Abstract
Caffeine (1,3,7-trimethylxanthine) is an habitual substance present in a wide variety of beverages and in chocolate-based foods and it is also used as adjuvant in some drugs. The antioxidant ability of caffeine has been reported in contrast with its pro- oxidant effects derived from its action mechanism such as the systemic release of catecholamines. The aim of this work was to evaluate the effect of caffeine on exercise oxidative stress, measuring plasma vitamins A, E, C and malonaldehyde (MDA) as markers of non enzymatic antioxidant status and lipid peroxidation respectively. Twenty young males participated in a double blind (caffeine 5mg·kg- 1 body weight or placebo) cycling test until exhaustion. In the exercise test, where caffeine was ingested prior to the test, exercise time to exhaustion, maximum heart rate, and oxygen uptake significantly increased, whereas respiratory exchange ratio (RER) decreased. Vitamins A and E decreased with exercise and vitamin C and MDA increased after both the caffeine and placebo tests but, regarding these particular variables, there were no significant differences between the two test conditions. The results obtained support the conclusion that this dose of caffeine enhances the ergospirometric response to cycling and has no effect on lipid peroxidation or on the antioxidant vitamins A, E and C.
Key Points.
Caffeine ingestion may improve maximal aerobic performance in non trained men.
Cellular oxidative damage is not altered by caffeine ingestion in maximal aerobic exercises.
Antioxidant response to exercise, vitamins A, E and C, is not modified by caffeine action in maximal aerobic efforts.
Key words: Trimethylxanthine, malonaldehyde, vitamins, catecholamines, VO2 max
Introduction
Caffeine, or 1,3,7- trimethylxanthine, is a widely used substance present in habitual beverages and chocolate-based foods and is also used for therapeutic purposes, being present in a wide variety of fixed combination prescription drugs. Moreover, caffeine is used as an ergogenic aid because multiple well-controlled experiments have found that moderate doses of caffeine (3-6 mg/kg) can improve performance in athletes (Graham, 2001, Flinn et al., 1990). Its use by athletes is actually favoured since the International Olympic Committee (IOC) has recently removed caffeine from its list of banned substances.
The ergogenic effect of caffeine ingestion before exercise has been reported above all in high intensity aerobic conditioning programs (Bruce et al., 2000; Jackman et al., 1996; Wiles et al., 1992). The mechanism for the caffeine-improved performance is not clear but several possibilities have been proposed such as the antagonism of adenosine receptors (Davis et al., 2003), the attenuation of effort perception or reduction of muscle pain (Doherty et al., 2004; O’connor et al., 2004) and the increase in catecholamine release (Greer et al., 2000; Graham et al., 2000; Jackman et al., 1996; Van Soeren et al., 1993).
Some of these caffeine-derived effects could favour the production of free radicals and a subsequent increase of oxidative stress such as the metabolic inactivation of catecholamines (Halliwell and Gutteridge, 1985; Jewett et al., 1989) and the increase of oxidative metabolism (Shigenaga et al., 1994; Thompson et al., 2001) including its own hepatic metabolism (Vistisen et al., 1992). There are also reports suggesting that caffeine is capable of inducing certain forms of oxidative damage by increasing lipid peroxidation (Dianzani et al., 1991)
Nevertheless, caffeine has been reported as a protective substance on cellular damage (Kamat et al., 2000; Krisko et al., 2005) with beneficial antioxidant effects (Nikolic et al., 2003); probably due to the main metabolites of caffeine, 1-methylxanthine and 1-methyluric acid, that are highly effective antioxidants and are able to prevent LDL oxidation in vitro (Lee, 2000).
So, what will be the behaviour of caffeine with regard to maximum aerobic effort? Will caffeine protect the organism from the deleterious effects of free radicals which are generated in these situations, or on the contrary, will caffeine act as a pro-oxidative substance by increasing cellular damage?
The present study examines the effect of caffeine on certain biomarkers of antioxidant status such as vitamins A, E and C and on a marker of lipid peroxidation such as malonaldehyde (MDA), in order to identify caffeine’s role regarding oxidative stress in an incremental cycling test until exhaustion.
Methods
Subjects
Participants were twenty young males who reported no caffeine consumption and who were not regularly performing exercise. The subject did not receive any vitamin or antioxidant supplementation before or during the study. They were informed of the procedure of this study and they all gave written consent. The participants’ characteristics are provided in Table 1 and values were calculated using cineanthropometric techniques.
Table 1.
Anthropometric data for the population studied (n = 20). Data are means (±SD).
| Age (years) | Height (m) | Body weight (kg) | % Lean body mass | % Body fat | % Body bone | % Residual |
|---|---|---|---|---|---|---|
| 20.9 (1.3) | 1.75 (.06) | 71.0 (5.5) | 53.5 (1.5) | 10.1 (1.1) | 12.3 (1.0) | 24.1 (0.0) |
Experimental design
Caffeine was administered using a single dose, double-blind and randomised procedure to protect against possible subject motivation. On the day of the experiment, the participants ingested capsules that contained caffeine (5mg·kg-1 body weight) or a placebo with water. One hour later they cycled until exhaustion on a cycle ergometer (Ergo-metrics 900 de Ergo-line®). The starting intensity for the test was set at 100 Watts and was increased by 50 Watts every two minutes until 300 Watts, when it was increased by 25 Watts until exhaustion. Expired gases, ventilation and heart rate were measured using a Medical Graphics gas analyser (MGC, model n° 762014-102) and a hear rate monitor (Polar ® S 720) with interface (Polar ® Advantage interface). Each subject performed the cycling test under two conditions, placebo and caffeine, with an interval of three days in order to favour recovery and to remove exercise adaptations. This research has been performed with the permission of legal and ethical committees in Spain.
Measurements
A blood sample was obtained from the anticubital vein in repose conditions before caffeine (or placebo) ingestion and immediately after the exercise. Haematocrite determination was made by microcentrifugation (Microcentrifugue Alresa) of 25 µl of blood contained in heparinised capillars, in order to correct the oxidative markers measured in blood due to a possible hemoconcentration. Plasma was obtained by centrifugation and was stored at -20°C until use, which was not longer than one week. Plasma samples were analysed by HPLC to determine the concentrations of vitamin A and E (Shearer, 1986) as lipid soluble no-enzymatic antioxidants, vitamin C (Manoharan and Schwille, 1994) as water soluble no enzymatic antioxidant and MDA (Esterbauer et al., 1984) as a measure of lipid peroxidation.
A urine sample was obtained in repose conditions before caffeine (or placebo) ingestion in order to guarantee no previous caffeine consumption and immediately after the exercise. Caffeine was determined by HPLC (Spectra SERIES P100 / UV 100) (Dobrocky et al., 1994).
Statistics
Statistical analysis was performed on SPSS version 11.0, performing the two way Anova test, in which the within group factor corresponds to the ergometer test and the inter group factor corresponds to the caffeine effect. A p value of < 0.05 was used to determine statistical significance.
Results
A comparison of the ergospirometric values between both tests (Table 2) showed that subjects in the caffeine trial cycled longer, supported more load, and their heart rate and relative oxygen consumption were higher than in the placebo trial, whereas the relative respiratory exchange ratio was lower. There were no differences between both tests in carbon dioxide production, expired volume or respiratory rate.
Table 2.
Effect of caffeine on time to exhaustion, load, heart rate (HR), relative oxygen consumption (VO2), carbon dioxide production (VCO2), expired volume (VE), respiratory rate (RR) and respiratory exchange ratio (RER). Data are means (±SD).
| Time (min) |
Load (W) |
HR (bpm) |
VO2 (ml·kg-1·min-1) |
VCO2 (ml·min-1) |
VE (l·min-1) |
RR (rpm) |
RER | |
|---|---|---|---|---|---|---|---|---|
| Placebo | 11.35 (1.45) | 328 (24) |
184 (5) |
38.5 (5.0) |
4050 (598) |
123.1 (22.3) | 47.7 (8.4) | 1.46 (.14) |
| Caffeine | 12.08 (1.59)** | 336 (24)* |
190 (6)** |
41.6 (6.5)* |
4136 (1183) |
136.2 (22.7)* | 51.7 (7.4) | 1.40 (.13)* |
* p< 0.05
** p< 0.01.
All plasma data have been corrected with the haematocrite values (Figure 1). Figures 2, 3 and 4 show plasma vitamin E, A, and C levels, before and after the cycling tests, while Figure 5 shows changes in MDA before and after the trials. Vitamins E and A, lipid soluble antioxidants, decreased significantly (p < 0.05) in both strenuous situations independently of caffeine ingestion (Figure 2 and 3), whereas vitamin C, water soluble antioxidant, increased in both tests with a more pronounced increase in the caffeine trial without reaching significance (Figure 4).
Figure 1.
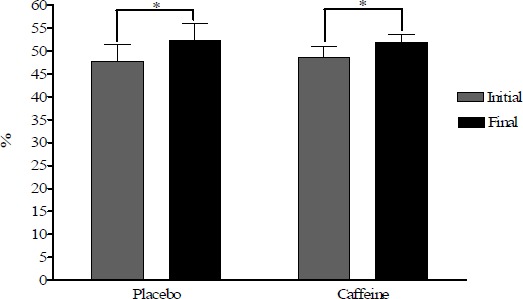
Haematocrite changes in placebo and caffeine trials. * p < 0.01.
Figure 2.
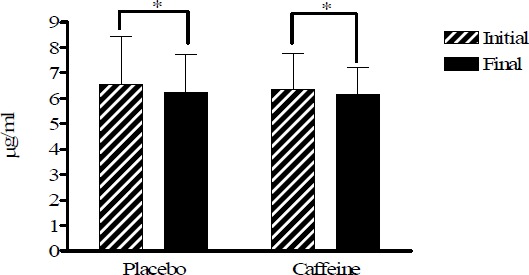
Vitamin E plasma levels in placebo and caffeine trials expressed in µg·ml-1. * p < 0.05).
Figure 3.
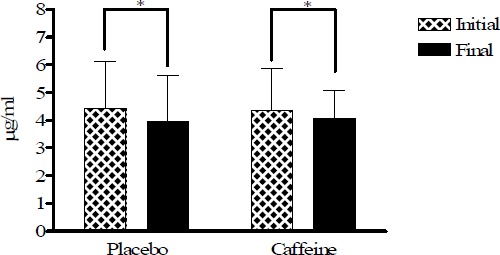
Vitamin A plasma levels in placebo and caffeine trials expressed in µg·ml-1. * p < 0.05).
Figure 4.
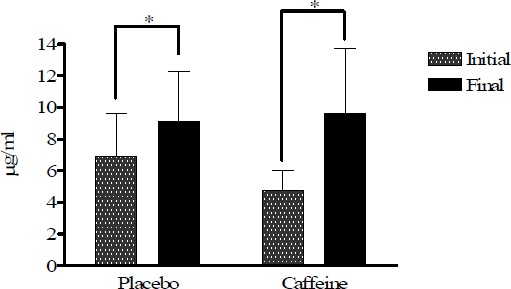
Vitamin C plasma levels in placebo and caffeine trials expressed in µg·ml-1. * p < 0.05).
Figure 5.
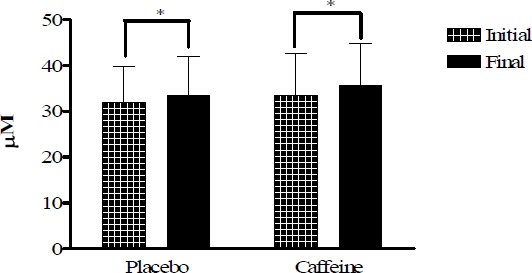
MDA plasma levels in placebo and caffeine trials expressed in µM. * p < 0.05).
Plasma MDA concentration as a measure of lipid peroxidation increased (p < 0.05) similarly in caffeine and placebo tests without differences between them (Figure 5).
While there were no urinary caffeine before, it was 5.61 ± 1.9 µg·ml-1 after exercise. Placebo samples had no caffeine concentration, while caffeine samples were below the former limit of 12 µg·ml-1 imposed by the International Olympic Committee until year 2003.
Discussion
Caffeine ingestion resulted in augmented exercise performance as indicated by a significantly higher time to exhaustion and maximal O2 consumption. These results are consistent with those reported by other authors (Doherty and Smith, 2004; Flinn et al., 1990; Kaminsky et al., 1998). The ergogenic ability of caffeine has been explained by increasing fat mobilization with the subsequent delay of muscle glycogen utilization (Doherty, 1998) reflected in our study by the lower RER values, and also could be explained either by a positive effect on the contractile property of skeletal muscle increasing the capacity for work, reducing leg muscle pain during exercise (O’connor et al., 2004) or by delaying fatigue through the central nervous system mechanisms, at least in part by blocking adenosine receptors (Doherty et al., 2004).
Subjects increased plasma MDA levels after graded cycle exercise until exhaustion on a cycle ergometer which means an increase of lipid peroxidation. These results were in accordance with others previously described in similar strenuous conditions (Ortenblad et al., 1997) and suggest the possibility of oxidative damage (Jammes et al., 2004). Since it has been reported that caffeine is a potent antioxidant capable of preventing lipid oxidation (Devasagayam et al., 1996), we expected lower values of MDA after the caffeine trial. Nevertheless, a caffeine dose of 5mg/kg bw ingested 1 hour prior to the exercise test does not seem to influence MDA production since there were no differences between both trials.
Organism response to lipid peroxidation during exercise was reflected with minor significant decreases on lipid soluble vitamins A and E after maximal test in both experimental groups, as it has been showed in other studies (Ortenblad et al., 1997; Sacheck and Blumberg, 2001), which indicates the consumption of plasma antioxidants protecting the plasma lipids against damage (Jammes et al., 2004). As Vitamin E can serve as a free radical scavenger and research has shown an increase in its utilization following exercise (Mastaloudis et al., 2001) it is possible to think that this decrease could be attributable to its actuation when other antioxidant systems are not capable of neutralizing oxygen free radicals responsible for lipid peroxidation (Bowles et al., 1991).
On the contrary, participants increased ascorbic acid plasma levels after both cycle tests. These results agree with previous works and they may have been due in part to a concomitant release of cortisol and ascorbic acid from adrenal glands (Gleeson et al., 1987; Umegaki et al., 2000) or to the ascorbate recycling and efflux from neutrophils induced by exercise (Tauler et al., 2002), reflecting enhanced antioxidant defences in response to the oxidative stress of exercise (Mastaloudis et al., 2001). The inconsistent base values of vitamin C in the two test conditions could be due to the complex metabolism of this substance after exercise (Peake, 2003). Despite randomisation of the tests, baseline vitamin C levels in caffeine conditions may have been lowered by the exercise carried out in the previously completed placebo test.
Nevertheless, a caffeine dose of 5mg·kg-1 bw ingested 1 hour prior to the exercise test does not seem to have any influence on non enzymatic antioxidant systems since there were no differences between both trials. These results suggest that caffeine does not have any influence on the oxidative stress in maximum incremental efforts.
The lack of caffeine in baseline urine samples indicates that only caffeine permitted by the study was ingested. Urine data from the caffeine trials show that a dose of 5 mg·bw-1 is permissible with regard to the expired antidoping regulation of 12 µg·ml-1 caffeine in urine.
Study limitations
This study was performed with a single dose of caffeine, so we can not know how different dosages of caffeine should affect to oxidative stress. Therefore, a suitable increase in oxidative stress could generate muscle damage. So for future studies, different dosages of caffeine, higher and lower, must be considered as a study of muscle damage markers.
Conclusions
The results of this study suggest that ingestion one hour prior to an incremental cycling test of a 5 mg/kg body weight dose of caffeine in subjects who do not usually perform exercise, allows them to increase time to exhaustion, work load, maximum oxygen consumption, as well as decreasing their respiratory exchange ratio. In spite of this increased performance, the oxidative stress is not modified by caffeine ingestion. We conclude that caffeine supplements at doses of 5mg·kg-1 have no effect on oxidative stress derived from maximum effort until exhaustion.
Biographies

Guillermo J. OLCINA
Employment
Lecturer and Vice-Dean of Coordination and International Relations in Sport Science Faculty of University of Extremadura, Spain.
Degree
PhD
Research interests
Combat sport and cycling.
E-mail: golcina@unex.es

Diego MUÑOZ
Employment
PhD studient in the University of Extremadura Physiology Department, Spain.
Degree
MSc
Research interests
Oxidative stress and exercise.
E-mail: diegomun@unex.es

Rafael TIMÓN
Employment
Lecturer in Sport Science Faculty of University of Extremadura, Spain.
Degree
PhD
Research interests
Exercise physiology and steroids.
E-mail: rtimon@unex.es

M. Jesús CABALLERO
Employment
Lecturer in Faculty of Medicine, Department of Pharmacology.
Degree
PhD
Research interests
Biochemical aspects of exercise, caffeine and pharmacological topics.
E-mail: mcaballe@unex.es

Juan I. MAYNAR
Employment
Lecturer in Sciences Faculty, Department of Analytical Chemistry and Electrochemistry.
Degree
PhD
Research interests
Biochemical aspects of exercise.
E-mail: jimaynar@unex.es

Alfredo CÓRDOVA
Employment
Researcher in the University of Valladolid, Spain.
Degree
PhD
Research interests
Oxidative stress and exercise.
E-mail: a.cordova@bio.uva.es

Marcos MAYNAR
Employment
Lecturer of Exercise Physiology in Sport Sciences Faculty, Department of Physiology.
Degree
PhD
Research interests
Exercise physiologye.
E-mail: diegomun@unex.es
References
- Bowles D., Torgan C., Ebner S., Kehrer J., Ivy J., Starnes J. W. (1991) Effects of acute, submaximal exercise on skeletal muscle vitamin E. Free Radical Research Communications 14, 139-143 [DOI] [PubMed] [Google Scholar]
- Bruce C., Anderson M., Fraser S., Stepto N., Klein R., Hopkins W., Hawley J. (2000) Enhancement of 2000-m rowing performance after caffeine ingestion. Medicine and Science in Sports and Exercise 32, 1958-1963 [DOI] [PubMed] [Google Scholar]
- Davis J., Zhao Z., Stock H., Mehl K., Buggy J., Hand G. (2003) Central nervous system effects of caffeine and adenosine on fatigue. American Journal of Physiology. Regulatory, Integrative and Comparatory Physiology 284, R399-404 [DOI] [PubMed] [Google Scholar]
- Devasagayam T., Kamat J., Mohan H., Kesavan P. (1996) Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochimica et Biophysica Acta 1282, 63-70 [DOI] [PubMed] [Google Scholar]
- Dianzani M., Muzio G., Biocca M., Canuto R. (1991) Lipid peroxidation in fatty liver induced by caffeine in rats. International Journal of Tissue Reactions 13, 79-85 [PubMed] [Google Scholar]
- Dobrocky P., Bennett P., Notarianni L. (1994) Rapid method for the routine determination of caffeine and its metabolites by high-performance liquid chromatography. Journal of Chromatography 652, 104-108 [DOI] [PubMed] [Google Scholar]
- Doherty M. (1998) The effects of caffeine on the maximal accumulated oxygen deficit and short-term running performance. International Journal of Sport Nutrition 8, 95-104 [DOI] [PubMed] [Google Scholar]
- Doherty M., Smith P., Hughes M., Davison R. (2004) Caffeine lowers perceptual response and increases power output during high-intensity cycling. Journal of Sports Sciences 22, 637-643 [DOI] [PubMed] [Google Scholar]
- Doherty M., Smith P. (2004) Effects of caffeine ingestion on exercise testing: a meta-analysis. International Journal of Sport Nutrition and Exercise Metabolism 14, 626-646 [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Lang J., Zadravec S., Slater T. (1984) Detection of malonaldehyde by high-performance liquid chromatography. Methods in Enzymology 105, 319-328 [DOI] [PubMed] [Google Scholar]
- Flinn S., Gregory J., Mcnaughton L., Tristram S., Davies P. (1990) Caffeine ingestion prior to incremental cycling to exhaustion in recreational cyclists. International Journal of Sports Medicine 11, 188-193 [DOI] [PubMed] [Google Scholar]
- Gleeson M., Robertson J., Maughan R. (1987) Influence of exercise on ascorbic acid status in man. Clinical and Science 73, 501-505 [DOI] [PubMed] [Google Scholar]
- Graham T. (2001) Caffeine and exercise: metabolism, endurance and performance. Sports Medicine 31, 785-807 [DOI] [PubMed] [Google Scholar]
- Graham T., Helge J., Maclean D., Kiens B., Richter E. (2000) Caffeine ingestion does not alter carbohydrate or fat metabolism in human skeletal muscle during exercise. The Journal of Physiology 529, 837-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer F., Friars D., Graham T. (2000) Comparison of caffeine and theophylline ingestion: exercise metabolism and endurance. Journal of Applied Physiology 89, 1837-1844 [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. (1985) Free radicals in biology and medicine. Clarendon Press, Oxford: [DOI] [PubMed] [Google Scholar]
- Jackman M., Wendling P., Friars D., Graham T. (1996) Metabolic catecholamine, and endurance responses to caffeine during intense exercise. Journal of Applied Physiology 81, 1658-1663 [DOI] [PubMed] [Google Scholar]
- Jammes Y., Steinberg J., Bregeon F., Delliaux S. (2004) The oxidative stress in response to routine incremental cycling exercise in healthy sedentary subjects. Respiratory, Physiology & Neurobiology 144, 81-90 [DOI] [PubMed] [Google Scholar]
- Jewett S., Eddy L., Hochstein P. (1989) Is the autoxidation of catecholamines involved in ischemia-reperfusion injury? Free Radical Biology and Medicine 6, 185-188 [DOI] [PubMed] [Google Scholar]
- Kamat J., Boloor K., Devasagayam T., Jayashree B., Kesavan P. (2000) Differential modification by caffeine of oxygen-dependent and independent effects of gamma-irradiation on rat liver mitochondria. International Journal of Radiation Biology 76, 1281-1288 [DOI] [PubMed] [Google Scholar]
- Kaminsky L., Martin C., Whaley M. (1998) Caffeine consumption habits do not influence the exercise blood pressure response following caffeine ingestion. The Journal of Sports Medicine and Physical Fitness 38, 53-58 [PubMed] [Google Scholar]
- Krisko A., Kveder M., Pifat G. (2005) Effect of caffeine on oxidation susceptibility of human plasma low density lipoproteins. Clinica Chimica Acta 355, 47-53 [DOI] [PubMed] [Google Scholar]
- Lee C. (2000) Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clinica Chimica Acta 295, 141-154 [DOI] [PubMed] [Google Scholar]
- Manoharan M., Schwille P. (1994) Measurement of ascorbic acid in human plasma and urine by high-performance liquid chromatography. Results in healthy subjects and patients with idiopathic calcium urolithiasis. Journal of Chromatography B, Biomedical Applications 654, 134-139 [DOI] [PubMed] [Google Scholar]
- Mastaloudis A., Leonard S., Traber M. (2001) Oxidative stress in athletes during extreme endurance exercise. Free Radical Biology and Medicine 31, 911-922 [DOI] [PubMed] [Google Scholar]
- Nikolic J., Bjelakovic G., Stojanovic I. (2003) Effect of caffeine on metabolism of L-arginine in the brain. Molecular and Cellular Biochemistry 244, 125-128 [PubMed] [Google Scholar]
- O’connor P., Motl R., Broglio S., Ely M. (2004) Dose-dependent effect of caffeine on reducing leg muscle pain during cycling exercise is unrelated to systolic blood pressure. Pain 109, 291-298 [DOI] [PubMed] [Google Scholar]
- Ortenblad N., Madsen K., Djurhuus M. (1997) Antioxidant status and lipid peroxidation after short-term maximal exercise in trained and untrained humans. The American Journal of Physiology 272, 1258-1263 [DOI] [PubMed] [Google Scholar]
- Peake J. M. (2003) Vitamin C: effects of exercise and requirements with training. International Journal of Sport Nutrition and Exercise Metabolism 13, 125-151 [DOI] [PubMed] [Google Scholar]
- Sacheck J., Blumberg J. (2001) Role of vitamin E and oxidative stress in exercise. Nutrition 17, 809-814 [DOI] [PubMed] [Google Scholar]
- Shearer M. (1986) Vitamins. HPLC of small molecules, a practical approach. IRL Press, Oxford [Google Scholar]
- Shigenaga M., Hagen T., Ames B. (1994) Oxidative damage and mitochondrial decay in aging. Proceeding of the National Academy of Sciences USA 91, 10771-10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauler P., Aguilo A., Fuentespina E., Tur J., Pons A. (2002) Diet supplementation with vitamin E, vitamin C and beta-carotene cocktail enhances basal neutrophil antioxidant enzymes in athletes. Pflugers Archiv 443, 791-797 [DOI] [PubMed] [Google Scholar]
- Thompson D., Williams C., Kingsley M., Nicholas C., Lakomy H., McArdle F., Jackson M. (2001) Muscle soreness and damage parameters after prolonged intermittent shuttle-running following acute vitamin C supplementation. International Journal of Sports Medicine 22, 68-75 [DOI] [PubMed] [Google Scholar]
- Umegaki K., Daohua P., Sugisawa A., Kimura M., Higuchi M. (2000) Influence of one bout of vigorous exercise on ascorbic acid in plasma and oxidative damage to DNA in blood cells and muscle in untrained rats. The Journal of Nutritional Biochemistry 11, 401-407 [DOI] [PubMed] [Google Scholar]
- Van Soeren M., Sathasivam P., Spriet L., Graham T. (1993) Caffeine metabolism and epinephrine responses during exercise in users and nonusers. Journal of Applied Physiology 75, 805-812 [DOI] [PubMed] [Google Scholar]
- Vistisen K., Poulsen H., Loft S. (1992) Foreign compound metabolism capacity in man measured from metabolites of dietary caffeine. Carcinogenesis 13, 1561-1568 [DOI] [PubMed] [Google Scholar]
- Wiles J., Bird S., Hopkins J., Riley M. (1992) Effect of caffeinated coffee on running speed, respiratory factors, blood lactate and perceived exertion during 1500-m treadmill running. British Journal of Sports Medicine 26, 116-120 [DOI] [PMC free article] [PubMed] [Google Scholar]


