Abstract
Objectively measured biomarkers will help to resolve the controversial role of sugar intake in the etiology of obesity and related chronic diseases. We recently validated a dual-isotope model based on RBC carbon (δ13C) and nitrogen (δ15N) isotope ratios that explained a large percentage of the variation in self-reported sugar intake in a Yup’ik study population. Stable isotope ratios can easily be measured from many tissues, including RBCs, plasma, and hair; however, it is not known how isotopic models of sugar intake compare among these tissues. Here, we compared self-reported sugar intake with models based on RBCs, plasma, and hair δ13C and δ15N in Yup’ik people. We also evaluated associations of sugar intake with fasting plasma glucose δ13C. Finally, we evaluated relations between δ13C and δ15N values in hair, plasma, RBCs, and fasting plasma glucose to allow comparison of isotope ratios across tissue types. Models using RBCs, plasma, or hair isotope ratios explained similar amounts of variance in total sugar, added sugar, and sugar-sweetened beverage intake (∼53%, 48%, and 34%, respectively); however, the association with δ13C was strongest for models based on RBCs and hair. There were no associations with fasting plasma glucose δ13C (R2 = 0.03). The δ13C and δ15N values of RBCs, plasma, and hair showed strong, positive correlations; the slopes of these relations did not differ from 1. This study demonstrates that RBC, plasma, and hair isotope ratios predict sugar intake and provides data that will allow comparison of studies using different sample types.
Introduction
Biomarkers of sugar intake will strengthen inferences from observational studies and will help to resolve the controversial role of sugar intake in the development of obesity and related chronic diseases (1). The carbon stable isotope ratio (δ13C) has been proposed as a low burden, economical measure of sugar intake, because this isotope ratio is elevated in corn-based sweeteners and cane sugar (2–5). We recently validated an improved isotopic model of sugar intake based on RBC carbon and nitrogen (δ15N) isotope ratios (2) that explains nearly 50% of the variation in self-reported intake in an Alaska Native (Yup’ik) population (6) and is improved because δ15N accounts for confounding dietary effects on δ13C. Furthermore, we and others have shown that δ13C and δ15N are correlated among different tissue types, including RBCs, clot, serum, and hair (3, 4), suggesting that this measure of sugar intake could also be applied to stored serum or plasma samples or used to noninvasively assay sugar intake by using hair. However, whether our model performs similarly in different tissue types is not known.
An alternative approach to isotopic measures of sweetener intake has been proposed by Cook et al. (5), who found that plasma glucose δ13C was strongly associated with postprandial corn and cane-based sweetener intake. However, the effect was not observed for fasted samples, limiting the utility of plasma glucose δ13C as a measure of longer-term or “usual” intake. Because the Cook study was based on a 7-d controlled feeding study, it is possible that the duration of dietary treatment was too short to sufficiently affect endogenous sources of fasting glucose. However, whether longer term differences in sugar intake, such as the differences in usual intake among individuals, could affect fasting plasma glucose δ13C has not been tested.
This study tested whether models of sugar intake based on stable isotope measurements from RBCs, plasma, and hair had similar validity in a Yup’ik study population. We also examined whether usual sugar intake was associated with fasting plasma glucose δ13C. Finally, we determined the associations between the δ13C and δ15N values of RBCs, plasma, fasting plasma glucose, and hair. Participants in this study were from 2 Yup’ik communities in rural southwest Alaska. This study will provide information to determine which sample types can be used to provide isotopic predictions of sugar intake in future studies and will enable comparison among existing stable isotope studies based on RBCs, plasma, and hair.
Methods
Participant recruitment and procedures.
Data are from the Center for Alaska Native Health Research Neqem Nallunailkutaa (“Foods’ Marker”) study. This study was approved by University of Alaska Fairbanks Institutional Review Board and the Yukon-Kuskokwim Health Corporation Human Studies Committee.
Between 2008 and 2009, a community-based sample of 68 Yup’ik participants aged 14–79 y was recruited from 2 coastal communities in southwest Alaska. At entry into the study, participants completed a demographic questionnaire and the first of four 24-h recall dietary interviews (24HR). Three more dietary interviews were conducted during the next 4 wk, as described in detail elsewhere (2).
Of the 68 participants enrolled in the Neqem Nallunailkutaa study, 65 had isotopic measurements for RBCs, plasma, and fasting plasma glucose and 54 had isotopic measurements for hair. Our comparisons of the dual isotopic models of sugar intake were based on 52 participants that had a full suite of isotopic measurements. Our evaluation of associations between fasting plasma glucose δ13C and sugar intake was based on the sample of 65 participants with fasting plasma glucose stable isotope measurements. Finally, associations between the δ13C and δ15N of RBCs, plasma, hair, and fasting plasma glucose values were based on the 52 participants who had a full suite of isotopic measurements.
Assessment of dietary intake.
24HRs were collected from each participant by certified interviewers using algorithm-driven, computer-assisted software [Nutrition Data System for Research (NDSR) software 2008; University of Minnesota]. Participants were asked to recall all food and beverages consumed the day prior to the interview using a multiple pass approach. All participants were given portion estimation tools (measuring cups, rulers, and food models or portion estimation guides; Fred Hutchinson Cancer Research Center). Although most participants were bilingual, a native Yup’ik speaker conducted interviews for participants who did not speak English. No recalls were excluded due to unreasonable intake (6).
The NDSR food and nutrient database (7) was used to calculate sugar intake. In this study, sugar intake is measured in 3 ways: as total sugars, added sugars, and SSBs. Total sugar intake (g/d) is defined as the total sum of all mono- and di-saccharides consumed and includes primarily fructose, glucose, and sucrose. Added sugar intake (g/d) was defined as the sum of sugars and syrups added to foods during food preparation or commercial food processing. SSB intake was calculated from the NDSR food codes as the sum of servings of sweetened soft drinks and sweetened fruit drinks [servings/d, 237 mL (8 fl oz)/serving].
Biological sample collection.
Fasting blood samples were collected into EDTA tubes and processed in rural communities using a portable centrifuge. RBCs and plasma were aliquotted and stored at −15°C in a portable freezer. Within 6 d, samples were shipped to the University of Alaska Fairbanks and stored at −80°C. Hair was collected by cutting ~50 hairs from the back of the head as close to the scalp as possible. Samples were taped with the cut end labeled and stored in plastic bags.
Biological samples were collected at least 2 wk after the completion of dietary interviews, so that their average age would approximately match the period during which interviews were conducted. RBCs have a lifespan of 90–120 d (8–10) and a mean age of 50 d (9). Hair grows at a rate of 1 cm/mo (11–13); we sampled the 2 cm of hair closest to the scalp to reflect the last 2 mo of intake (4). Plasma components turn over at differing rates, but the turnover rate of albumin, which comprises the bulk of plasma proteins, is 10%/d (14). Therefore, we assume that plasma isotope ratios represent approximately the last 2–3 wk of intake.
Stable isotope analysis.
RBCs and plasma aliquots were autoclaved and prepared for stable isotope analysis as described elsewhere (15). Neither autoclaving nor the use of EDTA-treated tubes affects RBC carbon or nitrogen isotope ratios (16). Hair samples were cleaned and prepared for stable isotope analysis as described elsewhere (4). RBC, whole plasma, and hair samples were analyzed at the Alaska Stable Isotope Facility by continuous-flow isotope ratio MS by using a Costech ECS4010 Elemental Analyzer (Costech Scientific) interfaced to a Finnigan Delta Plus XP isotope ratio mass spectrometer via the Conflo III interface (Thermo-Finnigan). The conventional means of expressing natural abundance isotope ratios is as δ values in permil (‰). This reflects the ratio of the abundance of the heavy and light isotopes relative to internationally recognized standards: δX = (Rsample – Rstandard)/(Rstandard) · 1000‰ (17). Here, R is the ratio of heavy:light isotope (15N:14N, 13C:12C) and the standards are Vienna PeeDee Belemnite for carbon and atmospheric nitrogen for nitrogen.
To assess analytical precision, an internal standard was analyzed for every 10 samples; precision was measured as the CV of these analyses (3.3% for δ15N and 0.6% for δ13C). The purity of the samples was assessed through the molar C:N ratios, which were 3.3 ± 0.1 for RBCs, 3.0 ± 0.1 for hair, and 3.7 ± 0.1 for plasma. The C:N ratios for hair and RBCs were consistent with those previously published (4, 18, 19). Expected C:N ratios for plasma have not been previously published; however, our values were consistent with that expected for a protein-rich blood fraction that also contains lipid.
We measured fasting plasma glucose δ13C values following the methods of Rembacz et al. (20), with modifications as described by Cook et al. (5). Briefly, 250 μL of plasma was added to a 10-mL Exetainer (Labco Limited). Plasma was then treated with 20 μL 5% acetic acid to remove circulating bicarbonate, vortexed, and dried under a steady stream of nitrogen. Samples were resuspended with 500 μL DI water, flushed with helium gas to provide anaerobic conditions, and capped with screw-top caps containing a rubber septum. Commercially available baker’s yeast (Red Star Yeast) was used as a 20-μL yeast/water suspension (5 g/L) and injected through the rubber septum. Exetainers were incubated at 30°C for 16 h and the 13C abundance of the resulting CO2 was analyzed at the Alaska Stable Isotope Facility using a Finnigan Delta Plus XP isotope ratio mass spectrometer equipped with a Gas Bench inlet system. Samples were analyzed in triplicate and the mean SD was 0.8‰.
Because biological samples from this study have a lower 13C:12C than Vienna PeeDee Belemnite, all δ13C values are negative. The term “δ13C values” is hereafter abbreviated as δ13C and the term “δ15N values” is abbreviated δ15N.
Statistical analyses.
SSBs (servings/d + 1) and total and added sugars (g/d +1) were log transformed for analyses. The ability of dual isotopic models based on δ13C and δ15N measured in RBCs, plasma, and hair to predict reported sugar intake was assessed using multiple linear regression models in which isotope ratios were the independent variables. How well fasting plasma glucose δ13C predicted sugar intake was assessed using simple linear regression, because plasma glucose does not contain any nitrogen. The β-coefficients for these models were back transformed and are interpreted as percentage change in the dietary variable for every 1‰ change in isotope ratio. To test relations among isotope ratios of RBCs, plasma, fasting plasma glucose, and hair, we used simple linear regression models. Agreement between measures was evaluated in 2 ways: first, whether the slope of their relation was equal to 1, and second, as the mean and SD of their differences, according to Bland and Altman (21). Agreement was considered good if the SD of the mean difference was within 0.5‰. All 2-sided tests of significance were set at an α of 0.05. All statistical analyses were performed using JMP version 8 (SAS Institute) and STATA/IC version 12 (StataCorp).
Results
The demographic and dietary intake characteristics for the 68 participants in the Neqem Nallunailkutaa study and the subset of participants who had a full suite of RBC, plasma, and hair isotopic measurements are given in Table 1 (n = 52). The mean reported total sugar intake was 89 g/d in the complete study population and 85 g/d in the isotope subset (n = 52), which is lower than has been reported for U.S. whites (22, 23) and other native circumpolar populations (24).
TABLE 1.
Participant characteristics and reported sugar intake for all participants in the Center for Alaska Native Health Research Neqem Nallunailkutaa study and in the set of participants with a full suite of RBC, plasma, and hair isotopic measurements1
| Neqem Nallunailkutaa study (n = 68) | RBC, plasma, and hair isotope subset (n = 52) | |
| Age, y | 40 ± 18 | 40 ± 17 |
| 14–20 | 11 (16) | 6 (12) |
| 21–40 | 22 (32) | 19 (37) |
| 41–60 | 27 (40) | 22 (42) |
| >60 | 8 (12) | 5 (10) |
| Sex | ||
| Male | 35 (51) | 22 (42) |
| Female | 33 (49) | 30 (58) |
| BMI, kg/m2 | 27 ± 6 | 28 ± 6 |
| <18.5 | 1 (1) | — |
| 18.5–<25 | 30 (44) | 21 (40) |
| 25–<30 | 16 (24) | 14 (27) |
| ≥30 | 21 (31) | 15 (29) |
| Sugar intake | ||
| Total sugar, g/d | 89 (77, 103) | 85 (72, 100) |
| Added sugar, g/d | 74 (62, 88) | 72 (61, 85) |
| SSB, servings/d | 1.09 (1.07, 1.10) | 1.40 (1.09, 1.76) |
Values are means ± SDs or n(%); geometric means (95% CIs) are given for log-transformed variables (sugar intake). SSB, sugar-sweetened beverage.
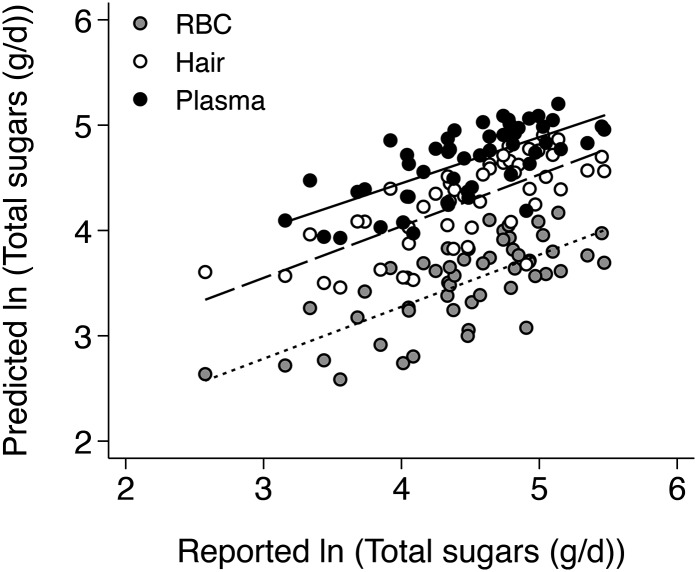
The associations of reported sugar intake with isotopic models of sugar intake for those participants who had RBC, plasma, and hair isotope measurements (n = 52) are given in Table 2. The strongest associations with sugar intake were observed for models based on isotope measurements in RBCs and hair, which both explained ~52%, 47%, and 34% of the variance in total sugars, added sugars, and SSBs, respectively. Models of sugar intake based on plasma isotope ratios were slightly less strong and the associations of plasma δ13C with sugar intake were only significant for SSBs, despite plasma δ15N showing strong inverse associations in all models. In the larger sample of 65 participants, however, plasma δ13C was associated with total sugars (β: 22.1; 95% CI: 4.1, 43.3; P = 0.0148) and SSBs (β: 25.4; 95% CI: 7.2, 46.6; P = 0.0054), and the association with added sugars was only marginally nonsignificant (β: 22.1; 95% CI: −0.69, 50.1; P = 0.06). Figure 1 shows the relation between reported total sugar intake and the predicted values using our dual isotope model for RBCs, plasma, and hair.
TABLE 2.
Associations of reported sugar intake with RBC, plasma, and hair isotope ratios for all participants in the Neqem Nallunailkutaa study with a full suite of RBC, plasma, and hair isotopic measurements (n = 52)
| Total sugars (g/d) |
Added sugars (g/d) |
SSB1 (servings/d) |
|||||||
| Isotope ratio | β2 | 95% CI | R2 | β2 | 95% CI | R2 | β2 | 95% CI | R2 |
| RBC | 0.53 | 0.48 | 0.34 | ||||||
| δ13C | 37.7** | 13.1, 67.6 | 31.4* | 6.8, 61.6 | 33.8** | 10.1, 62.7 | |||
| δ15N | −21.0*** | −26.1, −15.5 | −20.4*** | −25.8, −14.5 | −13.5*** | −19.1, −7.5 | |||
| Plasma | 0.48 | 0.43 | 0.28 | ||||||
| δ13C | 11.5 | −4.9, 30.8 | 10.3 | −6.6, 30.3 | 20.5* | 1.6, 42.8 | |||
| δ15N | −17.8*** | −23.1, −12.2 | −17.1*** | −22.7, −11.1 | −10.5** | −16.7, −3.9 | |||
| Hair | 0.52 | 0.47 | 0.34 | ||||||
| δ13C | 22.5* | 2.5, 46.3 | 21.1* | 0.49, 45.8 | 27.5** | 7.1, 51.7 | |||
| δ15N | −17.0*** | −22.0, −11.7 | −16.4*** | −21.6, −10.8 | −9.6** | −14.9, −4.0 | |||
SSB, sugar-sweetened beverage.
Indicates change in the dependent variable (sugar intake) for every 1‰ change in isotope ratio. All dietary variables were transformed for analysis; therefore, estimates of β are interpreted as percentage change in the dietary variable for every 1‰ change in isotope ratio. *P < 0.05, **P < 0.01, ***P < 0.0001.
FIGURE 1.
Associations of reported total sugar intake with predicted total sugar intake for all participants in the Neqem Nallunailkutaa study with a full suite of RBC, plasma, and hair isotopic measurements (n = 52). Total sugar intake was predicted using the formulae that were generated from the models of total sugar intake using RBC (dotted line), hair (dashed line), and plasma (solid line) δ13C and δ15N as predictors (Table 2).
There were no significant associations of sugar intake with fasting plasma glucose δ13C (n = 65). Fasting plasma glucose δ13C was not associated with intake of total sugars (β: 5.3; 95% CI: −1.7, 12.8; R2 = 0.03; P = 0.14), added sugars (β: 5.6; 95% CI: −2.4, 14.3; R2 = 0.03; P = 0.17), or SSBs (β: 5.4; 95% CI: −0.75, 12.0; R2 = 0.05; P = 0.09).
Table 3 gives means and distributions of δ13C and δ15N for all sample types (n = 52). In agreement with previous results from this population (4), hair δ13C values were consistently elevated over RBC δ13C values by 1.9 ± 0.3‰. Plasma and RBC δ13C values did not differ from one another but were elevated relative to fasting plasma glucose δ13C by 4.2 ± 2.1‰ and 4.0 ± 2.1‰, respectively. Hair δ15N was elevated 1.5 ± 0.5‰ relative to RBC δ15N (4) but did not differ from plasma δ15N. Plasma δ15N was elevated over RBC δ15N by 1.4 ± 0.4‰.
TABLE 3.
δ13C and δ15N for participants in the Neqem Nallunailkutaa study with a full suite of RBC, plasma, and hair isotopic measurements1
| δ13C | δ15N | |
| ‰ | ‰ | |
| RBC | −19.7 ± 0.6 | 9.3 ± 1.8 |
| Plasma | −19.9 ± 0.7 | 10.7 ± 1.8 |
| Hair | −17.8 ± 0.7 | 10.8 ± 2.0 |
| Fasting plasma glucose | −15.5 ± 2.3 | — |
Values are means ± SDs, n = 52.
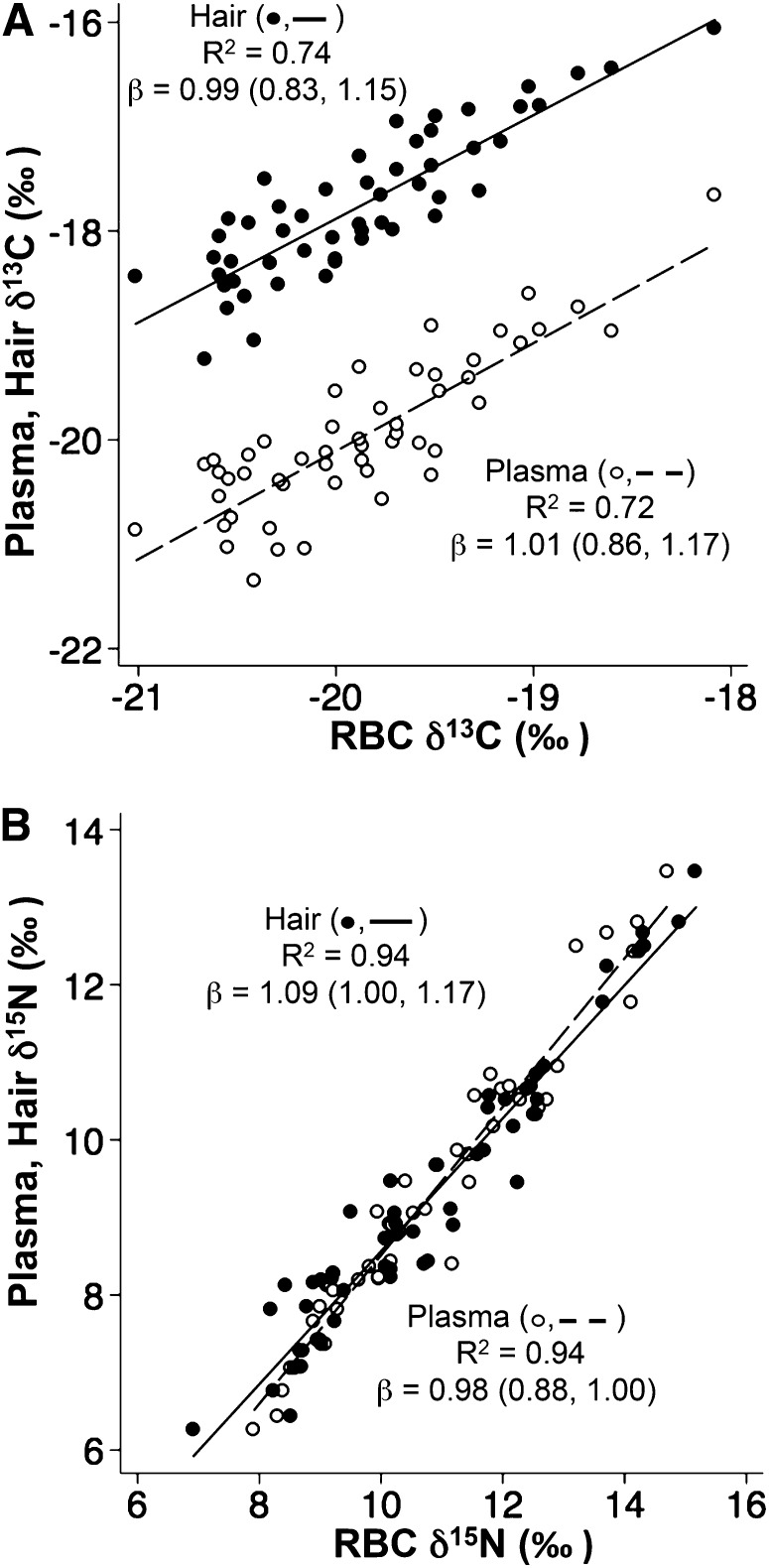
Figure 2 shows the associations between the δ13C and δ15N values of RBCs, plasma, and hair (n = 52). The δ13C and δ15N values of RBCs, hair, and plasma were strongly associated (all P < 0.0001) and the slopes of these relations did not differ from 1, with the exception of the association between hair and plasma δ15N (β: 0.87; 95% CI: 0.81, 0.93). Agreement among RBC, plasma, and hair isotope ratios was mostly good once the differences in δ13C and δ15N were accounted for. For δ13C, the SD of the mean difference was 0.39‰ for RBCs compared with plasma, 0.35‰ for RBCs compared with hair, and 0.39‰ for plasma compared with hair. For δ15N, the SD of the mean difference was 0.41‰ for RBCs compared with plasma, 0.53‰ for RBCs compared with hair, and 0.47‰ for plasma compared with hair. There were weak but significant associations of fasting plasma glucose δ13C with RBC δ13C (β: 0.08; 95% CI: 0.01, 0.16; r = 0.31; P = 0.03), plasma δ13C (β: 0.11; 95% CI: 0.02, 0.20; r = 0.33; P = 0.022), and hair δ13C (β: 0.14; 95% CI: 0.05, 0.22; r = 0.44; P = 0.002).
FIGURE 2.
Relations [R2; β (95% CI)] of RBC δ13C (A) and RBC δ15N (B) isotope ratios with hair and plasma isotope ratio intake for all participants in the Neqem Nallunailkutaa study with a full suite of RBC, plasma, and hair isotopic measurements (n = 52). The associations between δ13C and δ15N of hair and plasma were also strong and positive [R2 = 0.72; β: 0.90; 95% CI: 0.74, 1.06 (δ13C)] and [R2 = 0.94; β: 0.87; 95% CI: 0.81, 0.93 (δ15N)]. All P < 0.0001.
Discussion
This study tested whether isotope measurements from RBCs, plasma, and hair had similar validity when used to model sugar intake in a Yup’ik population. We found that that a dual isotopic model of sugar intake-based δ13C and δ15N explained nearly identical amounts of variance in total sugars, added sugars, and SSBs when isotope ratios were measured in hair or RBCs. These models slightly outperformed models based on plasma isotope ratios in which plasma δ13C was associated with SSBs but not total or added sugars. These dual isotope models can be used to generate predictive equations for estimating dietary sugar intake (2), which will have utility for assessing associations of sugar intake with chronic disease risk in this Alaska Native population. We also showed that fasting plasma glucose δ13C was not associated with differences in self-reported usual sugar intake, consistent with the findings of a recent 7-d controlled feeding study (5). Finally, we showed strong, positive associations and good agreement between δ13C and δ15N measured in RBCs, plasma, and hair. These results support the use of isotopes measured in RBCs, hair, and plasma to indicate sugar intake, although they suggest that measurements from RBCs and hair are preferable to those from plasma. Importantly, this study also provides a basis for comparing studies using these different sample types.
RBC and hair isotope ratios provided a better measure of reported sugar intake than did plasma isotope ratios. This difference in performance could be related to the difference in turnover time between these biological fractions; the timing of our dietary interviews, as well as our sampling strategy for the hair, was timed to reflect the average age of RBCs rather than plasma. However, despite the lack of association between total or added sugars and plasma δ13C, the variance explained by the dual isotope model using plasma isotope ratios was high for all sugar variables (28–48%). This is because of the strong inverse association between reported sugar intake and plasma δ15N (25), a measure of traditional food intake in Yup’ik people (15).
The finding that hair isotope ratios were similar to RBCs in their ability to predict sugar intake is important, because hair can be collected noninvasively and without the need for specialized personnel or storage (4). Such a noninvasive biomarker will be useful for studies involving children as well as in studies that do not otherwise require a blood draw. Furthermore, in rural study populations, trained community aides can easily collect hair samples, reducing the cost and difficulty of assessing dietary intake using this biomarker. Finally, hair has an additional advantage over blood and plasma: because hair grows continuously and does not remodel after growth, it provides a continuous record of the biomarker back through time. This may be useful to assess the impact of any intervention designed to reduce sugar consumption over time. The ability of hair isotope ratios to predict sugar intake in other, non-Native populations has not been assessed; however, we expect associations with hair to be similar to those previously reported for whole blood and serum (26, 27) based on our findings in this study.
Our finding that fasting plasma glucose δ13C was not associated with total sugars, added sugars, or SSBs is consistent with that from a recent controlled feeding study reported by Cook et al. (5), which demonstrated no effect of C4 sugar intake on fasting plasma glucose δ13C. This study built upon their work by testing whether fasting plasma glucose δ13C could be associated with dietary differences that are of a longer duration than a 7-d intervention. However, our results confirm that fasting plasma glucose δ13C does not reflect sugar intake even in the context of differences in usual consumption. This is likely due to plasma glucose carbon not solely deriving from dietary sugars; rather, we suggest that fasting plasma glucose incorporates carbon from other dietary sources through gluconeogenesis.
The differences between carbon and nitrogen isotope ratios in RBCs, plasma, and hair presented here were consistent with previous reports for RBCs and hair in Yup’ik people (4) and plasma protein and hair for a small sample (n = 10) of Chicago residents (28). To our knowledge, comparisons of RBCs and plasma isotope ratios have not been previously reported for humans; however, a recent study of Baltimore residents found very similar results for blood clot and serum (3). Associations among RBCs, hair, and plasma isotope ratios were strong and followed the expected 1:1 relations, which is also in agreement with previous work (3, 4). These findings will be useful in comparing isotopic studies conducted in hair, RBCs, and plasma in Yup’ik people and will provide a standard for comparison in similar studies in other, non-Native populations.
The primary limitation of this study is that we tested our proposed biomarkers against self-reported intakes, which are subject to random and systematic errors. Very little is known about factors that bias dietary self-report in Yup’ik people; however, sex and obesity have been identified as factors that may influence reporting bias in other populations (29). Neither sex nor BMI was associated with δ13C in this study population; therefore, these potential biases could not explain the associations we found with sugar intake. In this study, the assay for fasting plasma glucose δ13C was less precise than that of δ13C measured in bulk plasma, RBCs, and hair; however, intra-sample variation was significantly smaller than inter-sample variation, suggesting that the null result with sugar intake was not simply due to insufficient analytical precision.
Several limitations of these and other isotope biomarkers of sugar intake warrant further discussion. First, our models underestimated reported sugars at high levels of intake for plasma and hair and at all levels of intake for RBCs (Fig. 1). This underestimation was not observed in our initial validation of the RBC model in a larger sample (2); therefore, it may be an artifact of the smaller sample size presented here. Second, the δ13C biomarker has the potential to be affected by other sources of enriched 13C in the Yup’ik diet; our model controls for most, but not all, of these effects (2). Finally, one limitation of the δ13C biomarker of sugar intake more generally is that is it not associated with intake of sugars that are not 13C enriched, such as beet sugar, honey, and intrinsic sugars found in fruit and dairy products (30). Consumption of these sugars by Yup’ik people is low (31); therefore, we do not expect our results to be affected by their consumption. However, we anticipate associations of δ13C with total sugar intake will be attenuated in populations that consume sugars that are not 13C enriched (27).
In summary, this study demonstrated that the stable isotope ratios of RBCs, plasma, and hair, but not fasting plasma glucose, can be used to assess sugar intake in Yup’ik people and provided data that will allow comparison of isotope ratios in these different sample types. In particular, the finding that hair isotope ratios are as predictive of sugar intake as RBC isotope ratios is important, because it suggests that sugar intake can be measured noninvasively and without the need for specialized sampling equipment. Further studies are warranted to determine the utility of this dual isotope model of sugar intake in other, non-Native populations.
Acknowledgments
The authors thank Kristine Niles, Eliza Orr, and Jynene Black for their assistance with sample collection. The authors also thank Mat Wooller, Tim Howe, and Norma Haubenstock at the Alaska Stable Isotope Facility for their assistance with isotope analysis. This manuscript was improved by comments from Kyungcheol Choy and Justin Smith. D.M.O. designed research; S.H.N., B.B.B., S.E.H., and D.M.O. conducted research; S.H.N. analyzed data; S.H.N., D.M.O., and A.R.K. wrote the manuscript; and S.H.N. had primary responsibility for final content. All authors read and approved the final manuscript.
Literature Cited
- 1.Rippe JM, Kris Etherton PM. Fructose, sucrose, and high fructose corn syrup: modern scientific findings and health implications. Adv Nutr. 2012;3:739–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash SH, Kristal AR, Bersamin A, Hopkins SE, Boyer BB, O'Brien DM. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr. 2013;143:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraft RA, Jahren A, Saudek C. Clinical -scale investigation of stable isotopes in human blood: δ13C and δ15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun Mass Spectrom. 2008;22:3683–92. [DOI] [PubMed] [Google Scholar]
- 4.Nash SH, Kristal AR, Boyer BB, King IB, Metzgar JS, O'Brien DM. Relation between stable isotope ratios in human red blood cells and hair: implications for using the nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr. 2009;90:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook CM, Alvig A, Liu Y, Schoeller D. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr. 2010;140:333–7. [DOI] [PubMed] [Google Scholar]
- 6.Craig MR, Kristal AR, Cheney CL, Shattuck AL. The prevalence and impact of ‘atypical’ days in 4-day food records. J Am Diet Assoc. 2000;100:421–7. [DOI] [PubMed] [Google Scholar]
- 7.Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products: a research perspective. J Food Compost Anal. 2001;14:315–22. [Google Scholar]
- 8.Berlin NI, Waldmann T, Weissman S. Life span of red blood cell. Physiol Rev. 1959;39:577–616. [DOI] [PubMed] [Google Scholar]
- 9.Cohen RM, Franco R, Khera P, Smith E, Lindsell C, Ciraolo P, Palascak M, Joiner C. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eadie GS, Brown I. The potential life span and ultimate survival of fresh red blood cells in normal healthy recipients as studied by simultaneous Cr51 tagging and differential hemolysis. J Clin Invest. 1955;34:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cernichiari E, Toribara T, Liang L, Marsh D, Berlin M, Myers G, Cox C, Shamlaye C, Choisy O, Daivdson P, et al. The biological monitoring of mercury in the Seychelles study. Neurotoxicology. 1995;16:613–28. [PubMed] [Google Scholar]
- 12.Miyazawa N, Uematsu T. Analysis of ofloxacin in hair as a measure of hair growth and as a time marker for hair analysis. Ther Drug Monit. 1992;14:525–8. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh M, Uzuka M, Sakamoto M, Kobori T. Rate of hair growth. In: Montagne W, Dobson RL, eds. Hair Growth. Oxford: Pergamon Press; 1969. [Google Scholar]
- 14.Welle S. Human protein metabolism. New York: Springer-Verlag 1999. [Google Scholar]
- 15.Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL, Luick BR, Mohatt GV, Boyer BB, O'Brien DM. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr. 2012;142:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson MJ, Yai Y, O'Brien D. Age-related variation in red blood cell stable isotope ratios (δ13C and δ15N) from two Yupik villages in southwest Alaska: a pilot study. Int J Circumpolar Health. 2007;66:31–41. [DOI] [PubMed] [Google Scholar]
- 17.Fry B. Stable isotope ecology. New York: Springer; 2006. [Google Scholar]
- 18.O'Connell TC, Hedges R. Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol. 1999;108:409–25. [DOI] [PubMed] [Google Scholar]
- 19.O'Connell T, Hedges R, Healey M. Isotopic comparison of hair, nail and bone: modern analyses. J Archaeol Sci. 2001;28:1247–55. [Google Scholar]
- 20.Rembacz KP, Stellaard F, Faber KN. Selective conversion of plasma glucose into CO2 by Saccharomyces cerevisiae for the measurement of C-13 abundance by isotope ratio mass spectrometry: proof of principle. Rapid Commun Mass Spectrom. 2007;21:3169–74. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 22.Song WO, Wang Y, Chung CE, Song B, Lee W, Chun OK. Is obesity development associated with dietary sugar intake in the US? Nutr. 2012;28:1137–41. [DOI] [PubMed] [Google Scholar]
- 23.Sun SZ, Anderson GH, Flickinger BD, Williamson-Hughes PS, Empie MW. Fructose and non-fructose sugar intakes in the US population and their associations with indicators of metabolic syndrome. Food Chem Toxicol. 2011;49:2875–82. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Cao X, Roache C, Buchan A, Reid R, Gittelsohn J. Assessing dietary intake in a population undergoing a rapid transition in diet and lifestyle: the Arctic Inuit in Nunavut, Canada. Br J Nutr. 2010;103:749–59. [DOI] [PubMed] [Google Scholar]
- 25.Choy K, Nash SH, Kristal AR, Hopkins S, Boyer BB, O'Brien DM. The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. J Nutr. 2013;143:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davy BM, Jahren AH, Hedrick VE, Comber DL. Association of δ13C in fingerstick blood with added-sugar and sugar-sweetened beverage intake. J Am Diet Assoc. 2011;111:874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung EH, Saudek C, Jahren A, Kao W, Islas M, Kraft R, Coresh J, Anderson C. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol. 2010;172:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoeller D, Minagawa M, Slater R, Kaplan I. Stable isotopes of carbon, nitrogen and hydrogen in the contemporary North-American human food web. Ecol Food Nutr. 1986;18:159–70. [Google Scholar]
- 29.Macdiarmid J. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev. 1998;11:231–53. [DOI] [PubMed] [Google Scholar]
- 30.Jahren AH, Saudek C, Yeung E, Kao W, Kraft R, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84:1380–4. [DOI] [PubMed] [Google Scholar]
- 31.Bersamin A, Luick BR, Ruppert E, Stern JS, Zidenberg-Cherr S. Diet quality among Yup'ik Eskimos living in rural communities is low: the Center for Alaska Native Health Research pilot study. J Am Diet Assoc. 2006;106:1055–63. [DOI] [PubMed] [Google Scholar]