of most recombinant proteins was significantly reduced, suggesting that the majority of them contain heat-labile epitopes recognized by conformation-sensitive antibodies (Fig. 4). Only four proteins within the library (MSP3, MSP6, MSP11 and RAMA) exhibited strong immunoreactivity that was insensitive to heat denaturation (Fig. 4); strikingly, all four proteins are distinguished by the presence of repetitive acidic amino acids (50, 51). Three of these four proteins belong to the MSP3 family which are known to be dominated by a heat-stable coiled coil structure and a low complexity glutamic-rich region (52, 53). From these observations, we conclude that most recombinant ectodomains in the library are likely to be correctly folded.
Fig. 4.
Presence of heat-labile conformational epitopes across the P. falciparum merozoite protein library by systematic immunoreactivity profiling. The immunoreactivity of the recombinant P. falciparum merozoite proteins was tested using sera pooled from ten malaria-naïve adults from the UK as a control (green bar) or ten immune adults from Kenya (red bar). Reduced response of immune sera to heat-denatured antigens (blue bar) demonstrates the presence of heat-labile (conformational) epitopes. Bar charts show mean ± S.D.; n = 3.
DISCUSSION
One of the enduring technical challenges in malaria research has been the inability to routinely express Plasmodium proteins in a recombinant, functionally active form. This challenge has been generally acknowledged as a significant impeding factor for malaria research and the development of an effective vaccine (12). Here, we have used a mammalian expression system and a generic approach to develop a panel of soluble ectodomains representing the repertoire of abundant P. falciparum cell surface and secreted merozoite proteins. We have demonstrated their biochemical activity by recapitulating known interactions between parasite proteins and with host receptors.
Previous attempts at expressing P. falciparum proteins in heterologous expression systems have primarily relied on empirically identifying tractable fragments yielding soluble proteins in bacterial expression systems, which can be very time-consuming and does not necessarily lead to the production of a functional protein. In both bacterial and cell-free expression systems, proteins are usually produced under reducing conditions, precluding the formation of disulfide bonds that are structurally critical for cell-surface and secreted proteins. These approaches therefore do not produce extracellular proteins in their native fold and subsequently often require complex refolding procedures.
Mammalian expression systems have been previously used to express Plasmodium proteins (22, 54–56) but weren't widely adopted, possibly because of low yields. Recent improvements in mammalian expression plasmids and cell lines have led to the development of convenient and high-yielding systems that make this approach more practical (36, 57, 58). We (39, 40, 59) and others (60) have previously reported the successful expression of individual proteins using this system. Yields of the Plasmodium proteins did not significantly differ from other proteins libraries that we have constructed using the same approach; for example, zebrafish (38, 61, 62) and human erythrocyte receptors (39). The proteins were expressed at different levels, spanning several orders of magnitude, with MSP2 and AMA1 giving the highest levels of expression (up to ∼50 μg/ml). It should be pointed out that these proteins were expressed using a cost-effective transient transfection approach that typically results in ∼40–60% transfection efficiency; yields could therefore be significantly improved by selecting high-expressing stably transfected cell lines. Although the majority of selected merozoite proteins were expressed (Table I), we found that some, particularly those located in the rhoptry, were not expressed at detectable levels, if at all. Attempts to cotransfect multiple members of the same family (RAP1, 2, and 3, for example) with the idea that they may form a complex, did not lead to any further evidence of expression (data not shown). Some proteins trafficked to the rhoptry may require specific chaperones that are not provided by the HEK293 cells.
The inserts encoding each protein within the library are flanked by unique rare-cutting restriction enzymes (NotI and AscI) which enable facile shuttling of the ectodomain regions into a variety of different expression vectors containing useful C-terminal tags for biochemical manipulation. The enzymatically monobiotinylatable tag enables the orientated capture of the proteins on streptavidin-coated microtitre plates or microarray slides (63). Other vectors allow for the production of highly avid pentameric constructs or the purification of recombinant proteins through a C-terminal hexaHis tag, and several of these vectors are already available at plasmid resource providers (64).
We believe that this expression approach and protein library will be a valuable resource to investigate the functional roles of merozoite surface proteins in the biology and pathogenesis of P. falciparum malaria but could also be applied to study other aspects of malaria. Indeed, we have been able to express proteins from different stages of the lifecycle including sporozoites and gametes (CC, GJW unpublished) as well as from other Plasmodium species, such as P. vivax (J. Hostetler, GJW and JR, unpublished) using the same method. The merozoite protein resource will be useful for a wide range of investigations including immunoepidemiology, structural studies, cell-based assays and the identification of novel parasite-parasite and host-parasite interactions. Indeed, individual proteins from the resource have already been used to identify novel host receptors involved in erythrocyte invasion (39, 59). The production of large panels of correctly folded proteins in a systematic way enables the direct and unbiased comparison of proteins relative to each other, rather than the discrete approach using individual proteins that has been frequently used in the past. This will be particularly important for comparative immunoepidemiological studies and a systematic assessment of these proteins might lead to the identification of novel blood stage vaccine candidates.
Footnotes
* This work was supported by the Wellcome Trust (grant number 098051).
1 The abbreviations used are:
- AVEXIS
- avidity-based extracellular interaction screen
- EBA
- erythrocyte binding antigen
- HEK
- human embryonic kidney
- HBS
- hepes-buffered saline
- MSP
- merozoite surface protein
- RH
- reticulocyte-binding protein homolog.
REFERENCES
- 1. Murray C. J., Rosenfeld L. C., Lim S. S., Andrews K. G., Foreman K. J., Haring D., Fullman N., Naghavi M., Lozano R., Lopez A. D. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379, 413–431 [DOI] [PubMed] [Google Scholar]
- 2. Cowman A. F., Crabb B. S. (2006) Invasion of red blood cells by malaria parasites. Cell 124, 755–766 [DOI] [PubMed] [Google Scholar]
- 3. Agnandji S. T., Lell B., Soulanoudjingar S. S., Fernandes J. F., Abossolo B. P., Conzelmann C., Methogo B. G., Doucka Y., Flamen A., Mordmüller B., Issifou S., Kremsner P. G., Sacarlal J., Aide P., Lanaspa M., Aponte J. J., Nhamuave A., Quelhas D., Bassat Q., Mandjate S., Macete E., Alonso P., Abdulla S., Salim N., Juma O., Shomari M., Shubis K., Machera F., Hamad A. S., Minja R., Mtoro A., Sykes A., Ahmed S., Urassa A. M., Ali A. M., Mwangoka G., Tanner M., Tinto H., D'Alessandro U., Sorgho H., Valea I., Tahita M. C., Kabore W., Ouedraogo S., Sandrine Y., Guiguemde R. T., Ouedraogo J. B., Hamel M. J., Kariuki S., Odero C., Oneko M., Otieno K., Awino N., Omoto J., Williamson J., Muturi-Kioi V., Laserson K. F., Slutsker L., Otieno W., Otieno L., Nekoye O., Gondi S., Otieno A., Ogutu B., Wasuna R., Owira V., Jones D., Onyango A. A., Njuguna P., Chilengi R., Akoo P., Kerubo C., Gitaka J., Maingi C., Lang T., Olotu A., Tsofa B., Bejon P., Peshu N., Marsh K., Owusu-Agyei S., Asante K. P., Osei-Kwakye K., Boahen O., Ayamba S., Kayan K., Owusu-Ofori R., Dosoo D., Asante I., Adjei G., Chandramohan D., Greenwood B., Lusingu J., Gesase S., Malabeja A., Abdul O., Kilavo H., Mahende C., Liheluka E., Lemnge M., Theander T., Drakeley C., Ansong D., Agbenyega T., Adjei S., Boateng H. O., Rettig T., Bawa J., Sylverken J., Sambian D., Agyekum A., Owusu L., Martinson F., Hoffman I., Mvalo T., Kamthunzi P., Nkomo R., Msika A., Jumbe A., Chome N., Nyakuipa D., Chintedza J., Ballou W. R., Bruls M., Cohen J., Guerra Y., Jongert E., Lapierre D., Leach A., Lievens M., Ofori-Anyinam O., Vekemans J., Carter T., Leboulleux D., Loucq C., Radford A., Savarese B., Schellenberg D., Sillman M., Vansadia P. (2011) First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365, 1863–1875 [DOI] [PubMed] [Google Scholar]
- 4. Agnandji S. T., Lell B., Fernandes J. F., Abossolo B. P., Methogo B. G., Kabwende A. L., Adegnika A. A., Mordmüller B., Issifou S., Kremsner P. G., Sacarlal J., Aide P., Lanaspa M., Aponte J. J., Machevo S., Acacio S., Bulo H., Sigauque B., Macete E., Alonso P., Abdulla S., Salim N., Minja R., Mpina M., Ahmed S., Ali A. M., Mtoro A. T., Hamad A. S., Mutani P., Tanner M., Tinto H., D'Alessandro U., Sorgho H., Valea I., Bihoun B., Guiraud I., Kabore B., Sombie O., Guiguemde R. T., Ouedraogo J. B., Hamel M. J., Kariuki S., Oneko M., Odero C., Otieno K., Awino N., McMorrow M., Muturi-Kioi V., Laserson K. F., Slutsker L., Otieno W., Otieno L., Otsyula N., Gondi S., Otieno A., Owira V., Oguk E., Odongo G., Woods J. B., Ogutu B., Njuguna P., Chilengi R., Akoo P., Kerubo C., Maingi C., Lang T., Olotu A., Bejon P., Marsh K., Mwambingu G., Owusu-Agyei S., Asante K. P., Osei-Kwakye K., Boahen O., Dosoo D., Asante I., Adjei G., Kwara E., Chandramohan D., Greenwood B., Lusingu J., Gesase S., Malabeja A., Abdul O., Mahende C., Liheluka E., Malle L., Lemnge M., Theander T. G., Drakeley C., Ansong D., Agbenyega T., Adjei S., Boateng H. O., Rettig T., Bawa J., Sylverken J., Sambian D., Sarfo A., Agyekum A., Martinson F., Hoffman I., Mvalo T., Kamthunzi P., Nkomo R., Tembo T., Tegha G., Tsidya M., Kilembe J., Chawinga C., Ballou W. R., Cohen J., Guerra Y., Jongert E., Lapierre D., Leach A., Lievens M., Ofori-Anyinam O., Olivier A., Vekemans J., Carter T., Kaslow D., Leboulleux D., Loucq C., Radford A., Savarese B., Schellenberg D., Sillman M., Vansadia P. (2012) A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367, 2284–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Demanga C. G., Daher L.-J., Prieur E., Blanc C., Pérignon J.-L., Bouharoun-Tayoun H., Druilhe P. (2010) Toward the rational design of a malaria vaccine construct using the MSP3 family as an example: contribution of antigenicity studies in humans. Infection and Immunity 78, 486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richards J. S., Stanisic D. I., Fowkes F. J. I., Tavul L., Dabod E., Thompson J. K., Kumar S., Chitnis C. E., Narum D. L., Michon P., Siba P. M., Cowman A. F., Mueller I., Beeson J. G. (2010) Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infectious Dis. 51, e50–60 [DOI] [PubMed] [Google Scholar]
- 7. Osier F. H., Fegan G., Polley S. D., Murungi L., Verra F., Tetteh K. K., Lowe B., Mwangi T., Bull P. C., Thomas A. W., Cavanagh D. R., McBride J. S., Lanar D. E., Mackinnon M. J., Conway D. J., Marsh K. (2008) Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infection Immunity 76, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roussilhon C., Oeuvray C., Müller-Graf C., Tall A., Rogier C., Trape J.-F., Theisen M., Balde A., Pérignon J.-L., Druilhe P. (2007) Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Medicine 4, e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fowkes F. J., Richards J. S., Simpson J. A., Beeson J. G. (2010) The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Medicine 7, e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richards J. S., Beeson J. G. (2009) The future for blood-stage vaccines against malaria. Immunol. Cell Biol. 87, 377–390 [DOI] [PubMed] [Google Scholar]
- 11. Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., Paulsen I. T., James K., Eisen J. A., Rutherford K., Salzberg S. L., Craig A., Kyes S., Chan M. S., Nene V., Shallom S. J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M. W., Vaidya A. B., Martin D. M., Fairlamb A. H., Fraunholz M. J., Roos D. S., Ralph S. A., McFadden G. I., Cummings L. M., Subramanian G. M., Mungall C., Venter J. C., Carucci D. J., Hoffman S. L., Newbold C., Davis R. W., Fraser C. M., Barrell B. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birkholtz L. M., Blatch G., Coetzer T. L., Hoppe H. C., Human E., Morris E. J., Ngcete Z., Oldfield L., Roth R., Shonhai A., Stephens L., Louw A. I. (2008) Heterologous expression of plasmodial proteins for structural studies and functional annotation. Malar J. 7, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milek R. L., Stunnenberg H. G., Konings R. N. (2000) Assembly and expression of a synthetic gene encoding the antigen Pfs48/45 of the human malaria parasite Plasmodium falciparum in yeast. Vaccine 18, 1402–1411 [DOI] [PubMed] [Google Scholar]
- 14. Outchkourov N. S., Roeffen W., Kaan A., Jansen J., Luty A., Schuiffel D., van Gemert G. J., van de Vegte-Bolmer M., Sauerwein R. W., Stunnenberg H. G. (2008) Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc. Natl. Acad. Sci. U.S.A. 105, 4301–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stowers A. W., Zhang Y., Shimp R. L., Kaslow D. C. (2001) Structural conformers produced during malaria vaccine production in yeast. Yeast 18, 137–150 [DOI] [PubMed] [Google Scholar]
- 16. Aguiar J. C., LaBaer J., Blair P. L., Shamailova V. Y., Koundinya M., Russell J. A., Huang F., Mar W., Anthony R. M., Witney A., Caruana S. R., Brizuela L., Sacci J. B., Jr., Hoffman S. L., Carucci D. J. (2004) High-throughput generation of P. falciparum functional molecules by recombinational cloning. Genome Res. 14, 2076–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehlin C., Boni E., Buckner F. S., Engel L., Feist T., Gelb M. H., Haji L., Kim D., Liu C., Mueller N., Myler P. J., Reddy J. T., Sampson J. N., Subramanian E., Van Voorhis W. C., Worthey E., Zucker F., Hol W. G. (2006) Heterologous expression of proteins from Plasmodium falciparum: results from 1000 genes. Mol. Biochem. Parasitol. 148, 144–160 [DOI] [PubMed] [Google Scholar]
- 18. Vedadi M., Lew J., Artz J., Amani M., Zhao Y., Dong A., Wasney G. A., Gao M., Hills T., Brokx S., Qiu W., Sharma S., Diassiti A., Alam Z., Melone M., Mulichak A., Wernimont A., Bray J., Loppnau P., Plotnikova O., Newberry K., Sundararajan E., Houston S., Walker J., Tempel W., Bochkarev A., Kozieradzki I., Edwards A., Arrowsmith C., Roos D., Kain K., Hui R. (2007) Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol. Biochem. Parasitol. 151, 100–110 [DOI] [PubMed] [Google Scholar]
- 19. von Itzstein M., Plebanski M., Cooke B. M., Coppel R. L. (2008) Hot, sweet and sticky: the glycobiology of Plasmodium falciparum. Trends Parasitol. 24, 210–218 [DOI] [PubMed] [Google Scholar]
- 20. van Bemmelen M. X., Beghdadi-Rais C., Desponds C., Vargas E., Herrera S., Reymond C. D., Fasel N. (2000) Expression and one-step purification of Plasmodium proteins in dictyostelium. Mol. Biochem. Parasitol. 111, 377–390 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh S., Malhotra P., Lalitha P. V., Guha-Mukherjee S., Chauhan V. S. (2002) Expression of Plasmodium falciparum C-terminal region of merozoite surface protein (PfMSP119), a potential malaria vaccine candidate, in tobacco. Plant Sci. 162, 335–343 [Google Scholar]
- 22. Sim B. K., Chitnis C. E., Wasniowska K., Hadley T. J., Miller L. H. (1994) Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264, 1941–1944 [DOI] [PubMed] [Google Scholar]
- 23. Doolan D. L., Mu Y., Unal B., Sundaresh S., Hirst S., Valdez C., Randall A., Molina D., Liang X., Freilich D. A., Oloo J. A., Blair P. L., Aguiar J. C., Baldi P., Davies D. H., Felgner P. L. (2008) Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 8, 4680–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mu J., Awadalla P., Duan J., McGee K. M., Keebler J., Seydel K., McVean G. A., Su X. Z. (2007) Genome-wide variation and identification of vaccine targets in the Plasmodium falciparum genome. Nat. Genet. 39, 126–130 [DOI] [PubMed] [Google Scholar]
- 25. Tsuboi T., Takeo S., Iriko H., Jin L., Tsuchimochi M., Matsuda S., Han E. T., Otsuki H., Kaneko O., Sattabongkot J., Udomsangpetch R., Sawasaki T., Torii M., Endo Y. (2008) Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 76, 1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baca A. M., Hol W. G. (2000) Overcoming codon bias: a method for high-level overexpression of Plasmodium and other AT-rich parasite genes in Escherichia coli. Int. J. Parasitol. 30, 113–118 [DOI] [PubMed] [Google Scholar]
- 27. LaCount D. J., Schoenfeld L. W., Fields S. (2009) Selection of yeast strains with enhanced expression of Plasmodium falciparum proteins. Mol. Biochem. Parasitol. 163, 119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Angov E., Hillier C. J., Kincaid R. L., Lyon J. A. (2008) Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS One 3, e2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Z., Schnake P., Xiao L., Lal A. A. (2004) Enhanced expression of a recombinant malaria candidate vaccine in Escherichia coli by codon optimization. Protein Expr. Purif. 34, 87–94 [DOI] [PubMed] [Google Scholar]
- 30. Crompton P. D., Kayala M. A., Traore B., Kayentao K., Ongoiba A., Weiss G. E., Molina D. M., Burk C. R., Waisberg M., Jasinskas A., Tan X., Doumbo S., Doumtabe D., Kone Y., Narum D. L., Liang X., Doumbo O. K., Miller L. H., Doolan D. L., Baldi P., Felgner P. L., Pierce S. K. (2010) A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. U.S.A. 107, 6958–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trieu A., Kayala M. A., Burk C., Molina D. M., Freilich D. A., Richie T. L., Baldi P., Felgner P. L., Doolan D. L. (2011) Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Mol. Cell Proteomics 10, M111.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang V. T., Crispin M., Aricescu A. R., Harvey D. J., Nettleship J. E., Fennelly J. A., Yu C., Boles K. S., Evans E. J., Stuart D. I., Dwek R. A., Jones E. Y., Owens R. J., Davis S. J. (2007) Glycoprotein structural genomics: solving the glycosylation problem. Structure 15, 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonnhammer E. L., von Heijne G., Krogh A. (1998) A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6, 175–182 [PubMed] [Google Scholar]
- 34. Eisenhaber B., Bork P., Eisenhaber F. (1999) Prediction of potential GPI-modification sites in proprotein sequences. J. Mol. Biol. 292, 741–758 [DOI] [PubMed] [Google Scholar]
- 35. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004) Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783–795 [DOI] [PubMed] [Google Scholar]
- 36. Durocher Y., Perret S., Kamen A. (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 30, E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crosnier C., Staudt N., Wright G. J. (2010) A rapid and scalable method for selecting recombinant mouse monoclonal antibodies. BMC Biol. 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bushell K. M., Söllner C., Schuster-Boeckler B., Bateman A., Wright G. J. (2008) Large-scale screening for novel low-affinity extracellular protein interactions. Genome Res. 18, 622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crosnier C., Bustamante L. Y., Bartholdson S. J., Bei A. K., Theron M., Uchikawa M., Mboup S., Ndir O., Kwiatkowski D. P., Duraisingh M. T., Rayner J. C., Wright G. J. (2011) Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bustamante L. Y., Bartholdson S. J., Crosnier C., Campos M. G., Wanaguru M., Nguon C., Kwiatkowski D. P., Wright G. J., Rayner J. C. (2013) A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine 31, 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baum J., Chen L., Healer J., Lopaticki S., Boyle M., Triglia T., Ehlgen F., Ralph S. A., Beeson J. G., Cowman A. F. (2009) Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 39, 371–380 [DOI] [PubMed] [Google Scholar]
- 42. Brown M. H., Barclay A. N. (1994) Expression of immunoglobulin and scavenger receptor superfamily domains as chimeric proteins with domains 3 and 4 of CD4 for ligand analysis. Protein Eng. 7, 515–521 [DOI] [PubMed] [Google Scholar]
- 43. Stafford W. H., Gunder B., Harris A., Heidrich H. G., Holder A. A., Blackman M. J. (1996) A 22 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 80, 159–169 [DOI] [PubMed] [Google Scholar]
- 44. Pachebat J. A., Ling I. T., Grainger M., Trucco C., Howell S., Fernandez-Reyes D., Gunaratne R., Holder A. A. (2001) The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol. Biochem. Parasitol. 117, 83–89 [DOI] [PubMed] [Google Scholar]
- 45. Kauth C. W., Woehlbier U., Kern M., Mekonnen Z., Lutz R., Mücke N., Langowski J., Bujard H. (2006) Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 281, 31517–31527 [DOI] [PubMed] [Google Scholar]
- 46. Mello K., Daly T. M., Morrisey J., Vaidya A. B., Long C. A., Bergman L. W. (2002) A multigene family that interacts with the amino terminus of plasmodium MSP-1 identified using the yeast two-hybrid system. Eukaryotic Cell 1, 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taechalertpaisarn T., Crosnier C., Bartholdson S. J., Hodder A. N., Thompson J., Bustamante L. Y., Wilson D. W., Sanders P. R., Wright G. J., Rayner J. C., Cowman A. F., Gilson P. R., Crabb B. S. (2012) Biochemical and functional analysis of two Plasmodium falciparum blood-stage 6-cys proteins: P12 and P41. PLoS One 7, e41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maier A. G., Duraisingh M. T., Reeder J. C., Patel S. S., Kazura J. W., Zimmerman P. A., Cowman A. F. (2003) Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nature Medicine 9, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Douglas A. D., Williams A. R., Illingworth J. J., Kamuyu G., Biswas S., Goodman A. L., Wyllie D. H., Crosnier C., Miura K., Wright G. J., Long C. A., Osier F. H., Marsh K., Turner A. V., Hill A. V., Draper S. J. (2011) The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat. Commun. 2, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singh S., Soe S., Weisman S., Barnwell J. W., Pérignon J. L., Druilhe P. (2009) A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4, e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Topolska A. E., Lidgett A., Truman D., Fujioka H., Coppel R. L. (2004) Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J. Biol. Chem. 279, 4648–4656 [DOI] [PubMed] [Google Scholar]
- 52. Pearce J. A., Hodder A. N., Anders R. F. (2004) The alanine-rich heptad repeats are intact in the processed form of Plasmodium falciparum MSP3. Exp. Parasitol. 108, 186–189 [DOI] [PubMed] [Google Scholar]
- 53. Gondeau C., Corradin G., Heitz F., Le Peuch C., Balbo A., Schuck P., Kajava A. V. (2009) The C-terminal domain of Plasmodium falciparum merozoite surface protein 3 self-assembles into alpha-helical coiled coil tetramer. Mol. Biochem. Parasitol. 165, 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chattopadhyay R., Rathore D., Fujioka H., Kumar S., de la Vega P., Haynes D., Moch K., Fryauff D., Wang R., Carucci D. J., Hoffman S. L. (2003) PfSPATR, a Plasmodium falciparum protein containing an altered thrombospondin type I repeat domain is expressed at several stages of the parasite life cycle and is the target of inhibitory antibodies. J. Biol. Chem. 278, 25977–25981 [DOI] [PubMed] [Google Scholar]
- 55. Wickramarachchi T., Devi Y. S., Mohmmed A., Chauhan V. S. (2008) Identification and characterization of a novel Plasmodium falciparum merozoite apical protein involved in erythrocyte binding and invasion. PLoS One 3, e1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L., Menting J. G., Black C. G., Stowers A., Kaslow D. C., Hoffman S. L., Coppel R. L. (2000) Differences in epitope recognition, isotype and titer of antisera to Plasmodium falciparum merozoite surface protein 4 raised by different modes of DNA or protein immunization. Vaccine 19, 816–824 [DOI] [PubMed] [Google Scholar]
- 57. Durocher Y., Butler M. (2009) Expression systems for therapeutic glycoprotein production. Curr. Opin. Biotechnol. 20, 700–707 [DOI] [PubMed] [Google Scholar]
- 58. Pham P. L., Kamen A., Durocher Y. (2006) Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol. Biotechnol. 34, 225–237 [DOI] [PubMed] [Google Scholar]
- 59. Bartholdson S. J., Bustamante L. Y., Crosnier C., Johnson S., Lea S., Rayner J. C., Wright G. J. (2012) Semaphorin-7A is an erythrocyte receptor for P. falciparum merozoite-specific TRAP homolog, MTRAP. PLoS Pathogens 8, e1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Srivastava A., Gangnard S., Round A., Dechavanne S., Juillerat A., Raynal B., Faure G., Baron B., Ramboarina S., Singh S. K., Belrhali H., England P., Lewit-Bentley A., Scherf A., Bentley G. A., Gamain B. (2010) Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl. Acad. Sci. U.S.A. 107, 4884–4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martin S., Söllner C., Charoensawan V., Adryan B., Thisse B., Thisse C., Teichmann S., Wright G. J. (2010) Construction of a large extracellular protein interaction network and its resolution by spatiotemporal expression profiling. Mol. Cell Proteomics 9, 2654–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Söllner C., Wright G. J. (2009) A cell surface interaction network of neural leucine-rich repeat receptors. Genome Biol. 10, R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun Y., Gallagher-Jones M., Barker C., Wright G. J. (2012) A benchmarked protein microarray-based platform for the identification of novel low-affinity extracellular protein interactions. Anal. Biochem. 424, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Herscovitch M., Perkins E., Baltus A., Fan M. (2012) Addgene provides an open forum for plasmid sharing. Nat. Biotechnol. 30, 316–317 [DOI] [PubMed] [Google Scholar]