Abstract
Antipsychotic drugs provide limited efficacy for cognitive impairment in schizophrenia. Recent studies have found that the neurotensin NTS1 receptor agonist and putative atypical antipsychotic drug PD149163 reverses deficits in sensory-gating and novel object recognition, suggesting that this compound may have the potential to improve cognitive functioning in schizophrenia. The present study sought to extend these investigations by evaluating the effects of PD149163 on sustained attention using a visual signal detection operant task in rats. PD149163, the atypical antipsychotic drug clozapine, and the dopamine D2/3 receptor antagonist raclopride all significantly decreased percent “hit” accuracy, while none of these compounds altered “correct rejections” (compared to vehicle control). Clozapine and raclopride significantly increased response latency, while high doses of PD149163 and raclopride significantly increased trial omissions. Nicotine, which was tested as a positive control, significantly improved overall performance in this task and did not affect response latency or trial omissions. The present findings suggest that neurotensin NTS1 receptor agonists, like antipsychotic drugs, may inhibit sustained attention in this task despite having different pharmacological mechanisms of action.
Keywords: neurotensin, NTS1 receptor, PD149163, antipsychotic, attention, visual signal detection
1. Introduction
Neurocognitive impairments represent a core feature of schizophrenia (Heinrich and Zakzanis, 1998; Keefe et al. 2007) and are related to functional outcomes, such as an ability to gain employment or perform daily living activities (Green, 1996; Kaneda et al. 2010). Only modest cognitive gains are found from atypical antipsychotic drugs and these gains generally do not provide adequate improvements in functional outcomes (Woodward et al. 2005; Woodward et al. 2006). Given that currently available atypical antipsychotic drugs share a similar pharmacological profile (Kantrowitz et al. 2012; Schotte et al. 1996), novel pharmacological strategies have been pursued in an effort to develop antipsychotic drugs that engender suitable gains in cognitive functioning.
The development of brain-penetrant agonists for neurotensin NTS1 receptors (Cusack et al. 2000; Hadden et al. 2005; Wustrow et al. 2005) has led to one such novel approach for treating schizophrenia. This approach originates from early behavioral studies finding that intracerebroventricular administration of this neuropeptide neurotransmitter exhibited antipsychotic-like effects in behavioral models (Nemeroff, 1980) and from a series of neuropharmacological studies that identified interactions between neurotensin and dopamine, including the synthesis and release of neurotensin from mesocorticolimbic and nigrostrial dopamine neurons and the inhibition of dopamine D2 receptor binding and signaling by co-localized neurotensin NTS1 receptors (for reviews, see Binder et al. 2001; St-Galais et al. 2006).
The neurotensin NTS1 receptor agonists that have been most studied for antipsychotic efficacy are NT69L and PD149163. In general, acute administration of these agonists lead to antipsychotic drug-like effects, such as reversal of psychostimulant-induced behaviors (e.g., hyperactivity, rearing, and climbing) (Boules et al. 2001; Cusack et al., 2000) and inhibition of conditioned avoidance responding (Hertel et al. 2002, Holly et al. 2011) in rodents. Both compounds also fail to elicit catalepsy in rats (Cusack et al. 2000; Feifel et al. 2004; Holly et al. 2011), suggesting that they likely engender atypical, rather than typical, antipsychotic-like effects (Meltzer, 2004).
Recent studies have assessed the cognitive efficacy of neurotensin NTS1 receptor agonists. Both NT69L (Briody et al., 2010; Shilling et al. 2003) and PD149163 (Feifel et al. 1999; Feifel et al 2008) have attenuated psychotomimetic-induced deficits in prepulse inhibition, a putative model of sensory-gating deficiency in schizophrenia. Improvements in memory have been inferred from the ability of PD149163 to reverse memory deficits in vasopressin-deficient Brattleboro rats using a social discrimination paradigm (Feifel et al., 2009) and from the ability of centrally administered PD149163 to reverse scopolamine-induced deficits in novel object recognition (Azmi et al., 2006). Systemic administration of PD149163 has also improved conditioning in an aversive trace conditioning task (Grimond-Billa et al., 2008). However, no studies have yet to examine the effects that a neurotensin NTS1 receptor agonist may have on attention.
In the present study, the highly selective neurotensin NTS1 receptor agonist PD149163 (Petrie et al. 2004) was selected to evaluate the effects of NTS1 receptor activation on attention using a visual signal detection operant task. This task was chosen because it has been extensively used to study attention in rats, including studies that have assessed the effects antipsychotic drugs (Rezvani et al, 2008; Rezvani et al. 2004). In addition to testing PD149163, the dopamine D2/3 receptor antagonist and typical antipsychotic drug raclopride, a drug with relatively high selectivity for dopamine receptors compared to other monoaminergic receptors (Roth et al. 1994), and the atypical antipsychotic drug clozapine, which is commonly used as a representative for atypical antipsychotic drugs in pharmacological studies (Meltzer, 2013), were also studied in order to compare PD149163 to a typical and atypical antipsychotic drug. Finally, nicotine was tested as a positive control, based upon previous studies showing that nicotine improves performance in this task (e.g., Rezvani et al. 2002).
2. Material and methods
2.1. Subjects
Twelve adult, male Sprague-Dawley rats (Charles River, Portage, MI, USA) were individually housed in a temperature- and humidity-controlled vivarium kept on a 12-hour light/dark cycle (lights on at 0700 hours). All testing and training sessions occurred during the light cycle between 1100 and 1400 hours. All rats were given restricted access to food in order to maintain 85% of their ad libitum weights. Rats had free access to water in their home cages. All procedures were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at Northern Michigan University.
2.2. Apparatus
Rats were trained and tested in six identical operant chambers enclosed within a sound attenuating cabinet equipped with a fan for ventilation and masking noise (Med-Associates Inc., St. Albans. VT, USA). Each operant chamber contained a signal light, a house light, two retractable levers, and a food pellet dispenser. The levers were located on the front panel, equidistant from the signal light, which was positioned at the center of the wall above the food receptacle. Signal light intensity was adjusted by using a fader control that allowed for four different illumination levels (ENV-226A, Med-Associates), and background and signal illuminations were calibrated using a light meter (CEM, DT-1301, Metershack, Saratoga, CA, USA). Data were collected using MedPC version 4.0 (Med-Associates Inc.).
2.3. Drugs
The neurotensin NTS1 receptor agonist PD149163 (NIMH Drug Repository, Bethesda, MD, USA), the selective dopamine D2/3 receptor antagonist raclopride L-tartrate (Sigma-Aldrich, St. Louis, MO, USA), and the nicotinic receptor agonist (-)nicotine tartrate (Sigma-Aldrich) were dissolved in 0.9% physiological saline. The atypical antipsychotic clozapine (NIMH Drug Repository) was dissolved in sterile water with the aid of 1-2 drops of 85% lactic acid. Raclopride and nicotine were in salt form. All of the drugs were administered subcutaneously in a volume of 1.0 ml/kg. PD149163, raclopride, and clozapine were administered 30 minutes prior to each testing session, whereas nicotine was administered 10 minutes prior to testing sessions.
2.4. Visual Signal Detection Procedures
2.4.1. Training
After habituation to the study environment and completion of lever press training, rats were trained according to procedures adapted from previously published studies (e.g. Bushnell, 1999; Rezvani and Levin 2004; Rezvani et al. 2008). Lever assignments (i.e. blank and signal levers) were counter-balanced across animals. Sessions were conducted daily and consisted of 196 trials. Figure 1 shows the events for each trial. With the exception of a “timeout” period, described below, a chamber's house light and signal light remained on at all times. During blank conditions and intertrial periods, the signal light was kept at a low illumination, which provided, along with the house light, a background illumination of 10 lux.
Figure 1.
The compound structures of the neurotensin NTS1 receptor agonist PD149163 (left), atypical antipsychotic drug clozapine (center), and dopamine D2/3 antagonist and typical antipsychotic drug raclopride (right).
Each trial began with a “pre-signal” time delay of 1, 8, 16, or 24 s selected in random order. After a delay ended, either a “blank” or “signal” occurred for 300 ms. A blank consisted of no change in stimulus light illumination during the 300 ms period, while a signal consisted of an increased signal light intensity that provided for a 2.7 lux increase above background illumination for the 300 ms period. After a blank or signal period ended, a 2-4 s “post signal” interval commenced, which ended upon the extension of both levers. If a rat pressed the signal-lever after a signal occurred, then this was recorded as a hit. If a rat pressed the “blank” lever after no signal occurred, then this was recorded as a correct rejection. A correct response caused the delivery of a food pellet, whereas an incorrect response led to a “timeout” period, which consisted of deactivating both the house light and signal light for 2 s. If a lever response failed to occur within 5 s, then this was counted as a trial omission and a timeout period occurred. Levers retracted after a lever press or upon a trial omission. The training criteria were met when a rat achieved 75% or greater hits and correct rejections at a 1 s pre-signal delay for 2 out of 3 consecutive sessions.
2.4.2. Testing
Test sessions were identical to training sessions, including the distribution of pre-signal delays, except that three signal intensities (0.9, 1.8, and 2.7 lux) were used, rather than only the 2.7 lux intensity. Test sessions consisted of 96 blank trials, and 32 trials for each signal intensity. Once the training criterion was met, test sessions occurred twice a week with at least 2 days separating each test. A training session was conducted on the day immediately preceding a test session. After completing a dose response curve for a drug, there was a seven day washout period before testing the next drug. The order of drug testing began with assessments of PD149163 and clozapine, with half of the rats tested with PD149163 first and the remaining half tested with clozapine first. Tests were conducted next with raclopride and finally with nicotine.
2.5. Data analysis
The following dependent variables were used: 1) percent hits, 2) percent correct rejections, 3) response latency, and 4) response omissions. Percent hits were calculated by dividing the number of correct responses on signal trials by the number of signal trials completed, and then multiplying this value by 100. Percent correct rejections were calculated by dividing the number of correct responses on blank trials by the number of blank trials where a response occurred and then multiplying this value by 100. Response latency was defined as the total time elapsed from when the levers were extended to when a lever press occurred, divided by the total number of signal and blank trials completed. Response omissions were defined as total number of trials where no response occurred (collapsed across signal and blank trials). All data were reported as means +/- the standard error of the mean (SEM).
A two-factor repeated measures analysis of variance (ANOVA) test was conducted using light intensity as one factor and dose as a second factor for percent hits for each drug tested. The effect of dose on percent correct rejections, response latency, or trial omissions was assessed using a one-way repeated measures ANOVA. In addition, a two-factor repeated measures ANOVA was conducted to determine if differences were found in percent hit accuracy occurred between vehicle controls tests for each drug assessed in this study. This analysis used each drug's vehicle as different levels of the first factor and light intensity level as the second factor. Finally, a one-way repeated measures ANOVA was conducted to determine if differences were found for percent correct rejections, response latency, or trial omissions between vehicle control tests for each drug studied. Occasionally a dose was eliminated from these analyses if it caused a significant number of trial omissions. Fisher's LSD post hoc tests were conducted when appropriate. All statistical analyses were conducted using the GraphPad Prism 6.0 for Windows (La Jolla, CA, USA).
3. Results
3.1. Training and Baseline Performance
Eight of the twelve rats met criterion in 61.88 +/- 4.52 (mean +/- SEM) training sessions. The remaining four rats were removed from the study after failing to meet the training criteria within 85 trials, which were at least 2 standard deviations beyond the mean days to criterion for successfully trained rats. A two-factor repeated measures ANOVA using “drug vehicle” and “light intensity” as factors did not reveal statistically significant effects for either drug vehicle or an interaction between drug vehicle and light intensity. However, as noted below for all drugs tested, a significant main effect for light intensity was shown (F2,14 = 83.97, P < 0.001; data not shown). Moreover, a one-way ANOVA did not reveal significant differences between vehicle controls on percent correct rejections, omissions, or response latency (data not shown).
3.2. Percent hits
Percent hits for PD149163, clozapine, and raclopride are shown in figure 3. The 0.125 mg/kg dose of PD149163 caused a significant number of trial omissions, which precluded its inclusion in this analysis (see section 3.4 below). PD149163 (0.0156, 0.0312, and 0.0625 mg/kg; top-left panel) produced a significant main effect of dose (F3, 21 = 3.26, P < 0.05), which occurred at as a significant decrease at a 0.0625 mg/kg dose versus vehicle, and a significant main effect of light intensity (F2,14 = 105.20, P < 0.001), which was due to significant increases in percent hits following each increase (i.e., 0.9, 1.8, and 2.7 lux) in illumination. There was not a significant interaction effect. Clozapine (0.625, 1.25, and 2.5 mg/kg; middle-left panel) produced a significant main effect of dose (F3,21 = 16.63, P < 0.001), which resulted from significantly dose-dependent decrease in percent hits at a 1.25 or 2.5 mg/kg dose. Percent hits significantly improved for each increase in illumination (F(2,14) = 63.60, P<0.001), and there was not a significant interaction between these factors. Raclopride (0.025, 0.05, and 0.1 mg/kg; bottom-left) produced a significant main effect of dose (F3,21 = 5.58, P < 0.01), which occurred as a decrease in percent hits at a 0.05 and 0.1 mg/kg dose versus vehicle. Again, percent hits increased for each increment in light intensity (F2, 14 = 19.08, P < 0.001), and no interaction effect was found. The 0.2 mg/kg dose of raclopride produced a significant number of trial omissions, which precluded including this dose for this analysis (see section 3.4 below).
Figure 3.
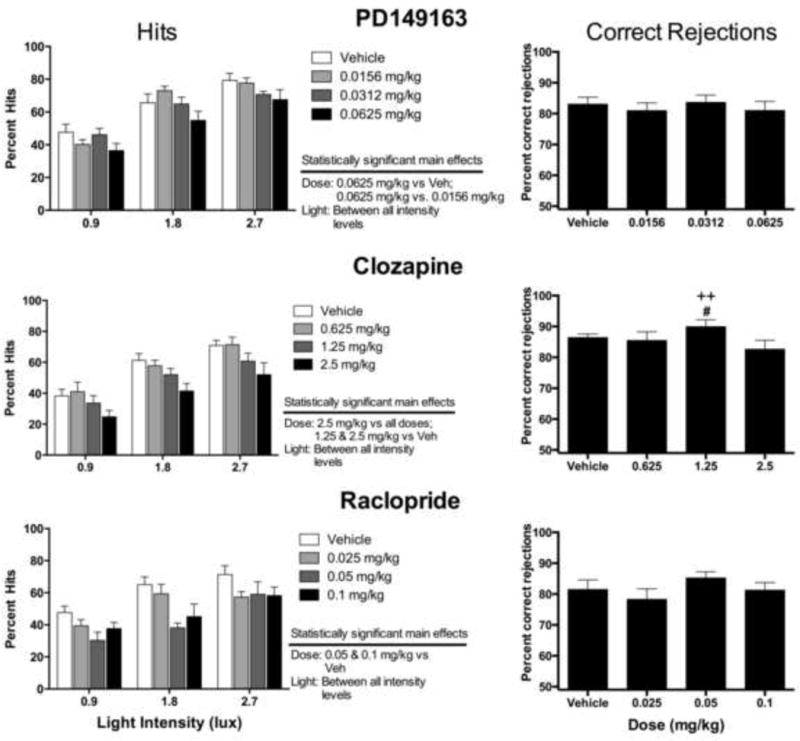
The effects of PD149163 (top), clozapine (middle), or raclopride (bottom) on percent hits (left panels) and percent correct rejections (right panels). Statistically significant main effects of dose for percent hits are noted. #P<0.05 versus 0.625mg/kg; ++P<0.01 versus 2.5 mg/kg. See figure 2 for a description of these procedures.
Percent hits for nicotine (0.05, 0.1, and 0.2 mg/kg) are shown in figure 4, left panel. Nicotine produced a significant main effect of dose (F3,21 = 7.31, P < 0.01), which occurred as an increase in percent hits at a 0.2 mg/kg dose versus vehicle and all other doses tested, and a significant main effect of light intensity (F2,14 = 26.44, P < 0.001), which occurred as an increase in percent hits for each elevation in illumination. Again, no the interaction effect was revealed between these factors.
Figure 4.
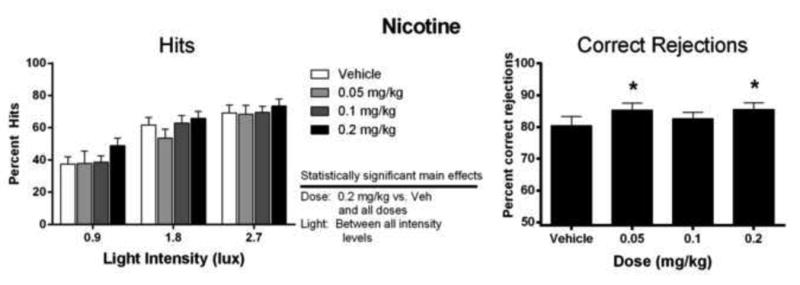
The effects of nicotine on correct percent hits (left) and percent correct rejections (right). Statistically significant main effects of dose for percent hits are noted. *P<0.05 versus vehicle.
3.3. Percent correct rejections
Percent correct rejections for PD149163, clozapine, and raclopride are shown in figure 3. PD149163 (0.0156, 0.0312, and 0.0625 mg/kg) failed to alter percent correct rejections (top-right panel). Clozapine (0.625, 1.25, and 2.5 mg/kg) produced a modest, but significant effect on correct rejections (F3,21 = 4.22, P < 0.05). The 0.625 and 2.5 mg/kg clozapine dose produced a significant decrease in percent correct rejection compared to the 1.25 mg/kg dose of clozapine; however these doses were not significantly different from vehicle control (middle-right panel). Raclopride (0.025, 0.05, and 0.1 mg/kg) failed to alter percent correct rejections (bottom-right). However, nicotine (0.05, 0.1, and 0.2 mg/kg; figure 4, right panel) significantly increased percent correct rejections (F3,21 = 7.31, P < 0.01), which occurred at a 0.05 mg/kg and a 0.2 mg/kg dose compared to vehicle control.
3.4. Response omissions
All response omission data are shown in table 1. PD149163 significantly increased response omissions (F4,28 = 3.47, P < 0.05), which occurred at a 0.125 mg/kg dose compared to vehicle. None of the doses of clozapine significantly increased omissions. The highest dose of raclopride, 0.2 mg/kg, significantly increased response omissions compared to vehicle and all other doses tested (F3,21 = 8.56, P < 0.001). Nicotine failed to significantly alter trial omissions.
Table 1. Response omissions and response latency data.
| Ligand | Dose | Omissions | Response Latency (ms) |
|---|---|---|---|
| PD149163 | Vehicle | 0 | 518 (±46) |
| 0.0156 | 0.13 (±0.13) | 571 (±56) | |
| 0.0312 | 0.25 (±0.25) | 563 (±42) | |
| 0.0625 | 3.38 (±3.23) | 653 (±67) | |
| 0.125 | 30.38 (±16.26)a | N/A | |
|
| |||
| Clozapine | Vehicle | 0.38 (±0.38) | 606(±43) |
| 0.625 | 0.38 (±0.38) | 533 (±54) | |
| 1.25 | 0.75 (±0.25) | 615 (±48) | |
| 2.5 | 3.00 (±1.45) | 746 (±74)c | |
|
| |||
| Raclopride | Vehicle | 1.00(±0.46) | 565 (±64) |
| 0.025 | 3.75 (±1.35) | 792 (±77) a | |
| 0.05 | 12.25 (±11.26) | 805 (±117)a | |
| 0.1 | 20.75 (±17.58) | 871(±121)b | |
| 0.2 | 98.88 (±26.06)d | N/A | |
|
| |||
| Nicotine | Vehicle | 0.38 (±0.38) | 554 (±55) |
| 0.5 | 0.13 (±0.13) | 564 (±61) | |
| 0.1 | 1.00 (±0.33) | 604 (±54) | |
| 0.2 | 0.50 (±0.27) | 614 (±77) | |
P<0.05,
P<0.01 vs vehicle;
P<0.01,
P<0.001 vs all doses (including vehicle)
3.5. Response latency
Response latency data are shown in table 1. PD149163 did not significantly affect response latency. Clozapine significantly increased response latency (F3,21 = 8.92, P < 0.001), which occurred at a 2.5 mg/kg dose compared to vehicle and all other doses of clozapine. Raclopride also significantly increased response latency (F3,21 = 4.91, P < 0.01), which occurred at a 0.1 mg/kg dose compared to all doses tested (including vehicle). Nicotine failed to significantly alter response latencies.
4. Discussion
To our knowledge, this study provides the first reported findings with a neurotensin NT1 receptor agonist using a signal detection task. In this study, the neurotensin NTS1 receptor agonist PD149163, the atypical antipsychotic drug clozapine, and dopamine D2/3 receptor antagonist and typical antipsychotic drug raclopride all significantly decreased the ability to detect changes in signal illumination. As reported in previous studies (Rezvani et al. 2002; Rezvani et al. 2005), nicotine improved both percent hits and correct rejections, although these effects were modest.
Neither typical nor atypical antipsychotic drugs have been shown to improve attention performance in this task using normal animals, but both classes generally produce reductions in percent hits. For example, the typical antipsychotic drug haloperidol and the atypical antipsychotic drugs clozapine and risperidone have been reported to decrease percent hits (Martinez and Sarter, 2008; Rezvani and Levin, 2004; Rezvani et al. 2008). However, clozapine has been shown to reduce signal detection deficits produced by repeated-amphetamine exposure or acute MK-801 administration (Martinez and Sarter, 2008; Rezvani et al. 2008). Occasional differences between the present and previous studies were observed between antipsychotic drugs for percent correct rejections. Rezvani and Levin (2004) reported a significant decrease in percent correct rejections after administration of a 2.5 mg/kg dose of clozapine, while no changes in percent correct rejections were observed for risperidone. In the present study, the 0.625 and 2.5 mg/kg dose of clozapine were found to significantly differ from the 1.25 mg/kg dose of clozapine; however, these doses were not found to be significantly different from vehicle control.
The results from Rezvani and Levin (2004) using the typical antipsychotic haloperidol were similar to the present results with raclopride. In this previous study, haloperidol produced a significant decrease in percent correct hits as well as a trend toward a significant decrease in percent correct rejections. Raclopride has also been shown to decrease correct responses and increase response omissions in the five choice serial reaction time test in rats (Shoaib and Bizarro, 2005).
In their work with antipsychotic drugs using the visual signal detection task, Rezvani and Levin (2004) suggested that dopamine D2 receptor blockade by antipsychotic drugs may account for reductions in percent hits. These suggestions are supported by the ability of the dopamine D2 receptor antagonists sulpiride (Harrison et al. 1997) and raclopride (Shoaib et al. 2001) to impair attention accuracy in five choice serial reaction time tasks. Further, dopamine D2 receptor knock-out mice exhibit impairments in attention (Glickstein et al. 2004), and in healthy human volunteers, an acute administration of 2.0 mg haloperidol impairs the ability to attend to changes in auditory cues (Kähkönen et al. 2001).
The present study may support this hypothesis, in that both clozapine and raclopride produce antagonism of dopamine D2 receptors, albeit with different affinities. Raclopride acts primarily as an antagonist at dopamine D2 and D3 receptors (Platania et al. 2012; Strange, 2001). Clozapine exhibits a weaker affinity for dopamine D2 receptors than raclopride and most other typical antipsychotic drugs, but also exhibits broad monoaminergic receptor antagonism that at least involves a preferential affinity for serotonin 5-HT2A receptors over dopamine D2 receptors (Schotte et al. 1996). PD149163 differs from clozapine and raclopride through acting as a highly selective agonist for neurotensin NTS1 receptors (Petrie et al. 2004), but may functionally exhibit antagonist-like pharmacological actions at dopamine D2 receptors, which are likely co-localized with D2 receptors on dopamine axonal terminals in the striatum, limbic system, and prefrontal cortex (Binder et al. 2001; Werkman et al. 2000). As noted in the introduction, the activation of neurotensin NTS1 receptors that are co-localized with dopamine D2-type receptors may diminish the ability of dopamine D2 receptors to bind to dopamine, which may occur through altering second messenger signaling cascades or direct receptor-receptor interactions (Binder et al. 2001). Thus, if PD149163, like neurotensin, causes a functional antagonism of dopamine D2 receptors, then this action may account for a decrease in percent hits observed in this study.
Given a relatively weak affinity of clozapine for dopamine D2 receptors, other receptor mechanisms for clozapine that may alter attention are worth mentioning. In particular, clozapine, unlike raclopride and PD149163, serves as a high-affinity antagonist for cholinergic muscarinic receptors (M1 – M4) (Arnt and Skarsfeldt; Bymaster et al., 1996; Bymaster et al., 2003). These affinities for muscarinic receptors are similar to those produced by the muscarinic receptor antagonist and cognitive disruptor scopolamine (Bolden et al., 1992), and clozapine shares discriminative stimulus effects with selective muscarinic receptor antagonists, including scopolamine and trihexyphenidyl (Kelley and Porter, 1997; Prus et al. 2004; Prus et al. 2005). Similar to the effect of clozapine on attention, scopolamine decreases hit accuracy in rats (Bushnell et al., 1997; McQuail and Burk, 2006; Mishima et al., 2002) and in mice (Dillion et al., 2009) in signal detection tasks. While clozapine also binds with a high affinity for serotonin 5-HT2 receptors, they likely fail to contribute to an impairment of attention in this task, given that the serotonin 5-HT2A/2B/2C receptor antagonist ketanserin failed to effect either percent correct hits or correct rejections in a study conducted by Rezvani et al. (2005).
In conclusion, the present study added to the cognitive profile of neurotensin NTS1 receptor agonists by examining the effects of the neurotensin NTS1 receptor agonist PD149163 using a standard model of attention in rats. PD149163 was generally comparable to the antipsychotic drugs studied, despite operating through a different receptor mechanism; however, prevention or inhibition of dopamine D2 receptor activation may account for their similar effects. Further studies evaluating the interplay between neurotensin receptors and monoaminergic transmission on attention and other cognitive domains may illuminate the common actions that neurotensin NTS1 receptor agonists and antipsychotic drugs may share. The failure of PD149163 to improve attention in this task, and in fact disrupt attention at the highest dose tested, suggests that PD149163 may have limited or deleterious effects on attention in schizophrenia. However, to better evaluate its treatment potential for cognitive impairment in schizophrenia, studies using deficit models that better approximate the neurocognitive deficits found in schizophrenia, including attention deficits induced by NMDA receptor antagonism (Rezvani et al. 2008), caused by repeated administration of amphetamine (Kondrad and Burk, 2004; Martinez and Sarter, 2008), or occurring in rats with innate cognitive deficits (e.g., Feifel et al. 2007; Feifel et al. 2011), are needed to better evaluate the validity of neurotensin NTS1 receptor agonism for this purpose.
Figure 2.
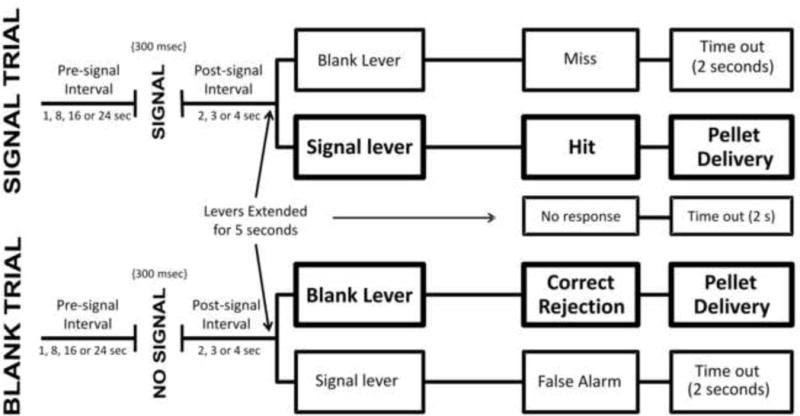
The procedures for either a signal trial (top), defined as a change in signal light intensity, or blank trial (bottom), defined as no change in signal light intensity, are shown. Both trial types started immediately following the completion of the previous trial. The signal trial (top) started with a pre-signal interval, followed by a brief increase in illumination of the signal light (i.e. signal). After the signal, there was a post-signal interval and then the levers were presented for the animal to emit a response. For signal trials, a correct response was defined as a “hit” and resulted in the delivery of food pellet, while an incorrect response was defined as a “miss” and resulted in a “timeout.” Blank trials (bottom) were identical to signal trials, except that no signal followed the pre-signal interval (i.e. no increase in illumination). For blank trials, a correct response was defined as a “correct rejection” and resulted in a food pellet, while an incorrect response was defined as a “false alarm” and resulted in a “timeout.” Response omissions resulted in a “timeout.”
Acknowledgments
The authors would like to thank Ashley Schmeling and Colette Armes for their technical assistance, as well as the NIMH Drug Repository for providing PD149163 and clozapine. The studies were supported by an NIH grant to AJP (1R15MH083241-01).
Source of funding: The study was funded by a NIH grant to AJP (1R15MH083241-01).
Footnotes
Conflicts of interest: There are no other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Azmi N, Norman C, Spicer CH, Bennett GW. Effects of a neurotensin analogue (PD149163) and antagonist (SR142948A) on the scopolamine-induced deficits in a novel object discrimination task. Behav Pharmacol. 2006;17:357–362. doi: 10.1097/01.fbp.0000224382.63744.20. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001;53:453–486. [PubMed] [Google Scholar]
- Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260:576–580. [PubMed] [Google Scholar]
- Boules M, Warrington L, Fauq A, McCormick D, Richelson E. A novel neurotensin analog blocks cocaine- and D-amphetamine-induced hyperactivity. Eur J Pharmacol. 2001;426:73–76. doi: 10.1016/s0014-2999(01)01197-9. [DOI] [PubMed] [Google Scholar]
- Briody S, Boules M, Oliveros A, Fauq I, Richelson E. Chronic NT69L potently prevents drug-induced disruption of prepulse inhibition without causing tolerance. Behav Brain Res. 2010;207:118–124. doi: 10.1016/j.bbr.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ. Detection of visual signals by rats: effects of signal intensity, event rate, and task type. Behav Processes. 1999;46:141–150. doi: 10.1016/s0376-6357(99)00030-3. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Padnos BK. Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacol. 1997;134:230–241. doi: 10.1007/s002130050446. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, McKinzie DL. Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Cusack B, Boules M, Tyler BM, Fauq A, McCormick DJ, Richelson E. Effects of a novel neurotensin peptide analog given extracranially on CNS behaviors mediated by apomorphine and haloperidol. Brain Res. 2000;856:48–54. doi: 10.1016/s0006-8993(99)02363-x. [DOI] [PubMed] [Google Scholar]
- Dillon GM, Shelton D, McKinney AP, Caniga M, Marcus JN, Ferguson MT, et al. Prefrontal cortex lesions and scopolamine impair attention performance of C57BL/6 mice in a novel 2-choice visual discrimination task. Behav Brain Res. 2009;204:67–76. doi: 10.1016/j.bbr.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Murray RJ, Tina-Tran DN, Rullan MA, Shilling PD. The reversal of amphetamine-induced locomotor activation by a selective neurotensin-1 receptor agonist does not exhibit tolerance. Psychopharmacol. 2008;200:197–203. doi: 10.1007/s00213-008-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;181:278–286. doi: 10.1016/j.bbr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacology. 2003;28:651–653. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29:731–738. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology. 2009;34:2011–2018. doi: 10.1038/npp.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. J Pharmacol Exp Ther. 1999;288:710–713. [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Melendez G. Clozapine and PD149163 elevate prepulse inhibition in Brown Norway rats. Behav Neurosci. 2011;125:268–272. doi: 10.1037/a0022691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein SB, Desteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb cortex. 2004;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Grimond-Billa SK, Norman C, GW B, Cassaday HJ. Selectively increased trace conditioning under the neurotensin agonist PD 149163 in an aversive procedure in which SR 142948A was without intrinsic effect. J Psychopharmacol. 2008;22:290–299. doi: 10.1177/0269881106081528. [DOI] [PubMed] [Google Scholar]
- Hadden MK, Orwig KS, Kokko KP, Mazella J, Dix TA. Design, synthesis, and evaluation of the antipsychotic potential of orally bioavailable neurotensin (8-13) analogues containing non-natural arginine and lysine residues. Neuropharmacology. 2005;49:1149–1159. doi: 10.1016/j.neuropharm.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Doubly dissociable effects of median- and dorsal-raphe lesions on the performance of the five-choice serial reaction time test of attention in rats. Behav Brain Res. 1997;89(1-2):135–149. doi: 10.1016/s0166-4328(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hermans E, Maloteaux JM. Mechanisms of regulation of neurotensin receptors. Pharmacol Ther. 1998;79:89–104. doi: 10.1016/s0163-7258(98)00009-6. [DOI] [PubMed] [Google Scholar]
- Hertel P, Olsen CK, Arnt J. Repeated administration of the neurotensin analogue NT69L induces tolerance to its suppressant effect on conditioned avoidance behaviour. Eur J Pharmacol. 2002;439:107–111. doi: 10.1016/s0014-2999(02)01414-0. [DOI] [PubMed] [Google Scholar]
- Holly EN, Ebrecht B, Prus AJ. The neurotensin-1 receptor agonist PD149163 inhibits conditioned avoidance responding without producing catalepsy in rats. European Neuropsychopharmacology. 2011;21:526–531. doi: 10.1016/j.euroneuro.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähkönen S, Ahveninen J, Jääskeläinen IP, Kaakkola S, Näätänen R, Huttunen J, et al. Effects of haloperidol on selective attention: a combined whole-head MEG and high-resolution EEG study. Neuropsychopharmacology. 2001;25:498–504. doi: 10.1016/S0893-133X(01)00255-X. [DOI] [PubMed] [Google Scholar]
- Kaneda Y, Jayathilak K, Meltzer H. Determinants of work outcome in neuroleptic- resistant schizophrenia and schizoaffective disorder: cognitive impairment and clozapine treatment. Psychiatry Res. 2010;178:57–62. doi: 10.1016/j.psychres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Citrome L. Lurasidone for schizophrenia: what's different? Expert Rev Neurother. 2012;12:265–273. doi: 10.1586/ern.12.7. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BM, Porter JH. The role of muscarinic cholinergic receptors in the discriminative stimulus properties of clozapine in rats. Pharmacol Biochem Behav. 1997;57:707–719. doi: 10.1016/s0091-3057(96)00342-5. [DOI] [PubMed] [Google Scholar]
- Kondrad R, Burk J. Transient disruption of attentional performance following escalating amphetamine administration in rats. Psychopharmacol. 2004;175:436–442. doi: 10.1007/s00213-004-1857-z. [DOI] [PubMed] [Google Scholar]
- Mazella J. Sortilin/neurotensin-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cell Signal. 2001;13:1–6. doi: 10.1016/s0898-6568(00)00130-3. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Burk JA. Evaluation of muscarinic and nicotinic receptor antagonists on attention and working memory. Pharmacol Biochem Behav. 2006;85:796–803. doi: 10.1016/j.pbb.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sarter M. Detection of the Moderately Beneficial Cognitive Effects of Low-Dose Treatment with Haloperidol or Clozapine in an Animal Model of the Attentional Impairments of Schizophrenia. Neuropsychopharmacology. 2008;33:2635–2647. doi: 10.1038/sj.npp.1301661. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. What's atypical about atypical antipsychotic drugs? Curr Opin Pharmacol. 2004;4:53–57. doi: 10.1016/j.coph.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Update on typical and atypical antipsychotic drugs. Ann Rev Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- Mishima K, Fujii M, Aoo N, Yoshikawa T, Fukue Y, Honda Y, et al. The Pharmacological Characterization of Attentional Processes Using a Two-lever Choice Reaction Time Task in Rats. Bio Pharm Bull. 2002;25:1570–1576. doi: 10.1248/bpb.25.1570. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurotensin: perchance an endogenous neuroleptic? Biol Psychiatry. 1980;15:283–302. [PubMed] [Google Scholar]
- Petri KA, Bubser M, Casey CD, Davis MD, Roth BL, Deutch AY. The neurotensin agonist PD149163 increases Fos expression in the prefrontal cortex of the rat. Neuropsychopharmacology. 2004;29:1878–1888. doi: 10.1038/sj.npp.1300494. [DOI] [PubMed] [Google Scholar]
- Platania CBM, Salomone S, Leggio GM, Drago F, Bucolo C. Homology Modeling of Dopamine D2 and D3 Receptors: Molecular Dynamics Refinement and Docking Evaluation. PLoS ONE. 2012;7:e44316. doi: 10.1371/journal.pone.0044316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, Baker LE, Meltzer HY. Discriminative stimulus properties of 1.25 and 5.0 mg/kg doses of clozapine in rats: examination of the role of dopamine, serotonin, and muscarinic receptor mechanisms. Pharmacol Biochem Behav. 2004;77:199–208. doi: 10.1016/j.pbb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Philibin SD, Pehrson AL, Stephens CL, Cooper RN, Wise LE, et al. Generalization testing with atypical and typical antipsychotic drugs in rats trained to discriminate 5.0 mg/kg clozapine from vehicle in a two-choice drug discrimination task. Drug Dev Res. 2005;64:55–65. [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, et al. Neuropsychological Function and Dysfunction in Schizophrenia and Psychotic Affective Disorders. Schizophr Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Bushnell PJ, Levin ED. Effects of nicotine and mecamylamine on choice accuracy in an operant visual signal detection task in female rats. Psychopharmacol. 2002;16:369–375. doi: 10.1007/s00213-002-1221-0. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Caldwell DP, Levin ED. Nicotinic–serotonergic drug interactions and attentional performance in rats. Psychopharmacol. 2005;179:521–528. doi: 10.1007/s00213-004-2060-y. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-antipsychotic drug interactions and attentional performance in female rats. Eur J Pharmacol. 2004;486:175–182. doi: 10.1016/j.ejphar.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Dawson E, Levin ED. Nicotine and clozapine effects on attentional performance impaired by the NMDA antagonist dizocilpine in female rats. Int J Neuropsychopharmacol. 2008;11:63–70. doi: 10.1017/S1461145706007528. [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5- hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharm Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacol. 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Richelson E, Feifel D. The effects of systemic NT69L, a neurotensin agonist, on baseline and drug-disrupted prepulse inhibition. Behav Brain Res. 2003;143:7–14. doi: 10.1016/s0166-4328(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacol. 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- St-Galais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: What is the evidence? J Psychatry Neurosci. 2006;31:229–245. [PMC free article] [PubMed] [Google Scholar]
- Strange PG. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev. 2001;53:119–133. [PubMed] [Google Scholar]
- Werkman TR, Kruse CG, Nievelstein H, Long SK, Wadman WJ. Neurotensin attenuates the quinpirole-induced inhibition of the firing rate of dopamine neurons in the rat substantia nigra pars compacta and the ventral tegmental area. Neuroscience. 2000;95:417–423. doi: 10.1016/s0306-4522(99)00449-2. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of cognitive change with haloperidol in clinical trials of atypical antipsychotics: dose effects and comparison to practice effects. Schizophr Res. 2006;89:211–224. doi: 10.1016/j.schres.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Wustrow DJ, Davis MD, Akunne HC, Corbin AE, Wiley JN, Wise LD, Heffner TG. Reduced amide bond neurotensin 8-13 mimetics with potent in vivo activity. Bioorg Med Chem Lett. 1995;5:997–1002. [Google Scholar]


