Summary
Background
The aim of this study was to compare the efficacy of radiofrequency ablation vs. cryoablation in the treatment of early breast cancer.
Patients and Methods
80 women (mean age 73 ± 5 years) with early breast cancer were retrospectively evaluated. 40 patients underwent cryoablation and 40 patients underwent radiofrequency ablation, both with sentinel lymph node excision. Tumor volume and histopatological data were compared by means of postprocedural 3.0-T magnetic resonance imaging (MRI). 30–45 days after the percutaneous ablation, all patients underwent surgical resection of the tumor. The mean follow-up was 18 months without any local recurrences.
Results
Both techniques allow good correlation with histopathological data. In 75 patients (93.8%) we observed complete necrosis; in 5 cases there was residual disease in the postprocedural MRI and postoperative histological examination. There was a good correlation between MRI volume and histologic samples. Cosmetic results were good in all patients but 2.
Conclusion
Both percutaneous radiofrequency ablation and cryotherapy are minimally invasive techniques with a good clinical and cosmetic outcome in selected cases. MRI examination is an ideal method to assess breast neoplasms in terms of quality and quantity as well as residual tumor extent after percutaneous ablation. Cryotherapy is the preferred method because of the analgesic effect of freezing with better patients compliance.
Key words: Cryoablation, Radiofrequency ablation, Breast cancer, Percutaneous ablation, MRI, Minimally invasive techniques
Zusammenfassung
Hintergrund
Ziel dieser Studie war es, die Effizienz der Radiofrequenzablation im Vergleich zur Cryoablation zur Behandlung des frühen Mammakarzinoms zu vergleichen.
Patientinnen und Methoden
80 Frauen (mittleres Alter 73 ± 5 Jahre) mit einem primären Mammakarzinom wurden retrospektiv evaluiert. Bei 40 Patientinnen wurde eine Cryoablation und bei weiteren 40 eine Radiofrequenzablation durchgeführt (jeweils mit Sentinelknoten-entfernung). Tumorvolumen und histopatologische Daten wurden mittels 3.0-T-Magnetresonanztomogra-phie (MRT) im Anschluss an den Eingriff bestimmt. 30–45 Tage nach der perkutanen Ablation wurde bei allen Patientinnen eine chirurgische Tumorentfernung vorgenommen. Das mittlere Follow-up war 18 Monate; während dieser Zeit traten keine Lokalrezidive auf.
Ergebnisse
Für beide Methoden konnte eine gute Korrelation mit histopathologischen Daten gezeigt werden. Bei 75 (93,8%) Patientinnen wurde eine komplette Nekro-tisierung beobachtet; in 5 Fällen zeigten das Kontroll-MRT und die postoperative histologische Untersuchung Resttumorgewebe. Es bestand eine gute Korrelation zwischen MRT-Volumen und den histologischen Proben. Das kosmetische Outcome war bis auf 2 Fälle gut.
Schlussfolgerung
Die perkutane Radiofrequenzablation und die Cryotherapie sind minimalinvasive Methoden mit einem guten klinischen und kosmetischen Outcome in ausgewählten Fällen. MRT ist eine ideale Methode zur qualitativen und quantitativen Charakterisierung von Mammakarzinomen sowie dem Ausmaß von Resttumorgewebe nach perkutaner Ablation. Cryotherapie wird der RFA vorgezogen, da der analgetische Effekt des Vereisens mit besserer Patientcompliance einhergeht.
Introduction
In the past decades, advanced techniques for breast-conserving surgery have been developed which provide good oncological and cosmetic results without altering the survival rate of patients [1]. As a standard of care, surgical biopsy has been replaced by percutaneous core needle biopsy, and axillary dissection has been replaced by sentinel lymph node mapping. Percutaneous excision without major surgery of a single, subclinical, invasive cancer in selected patients is a new goal. Being a superficial structure, the breast is a suitable organ for percutaneous treatment.
Breast cancer is a cause of morbidity and mortality also in older women, affecting up to 30% of the over-70s. With the increase in life expectancy, this figure is likely to exceed 30% in the next decade [2, 3]. Due to the common presence of co-morbidities, elderly patients are often treated with less aggressive approaches. Radiofrequency ablation (RFA), cryotherapy, interstitial laser ablation, focused ultrasound ablation, and focused microwave thermotherapy represent valid alternatives to open surgery with less psychological impact for the patient and good clinical and cosmetic outcome. Furthermore, these techniques are characterized by a low grade of complications, require shorter periods of hospitalization, and incur lower health care costs [4, 5, 6, 7]. We aimed to compare in vivo the efficacy of RFA and cryotherapy in the treatment of small invasive breast cancers in terms of tumor necrosis, pathological outcome, and cosmetic outcome.
Patients and Methods
Patients
This study was approved by our institutional review board. We retrospectively reviewed all the minimally invasive procedures performed on breast cancer patients in our Department between October 2008 and March 2011. 80 postmenopausal women (mean age 73 ± 5 years (standard deviation, SD); range 64–82 years) were enrolled (table 1). Inclusion criteria were biopsy-proved ductal invasive unifocal breast cancer 2 cm or smaller (T1), well differentiated tumor (G1 and G2) visible in both ultrasound (US) and magnetic resonance imaging (MRI) studies, and tumor located at least 1 cm from the skin and 1 cm from the chest wall at US examination. We excluded patients with multifocal or multicentric neoplasia, lobular neoplasia, intraductal carcinoma, retro-areolar tumors, and other non-ductal infiltrating cancers. All patients provided written informed consent. 40 patients underwent cryotherapy and 40 patients underwent RFA. In all patients, vacuum-assisted biopsy (VAB) was performed for histopathological analysis. After the percutaneous procedure, US-guided strand reperage and sentinel lymph-node biopsy were performed. A preoperative MRI 1 and 4 weeks after the procedure was performed as follow-up. 30–45 days after the percutaneous ablation, all patients received definitive surgery.
Table 1.
Patient characteristics and hormone status pattern
| Patients, n | 80 |
| Mean age ± SD (range), years | 73 ± 5 (64–82) |
| Hormone status, n | |
| ER/PR/HER2– | 72 |
| ER–/PR–/HER2 | 5 |
| ER–/PR–/HER2– | 3 |
ER = Estrogen receptor; PR = progesterone receptor; HER2 = human epidermal grow factor receptor 2.
Breast US
Ultrasonographic examinations were performed using a 5–12 MHz transducer and a US unit (ATL HDI 5000, Philips Medical Systems, Best, The Netherlands).
Breast MRI
Breast MRI examinations were performed with the patient placed in a prone position in a 3.0-T system (Achieva, Philips Medical Systems). A dedicated sensitivity-encoding breast coil was used for radiofrequency signal reception. Axial T2-weighted and T1-weighted images were obtained. The dynamic series consisted of a T1-weighted 3-dimensional fast-field echo sequence (repetition time/echo time, 5.1/2.4 ms; flip angle 20°). A total of 8 dynamic acquisitions with a temporal resolution of 70 s for a single dynamic acquisition were performed. Each dynamic acquisition consisted of 60 2.5-mm thick sections (gap, 0) with a 256×512 matrix and a field of view of 330 mm (adjusted to the size of the breast). Contrast agent bolus injection consisted of 0.2 mmol gadopentetate dimeglumine (Magnevist®, Schering, Berlin, Germany) per kilogram of body weight administered at an injection rate of 2.5 ml/s. Patients underwent 3 MRI examinations: 1 week before the procedure, 1 week after the procedure, and 4 weeks after the procedure.
Vacuum-Assisted Biopsy
Small invasive cancers were characterized based on histological samples obtained with the Mammotome® breast biopsy system (Ethicon Endo-Surgery, Cincinnati, OH, USA) or the Vacora® vacuum biopsy system (Bard Biopsy Systems, Tempe, AZ, USA) under US guidance. Specimens were subjected to standard histological and immunohistochemical evaluation (receptors for estrogen (ER), progesterone (PR), and HER2/ neu) (table 1). VAB involves the use of US guidance and needles of different calibers with a coaxial system of collection/aspiration that allows multiple samples to be taken through a single percutaneous insertion.
Cryotherapy
The procedure was performed in the angiography room. The devices were placed percutaneously under ‘real-time’ US guidance using a US probe at 5–12 MHz (ATL 5000, Philips Medical Systems). After disinfecting the skin of the breast, 10 ml of xilocaine 2% were administered sub-cutaneously, along the path of the probe and in the target area. After skin incision with a scalpel (3 mm), 1 cryoprobe (1.47 mm diameter, 17-gauge isotherms, IceRod® model, Galil Medical, Yokneam, Israel) and 2 thermocouples for temperature monitoring were positioned. The device was set with the tip of the exposed needle at 2–5 mm from the distal margin of the lesion. Light intravenous sedation was always given, but patients stayed awake during the procedure. If the distance between the lesion and the skin profile and/or muscle was equal to 1 cm, sterile saline solution heated at 40 °C was injected to avoid surrounding skin and chest wall damage. A cooling double-cycle (each 10 min) separated by 5 min of passive heating (stop of argon coolant flow), and active heating with helium of the cryoprobe after the second cooling cycle, determined complete cell necrosis at −40 °C. Further heating was then necessary to dissolve the iceball. At the end of the procedure, the probe and thermocouples were removed without the need for stitches, and a sterile patch and compression bandage were put in place. In the same session, surgical excision of the sentinel lymph node for subsequent histological evaluation was performed. Patients were strongly advised to report postprocedural symptoms at the clinical follow-up 1 week later. The appearance of the skin, the size of the lesion, and the level of comfort of the procedure for the patient were carefully recorded at the end of the session. Oral broad-spectrum antibiotic therapy was prescribed for the 5 days following the procedure.
Radiofrequency Ablation
An RFA system using a 460-KHz generator (TAG100 RF Generator, Fogazzi, Concesio, Italy) capable of 100 W maximum output at 200 Ω and a cool-tip single-needle electrode (Miras PTV, University Hospital Policlinico Tor Vergata, Rome, Italy, in collaboration with INVATEC ITALIA, Roncadelle, Brescia, Italy) were employed for the breast RFA procedures. The Miras PTV is a 15-gauge monopolar 200-mm double-lumen single electrode with active stainless steel tip exposure of 25 mm. The procedure was performed with patients in supine position and under general anesthesia, and the neoplasm was identified using US guidance. A small incision in the breast skin facilitated needle insertion. The cool-tip single-needle electrode was inserted into the core of the tumor. RF current was emitted, starting with the generator set to deliver energy according to the initial impedance value for a mean time of 5 min. The power was increased in 10-W intervals every 5 min until the readings on the external thermocouples indicated that the target temperature (90 °C) had been reached. Then, the power output was adjusted as needed to maintain the final temperature of 90 °C for 12 min. At the end of the procedure, a cool-down period of 60 s was applied to ensure necrosis of the tissue surrounding the needle tip. Upon completion of the procedure, the needle was withdrawn. Sterile skin closure strips were used to close the skin. Oral broad-spectrum antibiotic therapy was prescribed for the 5 days following the procedure.
Surgery
Lumpectomy was performed in all patients after a mean time of 34 days from percutaneous ablation (range 30–45 days). Axillary lymph node dissection was performed in 15 patients because of positivity of the sentinel lymph node biopsy.
Adjuvant Treatment
In accordance with the St Gallen oncologic criteria [8], patients underwent breast irradiation or adjuvant systemic chemotherapy. Hormonal therapy was administered according to grade, and ER, PR, and HER2/ neu status determined at the pretreatment biopsy (table 1).
Histopathology
VAB was performed to histologically characterize the lesions before treatment. Samples were formalin-fixed and paraffin-embedded. Histological sections were stained with hematoxylin and eosin for tumor staging. Predictive and prognostic markers were studied by immunohistochemistry, including ER (CONFIRM Anti-ER SP1, Ventana Medical Systems, Tucson, AZ, USA), PR (CONFIRM anti-PR 1E2, Ventana), and HER2 (PATHWAY HER2, Ventana); the proliferative activity of tumor cells was evaluated using the Ki-67 index (CONFIRM Ki-67, Ventana).
Cosmetic Evaluation
Cosmetic evaluation was performed at the end of ablation (Time 0) and at 4 weeks from the procedure (Time 1), prior to surgical excision. All patients were evaluated based on the following signs: appreciable nodule on palpation, skin bruising, skin rash, and skin hyperpigmentation. The absence of skin pigmentation was considered excellent cosmetic outcome (grade 1), breast with slight texture changes or mild pigmentation was considered good cosmetic outcome (grade 2), breast with moderate texture changes or pigmentation was regarded as acceptable outcome (grade 3), and the presence of marked texture or pigmentation changes was considered a poor cosmetic outcome (grade 4).
Follow-Up
All patients underwent a weekly US examination from the 1st to the 4th week after the ablation procedure. MRI was performed at the 1st and 4th week after cryotherapy or RFA.
Statistical Analysis
All data analysis was performed using the Statistical Package for the Social Sciences, version 15.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics consisted of the mean ± SD for variables with Gaussian distributions or median (min-max) for categorical parametric and non-parametric data. Comparisons of paired data were carried out with the t-test or Wilcoxon test for non-parametric data. Correlation analysis was carried out by calculating the Pearson coefficient (r). Statistical significance was set at p < 0.05.
Results
Biopsies revealed all tumors to be invasive ductal carcinomas, of which 72 were hormone-responsive (ER, PR, HER2-), 5 were not endocrine responsive (ER-, PR-, HER2), and 3 were triple-negative (ER-, PR-, HER2-). 73 were well differentiated (G1), 7 were moderately differentiated (G2).
Cryotherapy
All 40 patients undergoing cryotherapy were treated with lumpectomy. At the end of the second cooling cycle, the iceball around the probe at −40 °C was a mean 16 mm (SD ± 0.8 mm) × 31 mm (SD ± 1.2 mm) as shown by US. In 4 cases, postprocedural MRI showed residual enhancement which in 2 patients was no longer visible at the presurgerical MRI (expression of granulation tissue around avascular areas); in the other 2 cases both MRI and histopathology showed residual disease. In 38 lesions, there were no vital neoplastic cells. No local recurrence was registered.
Radiofrequency
All 40 patients undergoing RFA were treated with lumpectomy. The target temperature (90 °C) was reached after 15 ± 3.7 min (range 12–23 min). In 37 patients, we observed complete necrosis of the ablated tumor in both postprocedural MRI and after surgery, without enhancing lesions. In 5 cases, postprocedural MRI showed residual enhancement which in 2 patients was no longer visible at the presurgerical MRI (expression of granulation tissue around the coagulative necrosis areas); in the 3 remaining cases the persistent residual enhancement was an expression of residual disease as assessed by the presence of vital neoplastic cells (fig. 1). The RF volume obtained was 27 mm (SD ± 0.6 mm) ± 42 mm (SD ± 0.8 mm). No local recurrence was experienced.
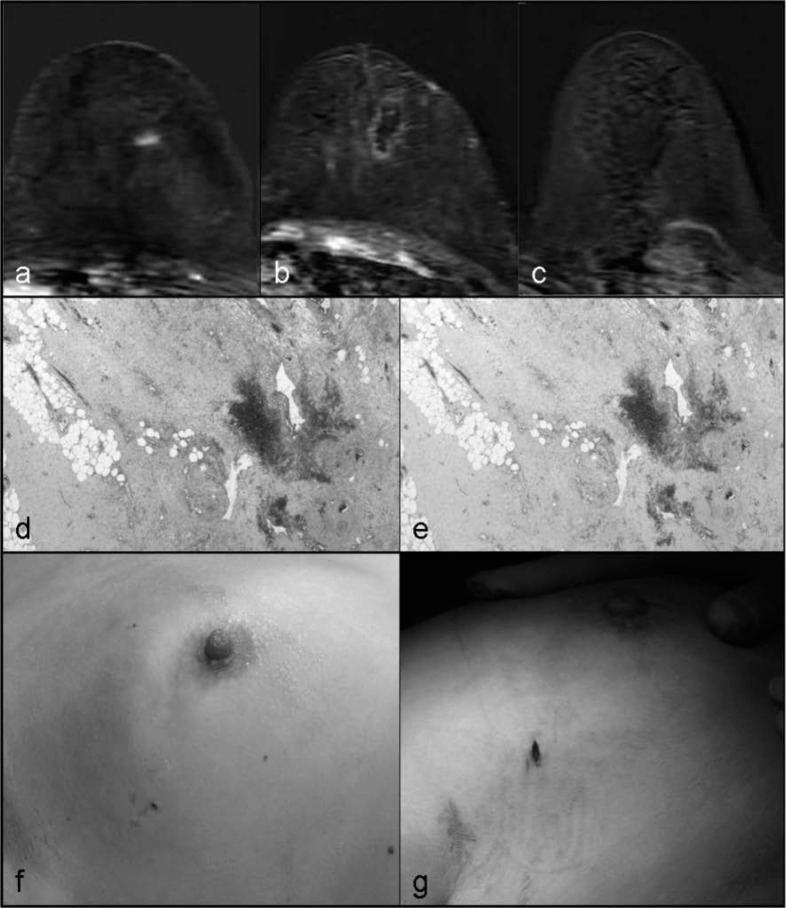
Fig. 1.
Radiofrequency ablation, histological examination, and cosmetic evaluation. a Axial T1-weighted magnetic resonance imaging (MRI) showing an enhancing lesion in the upper-outer left breast; b ablation volume after percutaneous ablation; c absence of enhancement at a delayed MRI; d hemorrhagic/necrotic areas (hematoxylin and eosin) without viable epithelial cells; e NADH diaphorase; f excellent cosmetic results at Time 0 after percutaneous ablation, g at Time 1 prior to surgical excision with evidence of mild pigmentation of the skin.
MRI Evaluation
After both procedures, MRI showed altered signal intensity and a minor degree of peripheral enhancement in the ablation areas. 1-week post-ablation MRI images displayed no suspicious residual enhancement in 71 (88.7%) of 80 lesions (36 for cryotherapy and 35 for RFA). The 4-week post-ablation MRI showed that 4 (5%) (2 for cryotherapy and 2 for RFA) of the 9 areas of residual enhancement were less conspicuous, suggesting reactive granulation tissue around the ablated areas. 5 (6.25%) of the 9 areas of residual enhancement increased in intensity on the 4-week postprocedural MRI images, suggesting residual tumor tissue which was confirmed by NADH diaphorase staining (table 2). There was a strong correlation between MRI volume and pathologic examination values (r = 0.896, p < 0.0001, 95% confidence interval (CI) 0.847–0.944).
Table 2.
Magnetic resonance imaging (MRI) evaluation: 1-week post-ablation MRI showed no residual enhancement in 71 (88.7%) of 80 lesions (36/35); 4-week post-ablation MRI showed increasing enhancement in 5 (6.25%) of the 9 areas of residual enhancement (2/3) suggesting residual tumor tissue which was confirmed by NADH diaphorase staining
| Cryotherapy (n = 40) |
RFA (n = 40) |
|||
|---|---|---|---|---|
| positive | negative | positive | negative | |
| MRI at 1 week | 4 | 36 | 5 | 35 |
| MRI at 4 weeks | 2 | 38 | 3 | 37 |
| Histology | 2 | 38 | 3 | 37 |
Histopathology
Specimen examination showed only necrosis or degenerative changes in 77 cases. The 5 enhancing lesions revealed by Time 1 MRI contained partial degenerative changes (2 for cryotherapy and 3 for RFA) with NADH diaphorase activity revealing vital cells.
Cosmetic Evaluation
Cosmetic outcome was evaluated immediately after percutaneous ablation and 4 weeks after the procedure. At Time 0, it was grade 1 in 49 patients (26 for cryotherapy and 23 for RFA), grade 2 in 18 (8 for cryotherapy and 10 for RFA), grade 3 in 12 (7 for cryotherapy and 5 for RFA), and grade 4 in 1 (0 for cryotherapy and 1 for RFA) (fig. 1). At Time 1, cosmetic evaluation was grade 1 in 71 patients (37 for cryotherapy and 34 for RFA), grade 2 in 5 (2 for cryotherapy and 3 for RFA), grade 3 in 2 (1 for cryotherapy and 1 for RFA), and grade 4 in 2 patients (0 for cryotherapy and 2 for RFA). The comparison between the 2 series did not reveal a significant prevalence of esthetic complications in any group.
Discussion
Minimally invasive ablative techniques for small breast cancer may be a desirable goal in breast-conserving therapy. These kinds of treatment could improve patient comfort and cosmetic results, and provide a treatment window for patients who are not surgical candidates.
To our knowledge, this is the first study comparing the effects of RFA and cryotherapy in a homogeneous population. In the past decade, some studies have been published showing the efficacy of both techniques in independent series. The most important achievement is real-time imaging with which complete ablation of the lesion can be ascertained. US guidance is useful for needle and thermocouple placement within the tumor in both techniques, even though it is not accurate for depicting the ablation volume. In US monitoring during RFA, the appearance of gas bubbles and a hyperechoic halo with a posterior acoustic shadow in the treated area indicates the thermal effect of the procedure. Moreover, it produces an increasing echogenicity, with a shadow effect (‘fog effect’) that hides the deep tissue layers and the mass itself [4]. In the future, it would be desirable to use MRI real-time monitoring which allows visualization of the entire boundaries of the ablation area. In 2008, van der Ploeg et al. [9] published the first case of MRI-guided breast RFA in 3 patients with invasive ductal carcinoma and no artifacts around the needle electrode [9, 10]. Although the iceball produced during cryoablation reduces the visibility in deeper tissues, it is much more regular, definite, and symmetric, allowing an easier comparison between the margins of the lesion and the ablation volume [11]. All RFAs were performed successfully, with no tissue impedance rising above 20 Ω from baseline during the procedure, independent of the breast composition pattern. Spherical shapes were obtained in all cases with the type of RFA needle used [6]. In our series, the first post-ablation MRI was performed to assess the short-term effect on tumor and tissues. In clinical practice, a post-ablation MRI may support the decision on further treatment if incomplete tumor ablation is suspected. As a matter of fact, 50% of focal enhancement at the first MRI after cryotherapy and 60% of positive cases after RFA were confirmed at the 4-week MRI and postsurgical histological evaluation. The 4-week post-ablation MRI images were helpful for further evaluation of residual tumor enhancement, without the bias related to inflammatory peripheral ring enhancement. We achieved 38 disease-free specimens after cryotherapy (94.84%) and 37 disease-free specimens after RFA (92.90%). There was no statistically significant difference between the cryotherapy and RFA groups (p = 0.082) so the results are substantially equivalent. A total of 5 failures occurred in terms of persistence of viable tumor cells. In both procedures, this failure can be ascribed to incorrect positioning of the device and insufficient size of the area of necrosis. In all failures, the necrosis area was displaced from the center of the tumor. Several studies showed that the absence of positive surgical margins does not guarantee complete removal of disease, and some studies have associated neoadjuvant endocrine therapy with improved treatment outcome in elderly women [12, 13]. In our series, a strong correlation was shown between postprocedural MRI and pathologic data. Both percutaneous ablation techniques provided good results in terms of cosmetic outcome and patients compliance. This is in accordance with the literature [10, 14], although cryotherapy is preferred because of the analgesic effect of freezing. Furthermore, cryotherapy only requires mild sedation compared to general anesthesia with RFA, which is more popular with patients [6, 10, 14, 15, 16].
In all cases but 2, the cosmetic results were acceptable for the patients. 1 RFA case with grade 4 outcome was complicated by a 1-cm2 skin necrosis, which was, however, remedied during lumpectomy. Another grade 4 RFA case progressed from grade 3 between Time 0 and Time 1 due to a retraction of the skin caused by fibrosis in a small-sized breast. We finally obtained 71 excellent results and good-mild results in 8 patients, with hyperpigmentation and/or subcutaneous thickening. The difference between cryotherapy and RFA regarding grade 4 cosmetic outcome may be related to a huge energy deposition in the tissue during RFA.
This study is limited by some bias; furthermore, it is a retrospective study without patient randomization, and the study population was small and highly selected for low-grade tumors in elderly patients. However, we can state that both RFA and cryotherapy resulted in a good level of patient satisfaction, although the analgesic effect of coldness is better endured than the heat from RFA. Minimally invasive ablation techniques may be useful in the treatment of single small breast cancers with complete necrosis of the lesion, and good cosmetic outcome and patient satisfaction are achieved in most cases.
References
- 1.Veronesi U, Cascinelli N, Mariani L. Twenty-years follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 2.Kimmick GG, Balducci L. Breast cancer and aging. Clinical interactions. Hematol Oncol Clin North Am. 2000;14:213–34. doi: 10.1016/s0889-8588(05)70285-9. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2000;37:S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 4.Fornage BD, Sneige N, et al. Small (< or = 2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology. 2004;231:215–24. doi: 10.1148/radiol.2311030651. [DOI] [PubMed] [Google Scholar]
- 5.Littrup PJ, Jallad B, Vorugu V. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size and number. J Vasc Interv Radiol. 2009;20:1343–51. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manenti G, Bolacchi F, Perretta T, et al. Small breast cancers: in vivo percutaneous US-guided radiofrequency ablation with dedicated cool-tip radiofrequency system. Radiology. 2009;251:339–46. doi: 10.1148/radiol.2512080905. [DOI] [PubMed] [Google Scholar]
- 7.Stijn van Esser M, van den Bosch AJ, van Diest P, et al. Minimally invasive ablative therapies for invasive breast carcinomas: an overview of current literature. World J Surg. 2007;31:2284–92. doi: 10.1007/s00268-007-9278-x. [DOI] [PubMed] [Google Scholar]
- 8.Carlson RW, Hudis CA, Pritchard KI. National Comprehensive Cancer Network Breast Cancer Clinical Practice Guidelines in Oncology. American Society of Clinical Oncology Technology Assessment on the Use of Aromatase Inhibitors; St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer: Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Canc Netw. 2006;4:971–9. doi: 10.6004/jnccn.2006.0082. [DOI] [PubMed] [Google Scholar]
- 9.Van der Ploeg IM, van Esser S, van den Bosch MA, et al. Radiofrequency ablation for breast cancer: a review of the literature. Eur J Surg Oncol. 2007;33:673–7. doi: 10.1016/j.ejso.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Van Esser S, van den Bosch MA, van Diest PJ, Mali WT, Borel Rinkes IH, van Hillegersberg R. Minimally invasive ablative therapies for invasive breast carcinomas: an overview of current literature. World J Surg. 2007;31:2284–92. doi: 10.1007/s00268-007-9278-x. [DOI] [PubMed] [Google Scholar]
- 11.Manenti G, Perretta T, Gaspari E, et al. Percutaneous local ablation of unifocal subclinical breast cancer: clinical experience and preliminary results of cryotherapy. Eur Radiol. 2011;21:2344–53. doi: 10.1007/s00330-011-2179-2. [DOI] [PubMed] [Google Scholar]
- 12.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–93. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 13.Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–8. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshinaga Y, Enomoto Y, Fujimitsu R. Image and pathological changes after radiofrequency ablation of invasive breast cancer: a pilot study of non surgical therapy of early breast cancer. World J Surg. 2013;37:356–63. doi: 10.1007/s00268-012-1820-9. [DOI] [PubMed] [Google Scholar]
- 15.Vlastos G, Verkooijen H. Minimally invasive approaches for diagnosis and treatment of early-stage breast cancer. Oncologist. 2007;12:1–10. doi: 10.1634/theoncologist.12-1-1. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3:212–8. doi: 10.1016/s1076-6332(96)80443-0. [DOI] [PubMed] [Google Scholar]