Abstract
Fish oil (FO) is a commonly used supplemental source of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), 2 n–3 (ω-3) polyunsaturated fatty acids (PUFAs) that have been shown to have a variety of health benefits considered to be protective against cardiometabolic diseases. Although the effects of EPA and DHA on lipid metabolism have been extensively studied, not all of the metabolic effects of FO-derived n–3 PUFAs have been characterized. Our laboratory recently showed that a high-fructose diet in rhesus monkeys induces the features of metabolic syndrome (MetS) similar to those observed in humans. Thus, we specifically wanted to evaluate the effects of FO in rhesus monkeys fed a high-fructose diet and hypothesized that FO supplementation would mitigate the development of fructose-induced insulin resistance, dyslipidemia, and other cardiometabolic risk factors. In this study, adult monkeys (aged 12–20 y) received either a standard unpurified diet plus 75 g fructose/d (control group; n = 9) or a standard unpurified diet, 75 g fructose/d, and 4 g FO (16% EPA + 11% DHA)/d (treatment group; n = 10) for 6 mo. Importantly, our results showed that daily FO supplementation in the monkeys prevented fructose-induced hypertriglyceridemia and insulin resistance as assessed by intravenous-glucose-tolerance testing (P ≤ 0.05). Moreover, FO administration in the monkeys prevented fructose-induced increases in plasma apolipoprotein (Apo)C3, ApoE, and leptin concentrations and attenuated decreases in circulating adropin concentrations (P ≤ 0.05). No differences between the control and FO-treated monkeys were observed in body weight, lean mass, fat mass, or fasting glucose, insulin, and adiponectin concentrations. In conclusion, FO administration in a nonhuman primate model of diet-induced MetS ameliorates many of the adverse changes in lipid and glucose metabolism induced by chronic fructose consumption.
Introduction
The incidence and prevalence of insulin resistance and metabolic syndrome (MetS)7 in the United States and worldwide have increased dramatically over the past decade (1–3). We and others have proposed that this increase may in part be attributable to increased consumption of fructose derived from dietary sucrose and high-fructose corn syrup (1–4). Individuals with MetS have a higher likelihood of developing atherosclerotic cardiovascular disease (ASCVD) and type 2 diabetes mellitus (T2DM) (1, 3, 5–8); thus, safe interventions to prevent or mitigate the conditions associated with MetS (including central adiposity, hypertension, hypertriglyceridemia, and impaired glucose tolerance) are needed.
To date, there are no U.S. Food and Drug Administration–approved pharmaceutical agents to treat or prevent MetS. As such, consumers often resort to over-the-counter nutraceutical therapies, many of which have little data to demonstrate their efficacy. One such agent is fish oil (FO). FO is a commonly used supplemental source of EPA (20:5n–3) and DHA (22:6n–3), 2 marine-derived n–3 PUFAs that have been shown to have a variety of protective effects against cardiometabolic diseases (9–11). The n–3 PUFAs are most widely known for their TG-lowering effects (12, 13), which are considered to be primarily mediated via peroxisome proliferator-activated receptor (PPAR)–dependent mechanisms (14). However, their potential ability to alleviate inflammation and increase insulin sensitivity has become an area of interest as well (11, 15).
Although the effects of EPA and DHA on lipid metabolism have been extensively studied (16), not all of the metabolic effects of FO-derived n–3 PUFAs have been characterized. Whereas EPA and DHA have been shown to prevent and reverse insulin resistance in obese rodents fed a high-fat (17–19) or high-sucrose (20) diet, they have not consistently shown insulin-sensitizing effects in obese humans and those with T2DM (21–23). Despite lowering circulating lipid concentrations, supplementation with EPA or FO for 11 mo also did not delay the onset of diabetes in University of California Davis–T2DM rats (24), a well-characterized rodent model of T2DM that exhibits polygenic adult-onset obesity and insulin resistance with inadequate β-cell compensation (25). However, other data suggest that FO-derived n–3 PUFAs may delay the development of MetS or its progression to T2DM (26).
Our laboratory recently demonstrated that a high-fructose diet in rhesus monkeys induces the features of MetS in humans, including obesity, dyslipidemia (particularly hypertriglyceridemia), and insulin resistance (27). Thus, we specifically designed a study to evaluate the effects of FO in rhesus monkeys fed a high-fructose diet and hypothesized that treating rhesus monkeys with a high-fructose diet combined with FO supplementation would mitigate the development of dyslipidemia, insulin resistance, and other associated cardiometabolic risk factors in this model. Herein, we report our findings demonstrating that FO prevents fructose-induced insulin resistance and hypertriglyceridemia in rhesus monkeys.
Materials and Methods
Animals.
The rhesus monkeys used for this study were provided by and maintained at the California National Primate Research Center at the University of California, Davis. Protocols for all the animal studies were approved by the University of California, Davis, Institutional Animal Care and Use Committee and were conducted in accordance with the USDA Animal Welfare Act and the NIH’s Guide for the Care and Use of Laboratory Animals. A total of 19 adult male rhesus monkeys, aged 12–20 y (body weight: 15.6 ± 0.4 kg), were studied. At baseline, all monkeys had fasting plasma glucose concentrations <100 mg/dL, fasting insulin concentrations <100 μU/mL, fasting plasma TG concentrations <100 mg/dL, and percentage body fat >20%.
Diet and energy intake measurements.
A commercial monkey unpurified diet (Advanced Protocol Old World Primate, LabDiet 5047) was provided ad libitum to all of the monkeys. This is a grain-based standard primate diet that provides 30% of energy as protein, 11% of energy as fat (n–3 FAs, 0.14% of unpurified diet; n–6 FAs, 1.68% of unpurified diet; total FAs, 1.83% of unpurified diet), and 59% of energy as carbohydrate. In addition, following baseline measurements, all monkeys were provided with 500 mL/d of a fruit-flavored (Kool-Aid; Kraft Foods), 15% fructose-sweetened beverage (75 g of fructose). Ten monkeys (treatment group) also received 4 g of FO supplementation/d. The FO (16% EPA/11% DHA; 32.1% n–3 FAs; 2.4% n–6 FAs; 77.3% total FAs; Jedwards International, validated by Covance) was mixed into a treat such as ice cream, yogurt, jelly, applesauce, or pudding; and 1% by weight of egg powder was added to make an emulsion. Then, 44 g of the mixture containing 4 g of FO was provided to the monkeys each day. The control monkeys received the same mixture with 4 g of safflower oil [0.05% FFAs; 6.54% palmitic acid (16:0); 2.62% stearic acid (18:0); 14.97% oleic acid (18:1); 73.47% linoleic acid (18:2); 0.05% linolenic acid (18:3); 0.15% behenic acid (22:0); Jedwards International] instead of FO. Beverage intake was recorded daily, and food intake was recorded for 1 wk at baseline, and then for another 1-wk period at 6 mo. The monkeys were randomly assigned by age equally to the FO-treated and control groups.
Body weight and body adiposity.
Body weights of the monkeys were determined monthly. At baseline and at 6 mo, percentage and total body fat were determined by DXA, a sensitive technique for measuring body fat (28) that has been previously validated for the determination of body composition in monkeys (29).
Intravenous-glucose-tolerance tests.
At baseline and at 6 mo, intravenous-glucose-tolerance tests (IVGTTs) were performed and the AUC for glucose and insulin calculated by using the trapezoidal method; glucose tolerance was estimated by the area above baseline under the glucose curve from 0 to 60 min, and an index of insulin sensitivity was calculated as described previously (27, 30).
Glucose, insulin, and HOMA-IR.
Plasma insulin concentrations were measured by using an RIA (Millipore), and plasma glucose concentrations were measured with a YSI Glucose Analyzer (YSI Life Sciences). HOMA-IR was calculated by using the following equation: ([fasting glucose (mmol/L) × fasting insulin (μU/mL)]/22.5) (31).
Lipid and lipoproteins.
Plasma total cholesterol, HDL cholesterol, LDL cholesterol, TG, apoA1, apoB, apoC3, and apoE concentrations were determined by using a Polychem Chemistry Analyzer (PolyMedCo).
Adipocyte and hepatic hormones.
Plasma adiponectin and leptin concentrations were measured by RIA (Millipore). Plasma adropin concentrations were measured by using a commercially available ELISA (Peninsula Laboratories, Bachem) (32). Before the analyses, all of the antibodies were tested to ensure their ability to measure the target hormone in samples from rhesus monkeys (27). The AUC for adropin during the 6-mo study was calculated by using the trapezoidal method.
Statistical analysis.
Descriptive statistics are provided for each of the outcomes as means ± SEMs. Within a given treatment group, Wilcoxon’s Signed Rank tests were used to compare baseline data for each outcome with the 6-mo measurements. Linear mixed models were used to assess the longitudinal trend of each outcome and to compare the changes in the FO treatment group compared with the control group. Unless otherwise noted, the P values reported represent the results of the linear mixed-model analyses. Each linear mixed model regressed a single outcome postbaseline on time (categorical for 1, 3, and 6 mo), group, group by time interaction, and the baseline value of the respective outcome. Random intercepts were included to account for repeated measures. Estimated mean differences between groups were calculated at 1, 3, and 6 mo, adjusting for baseline values. Before the analyses, the distributions of each outcome variable were also assessed for normality; for those outcome variables with skewed distributions, log transformations were applied to meet the normality assumptions of the linear mixed model. For each outcome requiring a transformation, the estimated differences between time points were back transformed to represent ratios of geometric means with corresponding 95% CIs and P values. One-tailed t tests were also performed to evaluate the AUC for the glucose and insulin excursions during the IVGTTs at baseline and 6 mo and the AUC for the adropin concentration changes from baseline to 6 mo. Statistical analyses with P values ≤0.05 were considered significant.
Results
Body weight and body composition.
In the control monkeys (n = 9), body weight increased from 15.8 ± 0.6 kg at baseline to 17.8 ± 0.8 kg at 6 mo (+13%; P = 0.004), whereas in the FO-treated monkeys (n = 10), body weight increased from 15.5 ± 0.5 kg at baseline to 16.9 ± 0.6 kg at 6 mo (+9%; P = 0.002). Although the increase in body weight from baseline to 6 mo was significant for each group, the mean difference in weight gain between the control monkeys and the FO-treated monkeys was not significant after adjusting for baseline values. Furthermore, there were no significant differences between the 2 groups with respect to lean body mass and fat mass.
Fasting lipid and lipoprotein concentrations.
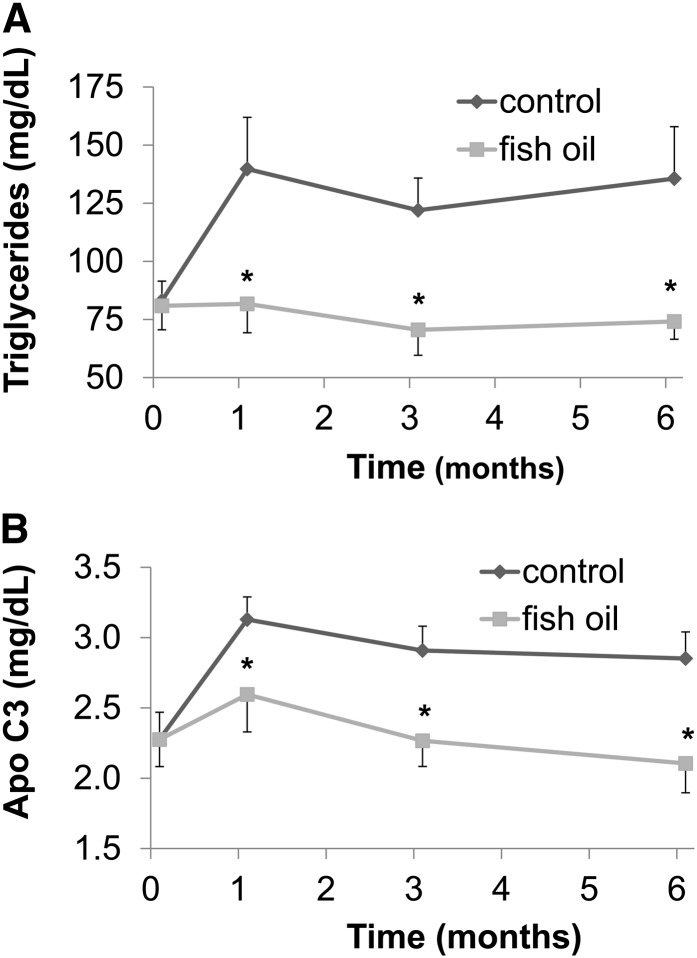
As shown in Table 1, fasting TG and apoC3 concentrations were significantly higher in the control group versus the FO treatment group at the completion of the 6-mo study (P = 0.005). Specifically, in the control monkeys, both fasting TG (+71 ± 25%; Fig. 1A) and fasting apoC3 (+28 ± 8%; Fig. 1B) concentrations increased. In contrast, both fasting TG concentrations (−3 ± 7%; Fig. 1A) and fasting apoC3 concentrations (−5 ± 9%; Fig. 1B) were unchanged in the monkeys that received the daily FO supplementation. ApoA1 concentrations decreased modestly in the FO-treated monkeys (−8 ± 2%; P = 0.018). ApoE concentrations also increased in the control group (+33 ± 9%) more than in the FO-treated group (+11 ± 6%) (P = 0.030). Moreover, cholesterol concentrations increased slightly in the control group (+4 ± 3%), whereas they decreased modestly in the monkeys treated with FO (−8 ± 5%) (P = 0.025, Wilcoxon’s rank-sum test).
TABLE 1.
Effects of FO on fasting plasma lipid and lipoprotein concentrations in rhesus monkeys fed a high-fructose diet1
| Control (n = 9) |
FO (n = 10) |
|||
| Result | Δ | Result | Δ | |
| mg/dL | mg/dL | |||
| Cholesterol | ||||
| Baseline | 142 ± 8 | — | 137 ± 9 | — |
| 6 mo | 146 ± 8 | +5 ± 4 | 125 ± 10 | −12 ± 6 |
| LDL-C | ||||
| Baseline | 70 ± 6 | — | 66 ± 7 | — |
| 6 mo | 64 ± 5 | −6 ± 4 | 62 ± 7 | −5 ± 5 |
| HDL-C | ||||
| Baseline | 55 ± 3 | — | 54 ± 4 | — |
| 6 mo | 55 ± 4 | 0 ± 3 | 49 ± 5 | −5 ± 2* |
| TGs | ||||
| Baseline | 83 ± 8 | — | 81 ± 10 | — |
| 6 mo | 136 ± 22 | +53 ± 21* | 74 ± 8 | −7 ± 7‡ |
| ApoA1 | ||||
| Baseline | 143 ± 6 | — | 144 ± 4 | — |
| 6 mo | 147 ± 8 | +4 ± 4 | 132 ± 6 | −11 ± 3† |
| ApoB | ||||
| Baseline | 55 ± 4 | — | 51 ± 5 | — |
| 6 mo | 56 ± 4 | +1 ± 3 | 50 ± 6 | −1 ± 3 |
| ApoC3 | ||||
| Baseline | 2.28 ± 0.19 | — | 2.28 ± 0.19 | — |
| 6 mo | 2.85 ± 0.19 | +0.57 ± 0.13** | 2.11 ± 0.21 | −0.17 ± 0.18‡ |
| ApoE | ||||
| Baseline | 2.12 ± 0.15 | — | 1.92 ± 0.13 | — |
| 6 mo | 2.74 ± 0.14 | +0.62 ± 0.14* | 2.09 ± 0.14 | +0.18 ± 0.11† |
Values are means ± SEMs. Differences in means from baseline: *P ≤ 0.05, **P ≤ 0.01. Differences in means from baseline to 6 mo between control and FO groups: †P ≤ 0.05, ‡P ≤ 0.01. FO, fish oil; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
FIGURE 1.
The effect of fish oil on fasting plasma TG (A) and apoC3 (B) concentrations in rhesus monkeys fed a high-fructose diet for 6 mo. Values are means ± SEMs; n = 9 (control) or 10 (fish oil). *Different from control, P ≤ 0.05.
Fasting glucose and insulin concentrations, glucose tolerance, and insulin sensitivity.
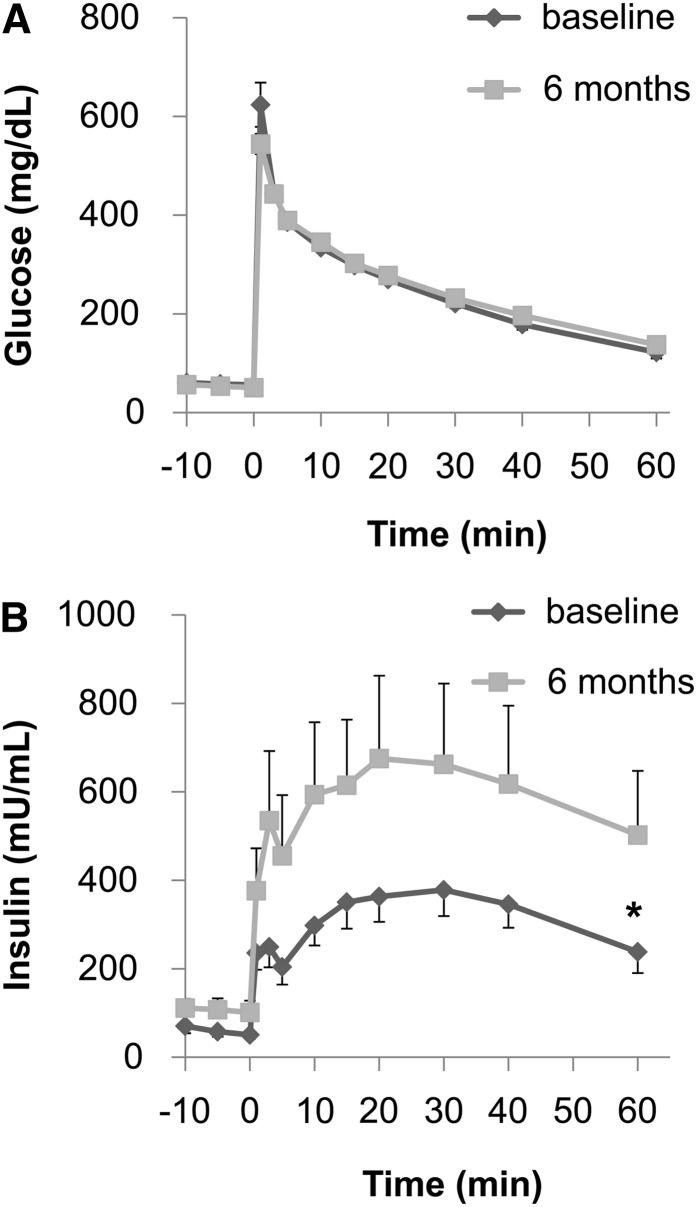
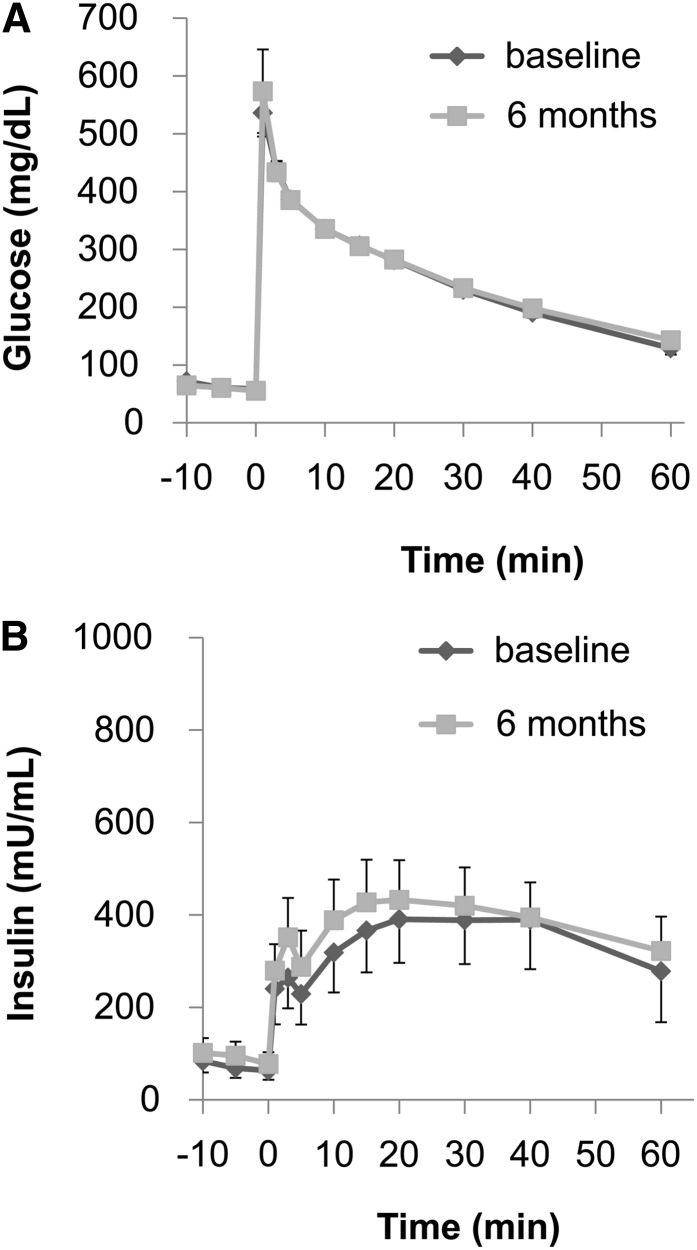
During the course of the study, none of the monkeys developed frank diabetes (defined by a fasting blood glucose concentration >125 mg/dL), and the fasting blood glucose concentrations remained fairly stable in both the control and FO treatment groups (Table 2). Both groups exhibited increased fasting insulin concentrations, suggesting β-cell compensation for insulin resistance; however, the increase of fasting insulin concentrations in the FO-treated monkeys tended to be smaller (+28 ± 27 mg/dL) than in the control monkeys (+57 ± 32 mg/dL). Furthermore, although the glycemic excursions and the AUC for glucose concentrations during the IVGTTs in the control and FO treatment groups were similar at the end of the 6-mo study (Figs. 2A and 3A), the insulin excursions and AUC for insulin concentrations were different (Figs. 2B and 3B). Specifically, the AUC insulin for the control group increased by 81 ± 39%, whereas the AUC insulin for the FO-treated monkeys increased only by 30 ± 27% (P = 0.037).
TABLE 2.
Effects of FO on fasting plasma glucose and insulin concentrations, glucose tolerance, and insulin sensitivity in rhesus monkeys fed a high-fructose diet1
| Control (n = 9) |
FO (n = 10) |
|||
| Result | Δ | Result | Δ | |
| Glucose, mg/dL | ||||
| Baseline | 68 ± 4 | — | 87 ± 5 | — |
| 6 mo | 69 ± 2 | +1 ± 3 | 81 ± 7 | −6 ± 7 |
| Insulin, μU/mL | ||||
| Baseline | 55 ± 13 | — | 61 ± 15 | — |
| 6 mo | 113 ± 44 | +57 ± 32* | 89 ± 26 | +28 ± 27 |
| AUC glucose, mg/dL × 60 min | ||||
| Baseline | 11,002 ± 536 | — | 11,021 ± 600 | — |
| 6 mo | 11,841 ± 392 | +839 ± 611 | 11,545 ± 332 | +524 ± 365 |
| AUC insulin, μU/mL × 60 min | ||||
| Baseline | 15,327 ± 2406 | — | 16,275 ± 4407 | — |
| 6 mo | 28,895 ± 8697 | +13,568 ± 7594* | 17,345 ± 3509 | +1070 ± 4280 |
| HOMA-IR | ||||
| Baseline | 10.04 ± 3.09 | — | 12.37 ± 2.79 | — |
| 6 mo | 19.46 ± 7.99 | +9.43 ± 5.10 | 18.20 ± 6.22 | +5.48 ± 6.71 |
| Insulin sensitivity index | ||||
| Baseline | 0.63 ± 0.11 | — | 0.76 ± 0.21 | — |
| 6 mo | 0.47 ± 0.11 | −0.16 ± 0.09 | 0.71 ± 0.24 | −0.06 ± 0.07 |
Values are means ± SEMs. *Different from baseline, P ≤ 0.05. FO, fish oil.
FIGURE 2.
Plasma glucose and insulin responses during an intravenous-glucose-tolerance test (IVGTT) in control monkeys at baseline and after 6 mo. The plasma glucose response (A) did not differ between the 2 time points. However, the plasma insulin response (B) during the IVGTT was significantly increased after 6 mo consuming fructose. Values are means ± SEMs; n = 9. *Different AUC from baseline, P ≤ 0.05.
FIGURE 3.
Plasma glucose and insulin responses during an intravenous-glucose-tolerance test in the FO–treated monkeys at baseline and after 6 mo. The plasma glucose response (A) did not differ between the 2 time points. Unlike in the control monkeys, the plasma insulin response (B) also did not differ between the 2 time points, demonstrating the insulin-sensitizing effects of FO. Values are means ± SEMs; n = 10. FO, fish oil.
Adipocyte and hepatic hormones.
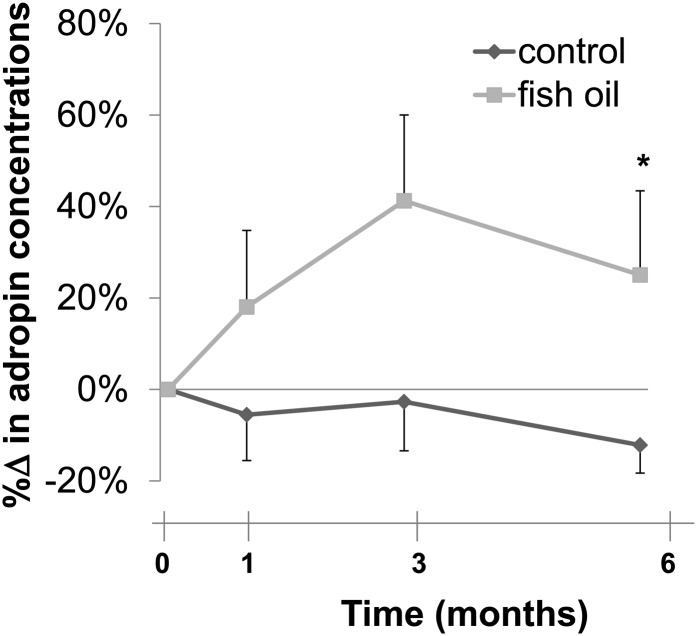
Fasting total adiponectin concentrations decreased comparably in both the control (−21 ± 8%) and the FO treatment (−27 ± 8%) groups at the completion of the study (Table 3). In contrast, the increase in fasting leptin concentrations was significantly greater in the control monkeys (+32 ± 8%) than in the monkeys treated with FO (+9 ± 5%) (P = 0.033). In addition, circulating adropin concentrations increased in the FO-treated group (+25 ± 18%), whereas they decreased in the control group (-12 ± 6%) (P = 0.042), a difference demonstrated by the AUC for plasma adropin concentrations over the course of the study (Fig. 4).
TABLE 3.
Effects of FO on fasting plasma adipocyte and hepatic hormones in rhesus monkeys fed a high-fructose diet1
| Control (n = 9) |
FO (n = 10) |
|||
| Result | Δ | Result | Δ | |
| Adiponectin, μg/mL | ||||
| Baseline | 5.87 ± 1.01 | — | 6.55 ± 0.71 | — |
| 6 mo | 4.63 ± 0.88 | −1.24 ± 0.45* | 4.71 ± 0.67 | −1.85 ± 0.68* |
| Leptin, μg/L | ||||
| Baseline | 19.01 ± 1.46 | — | 21.49 ± 2.12 | — |
| 6 mo | 24.63 ± 1.76 | +5.62 ± 1.18** | 22.88 ± 1.81 | +1.38 ± 0.83† |
| Adropin, μg/L | ||||
| Baseline | 1.92 ± 0.18 | — | 1.65 ± 0.34 | — |
| 6 mo | 1.66 ± 0.15 | −0.27 ± 0.11 | 1.83 ± 0.24 | +0.16 ± 0.29 |
Values are means ± SEMs. Differences in means from baseline: *P ≤ 0.05, **P ≤ 0.01. Difference in means from baseline to 6 mo between control and FO groups: †P ≤ 0.05. FO, fish oil.
FIGURE 4.
Effect of fish oil on percentage changes of fasting plasma adropin concentrations in rhesus monkeys fed a high-fructose diet. Values are means ± SEMs; n = 9 (control) or 10 (fish oil). *Different AUC from control at 6 mo, P ≤ 0.05.
Discussion
In this study, we demonstrated that daily FO supplementation in rhesus monkeys prevents fructose-induced hypertriglyceridemia and insulin resistance. Moreover, we report that FO administration in nonhuman primates prevents fructose-induced increases in apoC3 and apoE concentrations and attenuates the increase in leptin and the decrease in adropin concentrations.
Previous studies suggest that FO, particularly the long-chain n–3 PUFAs DHA and EPA, reduce plasma TG concentrations primarily from the decline in hepatic VLDL-TG production, and secondarily from an increase in VLDL-TG clearance (33). Specifically, FO counteracts intracellular lipolysis in adipocytes by suppressing adipose tissue inflammation, resulting in a decrease in the activity of hormone sensitive lipase, thereby decreasing the availability of nonesterified fatty acids for hepatic TG production (33). In addition, FO increases extracellular lipolysis by lipoprotein lipase, which is inhibited by apoC3. Thus, agents that lower apoC3 concentratons would be expected to also lower TG concentrations. Although a previous study reported that the TG-lowering effect of an ethyl ester preparation of FO in humans normalized elevated plasma TG concentrations independent of its effect on apoC3 (34), our data suggest that the FO-induced lowering of apoC3 may be a contributing factor to the effects of FO to prevent the increase in TG concentrations in response to dietary fructose.
In this study, we also demonstrate that FO supplementation prevents fructose-induced insulin resistance as demonstrated by the AUC insulin data during the IVGTTs, supporting an insulin-sensitizing action of n–3 PUFAs. Moreover, although others have reported that n–3 PUFAs lower increases in leptin expression in animal models of diet-induced obesity (35), we found that FO attenuates fructose-induced increases in leptin concentrations in a weight-independent manner, an interesting result that requires further investigation. Agonists of PPAR-γ have been demonstrated to increase the expression and secretion of adiponectin (36–38) and reduce the expression and secretion of leptin (39–41). Therefore, if FO supplementation were to elicit significant PPAR-γ activation in our rhesus monkey model, we would expect it to also prevent or attenuate the decrease in adiponectin observed during fructose feeding. However, the decreases in adiponectin concentrations were similar in the control and FO-treated monkeys. Although the results of studies investigating the effects of dietary n–3 PUFAs on circulating adiponectin concentrations in humans have been conflicting, we have reported that consumption of a diet high in n–3 PUFAs did not increase either total or high-molecular-weight adiponectin in humans over a 14-wk period (42). We also found that FO attenuates fructose-induced decreases in circulating adropin concentrations. Adropin is a peptide hormone produced by the liver that appears to have a role in metabolic and glucose homeostasis (43). Adropin deficiency is associated with increased adiposity and insulin resistance (44), and adropin concentrations increase with weight loss (32). Interestingly, our findings suggest that FO increases circulating adropin concentrations relative to controls and independent of changes in body weight/adiposity, another intriguing result that requires further investigation.
In addition, FO supplementation modestly decreased plasma apoA1 concentrations, a finding observed in previous studies evaluating the effect of the n–3 PUFAs on apo1 expression and concentrations (45, 46). In our study, FO supplementation also prevented fructose-induced increases in apoE concentrations, a positive finding with regard to cardiometabolic risk given that high plasma apoE concentrations strongly associate with cardiovascular mortality independent of APOE genotype and plasma lipids (47). In summary, because the ratio of n–6:n–3 PUFAs affects cardiovascular disease risk (48) and individuals with a low n–3 index (erythrocyte EPA+DHA as a percentage of total FAs) (49, 50) have a higher risk of cardiovascular events (51), the results of the present study indicating that FO ameliorates multiple lipid and metabolic variables in our fructose-fed rhesus monkey model (27) are consistent with FO's positive clinical attributes.
Importantly, the current study reinforces the use of rhesus monkeys as an animal model relevant to human metabolic diseases that allows for strict control of dietary variables and physical activity as well as the assurance of near 100% compliance with an intervention. As we have demonstrated previously and in this study (27), nutritional intervention studies conducted in rhesus monkeys can also be performed over an extended period of time (e.g., months to years), which is not practical nor feasible with human subjects. Moreover, this study is, to our knowledge, the first to assess circulating adropin concentrations in nonhuman primates and to demonstrate the prevention of fructose-induced hypertriglyceridemia by FO in a nonhuman primate model.
In conclusion, given the increasing incidence and prevalence of MetS, and the lack of approved pharmaceutical agents for its prevention, many individuals resort to the use of nutraceutical therapies for the prevention of the syndrome or at least its many components. FO is one of the most commonly used nutraceuticals and is well known as a rich source of n–3 PUFAs. Given the many health benefits of n–3 PUFAs, mainly EPA and DHA, interest in their biological mechanism(s) of action continues to grow. Because we previously demonstrated that, like in humans (52), consumption of a high-fructose diet in rhesus monkeys produces many of components of MetS within a relatively short (6-mo) period of time, we used this model to study the effects of FO on the development of diet-induced metabolic disease. In this study, we report the novel findings that FO prevents fructose-induced hypertriglyceridemia and insulin resistance in fructose-fed rhesus monkeys. We therefore demonstrate that the rhesus monkey model of fructose-induced MetS is directly translatable to the study of diet-induced MetS in humans and can be efficiently and effectively used to investigate the metabolic effects of pharmaceutical and nutraceutical agents and interventions for the prevention and treatment of diet-induced dyslipidemia and insulin resistance. New studies investigating the biochemical and molecular mechanisms underlying these metabolic effects of FO, DHA, and EPA are thus warranted.
Acknowledgments
The authors thank Vanessa Bakula, Marinelle Nunez, Guoxia Chen, and Sarah Davis for their technical and logistical contributions to the study. P.J.H. and K.L.S obtained funding for the study; A.A.B. and P.J.H. designed, drafted, and wrote the final manuscript and with P.J.H. and K.L.S. designed the study and supervised data collection and analysis; J.L.G. supervised sample collections from the California National Primate Research Center and with K.L.S. and B.P.C. performed the assays; and S.B.A. and B.R.S. supervised the statistical analyses. All authors edited the manuscript and approved the final version.
Footnotes
Abbreviations used: ASCVD, atherosclerotic cardiovascular disease; FO, fish oil; IVGTT, intravenous-glucose-tolerance test; MetS, metabolic syndrome; PPAR, peroxisome proliferator-activated receptor; T2DM, type 2 diabetes mellitus.
Literature Cited
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36. [DOI] [PubMed] [Google Scholar]
- 3.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–57. [DOI] [PubMed] [Google Scholar]
- 5.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab. 2003;88:2399–403. [DOI] [PubMed] [Google Scholar]
- 7.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am. 2004;33:283–303. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: the price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol Metab Clin North Am. 2005;34:49–62. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–67. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Wu JH. (n-3) Fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142 Suppl:614S–25S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res. 2012;65:211–22. [DOI] [PubMed] [Google Scholar]
- 12.Harris WS. n-3 Fatty acids and lipoproteins: comparison of results from human and animal studies. Lipids. 1996;31:243–52. [DOI] [PubMed] [Google Scholar]
- 13.Harris WS. N–3 Fatty acids and serum lipoproteins: animal studies. Am J Clin Nutr. 1997;65 Suppl:1611S–6S. [DOI] [PubMed] [Google Scholar]
- 14.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–93. [DOI] [PubMed] [Google Scholar]
- 15.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13:474–83. [DOI] [PubMed] [Google Scholar]
- 17.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–8. [DOI] [PubMed] [Google Scholar]
- 18.González-Périz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–22. [DOI] [PubMed] [Google Scholar]
- 20.Ghafoorunissa IA, Rajkumar L, Acharya V. Dietary (n-3) long chain polyunsaturated fatty acids prevent sucrose-induced insulin resistance in rats. J Nutr. 2005;135:2634–8. [DOI] [PubMed] [Google Scholar]
- 21.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–9. [DOI] [PubMed] [Google Scholar]
- 22.Rivellese AA, Maffettone A, Iovine C, Di Marino L, Annuzzi G, Mancini M, Riccardi G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996;19:1207–13. [DOI] [PubMed] [Google Scholar]
- 23.Hendrich S. (n-3) Fatty acids: clinical trials in people with type 2 diabetes. Adv Nutr. 2010;1:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings BP, Stanhope KL, Graham JL, Griffen SC, Havel PJ. Supplementation with EPA or fish oil for 11 months lowers circulating lipids, but does not delay the onset of diabetes in UC Davis-type 2 diabetes mellitus rats. Br J Nutr. 2010;104:1628–34. [DOI] [PubMed] [Google Scholar]
- 25.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nettleton JA, Katz R. n-3 Long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105:428–40. [DOI] [PubMed] [Google Scholar]
- 27.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011;4:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–4. [DOI] [PubMed] [Google Scholar]
- 29.Narita H, Ohkubo F, Yoshida T, Cho F, Yoshikawa Y. [Measuring bone mineral content and soft tissue mass in living the cynomolgus monkey.] Jikken Dobutsu. 1994;43:261–5. [DOI] [PubMed] [Google Scholar]
- 30.Swarbrick MM, Havel PJ, Levin AA, Bremer AA, Stanhope KL, Butler M, Booten SL, Graham JL, McKay RA, Murray SF, et al. Inhibition of protein tyrosine phosphatase-1B with antisense oligonucleotides improves insulin sensitivity and increases adiponectin concentrations in monkeys. Endocrinology. 2009;150:1670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 32.Butler AA, Tam CS, Stanhope KL, Wolfe BM, Ali MR, O'Keeffe M, St-Onge MP, Ravussin E, Havel PJ. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J Clin Endocrinol Metab. 2012;97:3783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shearer GC, Savinova OV, Harris WS. Fish oil—how does it reduce plasma triglycerides? Biochim Biophys Acta 2012;1821:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swahn E, von Schenck H, Olsson AG. Omega-3 ethyl ester concentrate decreases total apolipoprotein CIII and increases antithrombin III in postmyocardial infarction patients. Clin Drug Investig. 1998;15:473–82. [DOI] [PubMed] [Google Scholar]
- 35.Fan C, Liu X, Shen W, Deckelbaum RJ, Qi K. The regulation of leptin, leptin receptor and pro-opiomelanocortin expression by n-3 PUFAs in diet-induced obese mice is not related to the methylation of their promoters. Nutr Metab (Lond). 2011;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. [DOI] [PubMed] [Google Scholar]
- 37.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–74. [DOI] [PubMed] [Google Scholar]
- 38.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallen CB, Lazar MA. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1996;93:5793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe S, Takeuchi Y, Fukumoto S, Fujita H, Nakano T, Fujita T. Decrease in serum leptin by troglitazone is associated with preventing bone loss in type 2 diabetic patients. J Bone Miner Metab. 2003;21:166–71. [DOI] [PubMed] [Google Scholar]
- 41.Zheng H, Li S, Ma L, Cheng L, Deng C, Chen Z, Xie C, Xiang M, Jiang W, Chen L. A novel agonist of PPAR-gamma based on barbituric acid alleviates the development of non-alcoholic fatty liver disease by regulating adipocytokine expression and preventing insulin resistance. Eur J Pharmacol. 2011;659:244–51. [DOI] [PubMed] [Google Scholar]
- 42.Kratz M, Swarbrick MM, Callahan HS, Matthys CC, Havel PJ, Weigle DS. Effect of dietary n–3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr. 2008;87:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA, Thearle M, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganesh Kumar K, Zhang J, Gao S, Rossi J, McGuinness OP, Halem HH, Culler MD, Mynatt RL, Butler AA. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring). 2012;20:1394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuang YL, Paulson KE, Lichtenstein AH, Matthan NR, Lamon-Fava S. Docosahexaenoic acid suppresses apolipoprotein A-I gene expression through hepatocyte nuclear factor-3β. Am J Clin Nutr. 2011;94:594–600. [DOI] [PubMed] [Google Scholar]
- 46.Lee SP, Dart AM, Walker KZ, O'Dea K, Chin-Dusting JP, Skilton MR. Effect of altering dietary n-6:n-3 PUFA ratio on cardiovascular risk measures in patients treated with statins: a pilot study. Br J Nutr. 2012;108:1280–5. [DOI] [PubMed] [Google Scholar]
- 47.Mooijaart SP, Berbee JF, van Heemst D, Havekes LM, de Craen AJ, Slagboom PE, Rensen PC, Westendorp RG. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med. 2006;3:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris WS, Von Schacky C. The Omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. [DOI] [PubMed] [Google Scholar]
- 50.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. [DOI] [PubMed] [Google Scholar]
- 51.Aarsetoey H, Aarsetoey R, Lindner T, Staines H, Harris WS, Nilsen DW. Low levels of the omega-3 index are associated with sudden cardiac arrest and remain stable in survivors in the subacute phase. Lipids. 2011;46:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]