Abstract
Purpose
We report a case of methicillin-resistant Staphylococcus aureus (MRSA) keratitis after Descemet's stripping automated endothelial keratoplasty (DSAEK).
Case Report
An 87-year-old woman who had undergone a DSAEK 4 months previously was referred to Tokushima University Hospital with a diagnosis of infectious keratitis after DSAEK. A white abscess and infiltration in the inferior cornea of the right eye were observed. We started an empiric therapy using topical levofloxacin and chloramphenicol on the basis of the microscopic findings of the corneal scraping concurrently with cultivation of the cornea.
Results
A strain of MRSA was isolated from the corneal sample. Although the strain was susceptible to chloramphenicol, it was resistant to quinolone. The keratitis improved rapidly due to empiric therapy, and topical steroids could be resumed 6 days after initiation of the empiric therapy.
Conclusions
To our knowledge, this is the first case of MRSA keratitis, and the second case of bacterial keratitis, after DSAEK. MRSA keratitis can occur following uneventful DSAEK. The empiric therapy on the basis of results from a light microscopic examination of a Gram-stained corneal scraping and restarting topical steroids in the early stages of medication contributed to the good clinical course of this case.
Key words: Descemet's stripping automated endothelial keratoplasty, Methicillin-resistant Staphylococcus aureus, Keratitis
Introduction
Descemet's stripping automated endothelial keratoplasty (DSAEK) has become the main keratoplasty procedure for bullous keratopathy worldwide, because it has several advantages compared with conventional penetrating keratoplasty, such as less postoperative astigmatism, decreased frequency of rejection, and no possibility of suture-related corneal infections. Although most reports have ascribed postoperative complications to endothelial graft detachment and rejection, several recent articles have reported infectious keratitis, mostly due to fungal infection, following DSAEK. To our knowledge, there is only one article by Sharma et al. [1] on bacterial keratitis following DSAEK. Herein, we report the first case of MRSA keratitis, and the second case of bacterial keratitis, following DSAEK.
Case Report
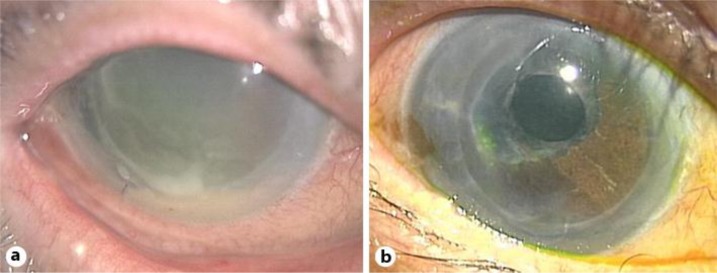
An 87-year-old woman who had undergone a DSAEK 4 months previously and received topical steroids as one of the perioperative medications presented to a medical practitioner with discharge and conjunctival hyperemia of the right eye. During the period between 3 days before surgery and 1 month after surgery, topical gatifloxacin ophthalmic solution (Gatiflo® 0.5% ophthalmic solution, Senju Pharmaceutical, Co., Ltd., Osaka, Japan) had been administered 4 times daily. The topical steroids were withheld and she was referred to Tokushima University Hospital with a diagnosis of infectious keratitis after DSAEK. At the first visit, slit-lamp microscopy revealed a white abscess and infiltration in the inferior cornea of the right eye (fig. 1a). Examination of a cross-section of the infected cornea on anterior-segment optical coherence tomography suggested that the abscess was located only in the recipient cornea (fig. 2). The best-corrected visual acuity (BCVA) was hand motion. A corneal scraping revealed Gram-positive cocci, and empiric therapy was started with frequent administration of both 1.5% levofloxacin (LVFX; Cravit® 1.5% ophthalmic solution, Santen Pharmaceutical, Co., Ltd., Osaka, Japan) and 0.25% chloramphenicol with 100,000 U/ml of colistin sodium methanesulfonate ophthalmic solutions (CP/CL; Ophthalon® ophthalmic solution, Wakamoto, Co., Ltd., Tokyo, Japan). Methicillin-resistant Staphylococcus aureus (MRSA) was isolated from the corneal scraping. Although the strain of MRSA was resistant to quinolone, it was susceptible to chloramphenicol (table 1). Since the empiric treatment worked and minimal inhibitory concentrations (MICs) were determined for systemic administration, the treatment was continued. The corneal infectious lesion rapidly decreased in size, allowing resumption of topical steroids 6 days after initiation of the empiric therapy. Although the corneal endothelial cell densities before and after medication were 2,193 and 813 cells/mm2, respectively, both the endothelial graft and the recipient cornea were thin and clear (fig. 1b). The BCVA improved to 6/20. The infection has not recurred so far.
Fig. 1.
Anterior segment photographs before and after medication. a Marked conjunctival hyperemia and corneal edema together with a small abscess in the inferior cornea and hypopyon are shown. b The cornea is clear and the infection did not recur.
Fig. 2.
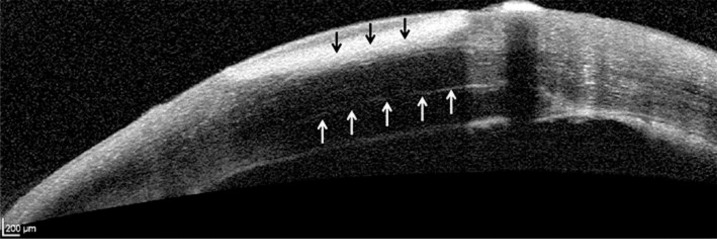
Cross-sectional image of the infected cornea on optical coherence tomography. The abscess is located only in the recipient cornea. White arrows indicate host-graft interface. Black arrows indicate the abscess.
Table 1.
MICs and drug susceptibilities of isolated MRSA
| Antibiotics | MIC, μg/ml | Susceptibility |
|---|---|---|
| Penicillin G | 64 | resistant |
| Oxacillin | 64 | resistant |
| Ampicillin | 64 | resistant |
| Ampicillin/sulbactam | 64 | resistant |
| Amoxicillin/clavulanic acid | 64 | resistant |
| Cefazolin | 256 | resistant |
| Cefpirome | 256 | resistant |
| Cefozopran | 32 | resistant |
| Cefdinir | 16 | resistant |
| Cefditoren pivoxil | 16 | resistant |
| Flomoxef | 32 | resistant |
| Imipenem/cilastatin | 32 | resistant |
| Meropenem | 32 | resistant |
| Gentamicin | 64 | resistant |
| Amikacin | 16 | susceptible |
| Arbekacin | 4 | susceptible |
| Erythromycin | >256 | resistant |
| Clarithromycin | 4 | resistant |
| Clindamycin | 4 | resistant |
| Minocycline | 16 | resistant |
| Vancomycin | 2 | susceptible |
| Teicoplanin | 2 | susceptible |
| Fosfomycin | >256 | resistant |
| Levofloxacin | >256 | resistant |
| Sulfamethoxazole-trimethoprim | 2 | susceptible |
| Rifampicin | 1 | susceptible |
| Linezolid | 2 | susceptible |
| Chloramphenicol | 4 | susceptible |
Discussion
In the report by Sharma et al. [1], keratitis caused by Staphylococcus aureus occurred 5 weeks postoperatively. The localization of the corneal abscess resembled that of our patient and was limited within the recipient cornea. In contrast to our patient, the keratitis of their case did not resolve and penetrating keratoplasty was needed because of corneal perforation. We propose the following factors as an explanation of the difference in post-medication courses between their patient and ours. First, the corneal abscess might have been smaller in our case. Second, we selected high-concentration LVFX and CP/CL for an empiric therapy on the basis of light microscopic findings of the corneal smear, while Sharma et al. [1] used topical cephem and aminoglycoside. In developed countries, elderly patients with a history of hospitalization or repeated visits to hospitals are likely to be infected by MRSA as a nosocomial infection [2, 3]. Therefore, we considered that an empiric therapy for infectious keratitis in an elderly patient should cover MRSA.
The isolated strain of MRSA was resistant to quinolone, but we hypothesize that 1.5% LVFX had a bactericidal effect because the concentration in the cornea might have exceeded the MIC [4] or reached the mutant prevention concentration. With regard to CP/CL, Fukuda et al. [3] reported that most clinical isolates of MRSA from ophthalmic samples in elderly patients were susceptible to chloramphenicol. The abovementioned factors suggest that our empiric therapy resulted in bactericidal effects on the MRSA strain in the very early stages of medication.
We consider that the intensive therapy prevented a recurrence of the infection even though topical steroids were restarted earlier than usual in cases of infectious keratitis following keratoplasty. Early resumption of topical steroids might have helped minimize endothelial damage due to cessation of steroids in the comparatively early postoperative stage of DSAEK and severe inflammation in the anterior chamber.
In conclusion, MRSA keratitis can occur following uneventful DSAEK. Epidemiological data and results of a light microscopic examination of a Gram-stained corneal scraping should be considered in determining empiric therapy. In terms of medication, frequent application of topical chloramphenicol is effective. High-concentration quinolone may also be effective. Restarting topical steroids in the early stages of medication contributes to a good clinical course. However, restarting topical steroids in bacterial keratitis following DSAEK is advisable only in cases in which empiric therapy covers pathogens.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Sharma N, Agarwal PC, Kumar CS, Mannan R, Titiyal JS. Microbial keratitis after Descemet stripping automated endothelial keratoplasty. Eye Contact Lens. 2011;37:320–322. doi: 10.1097/ICL.0b013e31820e7144. [DOI] [PubMed] [Google Scholar]
- 2.Zilberbeng MD, Chaudhari P, Nathanson BH, Campbell RS, Emons MF, Fiske S, Hays HD, Shorr AF. Development and validation of a bedside risk score for MRSA among patients hospitalized with complicated skin and skin structure infections. BMC Infect Dis. 2012;12:154. doi: 10.1186/1471-2334-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda M, Ohashi H, Matsumoto C, Mishima S, Shimomura Y. Methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococcus ocular surface infection efficacy of chloramphenicol eye drops. Cornea. 2002;21:S86–S89. doi: 10.1097/01.ico.0000263125.99262.42. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M, Yamada M, Kinoshita S, Inatomi T, Ohashi Y, Uno T, Shimazaki J, Satake Y, Maeda N, Hori Y, Nishida K, Kubota A, Nakawawa T, Shimomura Y. Comparison of corneal and aqueous humor penetration of moxifloxacin, gatifloxacin, and levofloxacin during keratoplasty. Adv Ther. 2012;29:339–349. doi: 10.1007/s12325-012-0016-x. [DOI] [PubMed] [Google Scholar]