Abstract
Purpose of review
Individuals practicing unprotected receptive anal intercourse are at particularly high risk of HIV infection. Men who have sex with men (MSM) in the developed and developing world continue to have disproportionate and increasing levels of HIV infection. The last few years have seen important progress in demonstrating the efficacy of oral pre-exposure prophylaxis (PrEP), vaginal microbicides, and treatment as prevention but there has also been significant progress in the development of rectal microbicides (RM). The purpose of this review is to summarize the status of RM research and to identify opportunities, challenges, and future directions in this important field of HIV prevention.
Recent findings
Recent Phase 1 RM studies have characterized the safety, acceptability, compartmental pharmacokinetics (PK), and pharmacodynamics (PD) of both UC781 and tenofovir gels. The tenofovir gel formulation used in vaginal studies was not well tolerated in the rectum and newer rectal specific formulations have been developed and evaluated in Phase 1 studies.
Summary
Complex Phase 1 studies have provided important data on candidate RMs. Tenofovir gel is poised to move into Phase 2 evaluation and it is possible that a Phase 2B/3 effectiveness study could be initiated in the next 2–3 years.
Keywords: Rectal, microbicides, HIV prevention
Introduction
The field of vaginal microbicide (VM) development began as researchers in women’s health and contraception contemplated whether a virucidal gel could be developed that would have activity against sexually transmitted diseases including HIV-1 (1). Nonoxynol-9 (N9) had demonstrated these properties in laboratory experiments (2) and was rapidly advanced into effectiveness studies. In addition it was also evaluated as a potential RM (3;4). Unfortunately, N9 was not found to be satisfactory as a VM (5) and was not further developed as an RM. However, these early studies set the framework for how future microbicides were evaluated and emphasized the importance of characterizing safety and acceptability in Phase 1 studies. Following the early N9 studies, the field focused, without much success, on surfactant and polyanion VM candidates (6). During this period there was no research on specific RM. However it was clear that MSM were interested in the concept of RM and would be willing to enroll in clinical trials evaluating the safety and effectiveness of these products (7;8). It was also apparent that sexual lubricant use, a possible model for RM use, was common among MSM (9). Other important early studies included exploring product formulation preferences in MSM (10;11) and assessment of the acceptability of different volumes of rectal gels (12). The HPTN-056 study provided data on the variability of rectal mucosal safety parameters that might be measured in future RM studies (13) and set the stage for the first RM Phase 1 studies evaluating antiretroviral products.
Review
The biology of HIV infection associated with anal sex
Unprotected receptive anal intercourse (URAI) between HIV serodiscordant partners is an extremely efficient way of transmitting HIV infection and this risk is likely to be significantly increased in situations where one or both of the partners have STIs such as rectal gonorrhea or herpes simplex infection. In addition, the extremely high viral loads seen during primary HIV infection will further exacerbate the risk of HV transmission (14). As a consequence it is estimated that the risk of HIV associated with heterosexual URAI is 1.7% per act whereas it is only 0.08% during unprotected vaginal intercourse (15). This situation is partially due to the rectal environment. A single layer of columnar epithelium and a preponderance of activated CD4+ T cells expressing the CCR5 co-receptor (16) provide a uniquely vulnerable environment for HIV acquisition.
Epidemiology of HIV infection associated with URAI
Recent epidemiological studies have confirmed that URAI is a common practice among MSM in the Americas and Europe (17;18) but have also clearly identified at risk MSM in the developing world (19) as well as women across the globe (20;21). Unfortunately this prevalence of URAI also appears, at least in MSM, to be associated with high prevalence and incidence of HIV infection as well as other STIs. These observations emphasize the need to develop new prevention strategies, including RM, in populations at risk of HIV infection through URAI who are unable or unwilling to use a condom.
The RM pipeline
In theory, the RM pipeline could be derived from the VM pipeline. However the majority of RM candidates have only been evaluated in preclinical studies and the only products to have been evaluated in clinical studies are the non-nucleoside reverse transcriptase inhibitor (NNRTI) UC781 (22**) and the nucleotide inhibitor, tenofovir (23). The first generation of antiretroviral rectal microbicides only contained one active pharmaceutical ingredient (API) but there is increasing interest in evaluating combination products with two, three, or even four API (24;25*). The majority of research has been conducted on nucleotides (tenofovir) or NNRTIs (UC781 and dapivirine (TMC120)). However, recent studies have also evaluated L’644, a fusion inhibitor, that demonstrated post-exposure activity in both colorectal and cervical explants as well as activity against reverse transcriptase resistant isolates (26*). Protease inhibitors are also being considered as candidate RM (27*;28*). Potential RM candidates are listed in Table 1 together with their current stage of development.
Table 1.
Products that have been evaluated as candidate rectal microbicides*
| Product | Development Stage | Study | Status | ClinTrials.gov | Reference |
|---|---|---|---|---|---|
| Cellulose acetate PRO2000 SPL7013 Vena Gel UC781 |
Preclinical | Explant model | Completed | (29) | |
| PRO2000 Dextrin sulfate |
Preclinical | Explant model | Completed | (30) | |
| Tenofovir | Preclinical | Explant model | Completed | (31) | |
| C34 T20 T1249 L’644 |
Preclinical | Explant model | Completed | (26) | |
| Tenofovir Emtricitabine UC781 TMC120 |
Preclinical | Explant model | Completed | (25) | |
| Saquinavir | Preclinical | Explant model | Completed | (28) | |
| Maraviroc Griffithsin MIV-150 Carageenan Zinc acetate |
Preclinical | Explant model | Ongoing | ||
| BufferGel® Nonoxynol-9 C31G Octylglycerol Polystyrene sulfate Cellulose sulfate SPL7013 Carraguard UC781 |
Preclinical | NHP/safety | Completed | Summarized in (32) | |
| Cyanovirin gel | Preclinical | NHP/Efficacy | Completed | (33) | |
| Tenofovir gel | Preclinical | NHP/Efficacy | Completed | (34) | |
| MIV-150 gel | Preclinical | NHP/Efficacy | Completed | (35) | |
| UC781 gel | Phase 1 | RMP-01 | Completed | NCT00408538 | (22;36) |
| Tenofovir 1% gel (VF) | Phase 1 | RMP-02/MTN-006 | Completed | NCT00984971 | (23) |
| Tenofovir 1% gel (RGF) | Phase 1 | MTN-007 | Completed | NCT01232803 | (37) |
| Tenofovir 1% gel (RGF) | Phase 1 | Project Gel | Ongoing | NCT01283360 | N/A |
| Tenofovir 1% gel (VF, RGF, RF) | Phase 1 | CHARM-01 | Q3 2012 | NCT01575405 | N/A |
| Tenofovir 1% gel (VF, RGF, RF) | Phase 1 | CHARM-02 | Q3 2012 | NCT01575418 | N/A |
| Tenofovir 1% gel (RGF) | Phase 2 | MTN-017 | Q3 2012 | Pending | N/A |
Products that have been studied in colorectal explant systems, animal models, or humans
VF: vaginal formulation, RGF: reduced glycerin formulation, RF: rectal specific formulation
NHP: non-human primate
Formulation considerations
Qualitative and clinical studies have suggested that acceptable RM formulations could include gels, suppositories, or douches (10;11). Gels were considered more acceptable than suppositories (11) and a volume escalation of a placebo gel demonstrated that up to 35 mL of gel with the physical properties of Femglide® (transparent and odorless) was acceptable to the majority of participants (12). The first RM clinical trials evaluated the rectal safety of gels that were being developed as VM. These products had a number of characteristics that were suboptimal for an RM. They were extremely hyperosmolar and had an acid pH. Hyperosmolar products are known to induce mucosal damage (38;39*) and may be associated with increased risk of acquiring STIs (40*). Rectal use of the vaginal formulation of tenofovir 1% gel was associated with gastrointestinal adverse events including diarrhea, bloating, urgency, and abdominal pain (23). Consequently, efforts are under way to develop rectal specific microbicide formulations that are iso-osmolar with a neutral pH (41**;42). Initial preclinical evaluation of a reduced glycerin formulation of tenofovir (RG-TFV) 1% gel suggested that the new gel induced less mucosal damage than the original formulation but was equally effective in explant models of HIV infection (31**). The RG-TFV 1% also appeared to have improved in vivo safety and acceptability in Phase 1 studies (37).
Preclinical evaluation of rectal microbicides
The preclinical and clinical evaluation of candidate microbicides encompasses steps that are required by regulatory authorities such as the United States Food and Drug Administration (FDA) as well as guidelines developed by groups working within the field of microbicide development (43;44). The role of preclinical evaluation is to characterize product safety, stability, and effectiveness and to determine whether products should be advanced into Phase 1 clinical trials. Buckheit and Buckheit have recently published updated recommendations for the preclinical development of both rectal and vaginal microbicide candidates that places greater emphasis on evaluating products in the context of relevant biological fluids and in human tissue systems (45). In vitro colorectal explant models play an important role in evaluating the safety and efficacy of RM (29;30) and have been evaluated for use in multicenter studies (46). Non-human primate (NHP) models have also been used to evaluate product safety and efficacy (32;47). Cyanovirin, tenofovir, and the NNRTI MIV-150 have all protected against rectal challenge with SIV and SHIV (33–35**). In addition, a zinc acetate carageenan gel has been shown to protect against rectal challenge with herpes simplex virus (HSV)-2 (48). Humanized murine models of HIV infection (49) have been used to evaluate vaginal microbicides (50) and are also being used to evaluate rectal microbicides (Garcia-Martinez JW, personal communication). One note of caution has been raised about the suitability of tenofovir as an RM. GarcÍa-Lerma et al. recently reported results of a NHP study in which the animals received oral GS7340, a tenofovir prodrug, before rectal challenge with SHIVSF162P3. Despite achieving high systemic and mucosal levels of tenofovir, GS7340 did not afford protection from infection with SHIVSF162P3. One possible explanation was that endogenous dATP in rectal lymphocytes competed with, and reduced the antiviral efficacy of, the nucleotide reverse transcriptase inhibitor (51**). It remains to be seen whether the higher concentrations of tenofovir achieved through topical administration with an RM will be sufficient to circumvent this phenomenon.
Clinical development of rectal microbicides
As with VM, the purpose of Phase 1 RM studies is to generate preliminary data on the safety, acceptability, PK, and PD activity of the candidate microbicide. However, in contrast to VM development where there have been multiple Phase 1 studies of surfactant, polyanion, and antiretroviral candidates, there have only been four Phase 1 rectal microbicide studies conducted to date; HIVNET-008 (N9 gel) (3), RMP-01 (UC781 0.1 and 0.25% gel) (22;36), RMP-02/MTN-006 (oral tenofovir and tenofovir 1% gel (original formulation) (23)), and MTN-007 (N9 gel, HEC placebo gel (52), and tenofovir 1% gel (reduced glycerin formulation) (37). These studies are discussed in more detail below.
HIVNET-008
The HIVNET-008 study was designed to assess the safety of N9 when applied one to four times daily to the rectum and penis. Twenty five HIV-negative and ten HIV-positive, monogamous gay male couples were enrolled in Seattle, WA. Each partner was exclusively insertive or receptive while using N9 gel and served as his own control during placebo gel use compared to during N9 gel use. The study was conducted over 7 weeks. During the first week participants used the placebo gel. Thereafter, couples used the N9 gel and the frequency of use was escalated from once daily to two applicators twice daily in the final week of the study. Despite the frequency of administration, adverse events (AEs) were generally mild and transient. No rectal ulcers were detected; superficial rectal erosions were noted in two HIV-negative participants. Abnormal or slightly abnormal histologic abnormalities of rectal biopsies were detected in 31 (89%) of receptive participants after N9 gel use compared to 24 (69%) of participants after 1 week of placebo gel use. Excluding participants who felt no need for an HIV prevention method, 58% said they would use N9 if approved for rectal use; 69% of receptive users reported rectal fullness and related side effects after insertion of the gel, and 68% reported applicator-related discomfort; 59% of insertive participants found the gel too sticky (53).
RMP-01
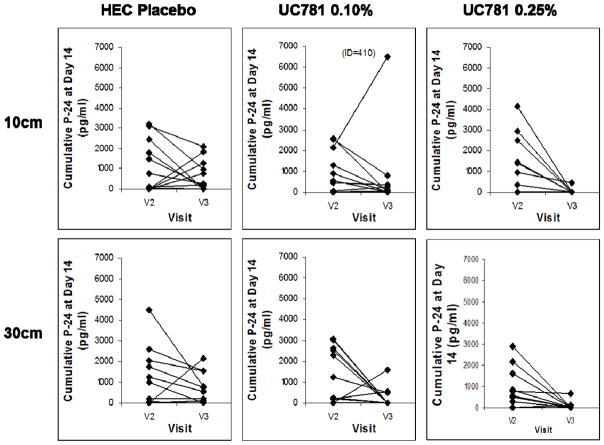
Thirty six HIV-1 seronegative, sexually-abstinent men and women were enrolled in Los Angeles, CA and randomized into a double-blind, placebo-controlled trial comparing UC781 gel at two concentrations (0.10%, 0.25%) with a placebo gel (1:1:1). Safety and acceptability were primary study endpoints. Changes in colorectal mucosal safety biomarkers and UC781 plasma drug levels were secondary endpoints. Ex vivo explant infectibility with HIV-1 was an ancillary study endpoint. Samples were collected at enrollment, after a single rectal dose of study product, and after seven daily doses. The majority of AEs were mild. Product acceptability was high, including likelihood of future use. No changes in mucosal safety biomarkers were identified. Plasma levels of UC781 were not detected. Ex vivo infection of biopsies using two titers of HIV-1BaL showed marked suppression of HIV-1 p24 in tissues exposed in vivo to 0.25% UC781 (Figure 1). Ideally the product would have been advanced into Phase 2 development but the IND sponsor (CONRAD; http://www.conrad.org/) terminated the UC781 development program.
Figure 1.
Suppression of HIV replication of HIV-1 in colorectal explants following in vivo application of UC781 gel or placebo gel
UC781 gel (0.10% or 0.25%) or placebo gel was applied rectally to a total of 36 participants (12 per study arm). After approximately 30 minutes colorectal tissue samples were collected at 10 or 30 cm from the anal margin. Colorectal explants were challenged with HIV-1BaL and explant supernatant collected every 3–4 days out to day 14. Cumulative day 14 HIV-1 p24 at Visit 2 (enrollment) and Visit 3 (single dose exposure) are presented here. Significant inhibition of viral replication was seen with the 0.25% gel concentration but not the 0.10% gel. Figure 1 adapted from Anton PA et al. (22).
RMP-02/MTN-006
Eighteen participants were enrolled from Pittsburgh, PA and Los Angeles, CA. All participants received a single 300mg dose of oral tenofovir and were then randomized 2:1 to receive a single then seven daily doses of tenofovir 1% gel or the HEC placebo gel. Safety endpoints included clinical AEs and mucosal safety biomarkers. Participants were assessed at enrollment, after single doses of oral tenofovir and study gel, and after seven daily doses of study gel. Blood and colonic biopsies were collected for PK analysis and ex vivo challenge with HIV-1. No serious AEs were reported. However, AEs, especially gastrointestinal AEs, were significantly increased with seven-day use of the tenofovir 1% gel. Only 25% of participants liked the tenofovir gel; however, likelihood of use, if the product was somewhat protective, was high (75%). No significant mucosal injury was detected. Tissue TFV diphosphate (TFV-DP) Cmax 30-minutes after single rectal exposure was 112-times greater than single oral-exposure with tissue 7-day exposure 5-times greater than single rectal-exposure. Seven-day exposure to rectal TFV was associated with significant suppression of explant infection. Increased AEs suggested that the vaginal formulation of tenofovir 1% gel used rectally was not entirely safe or fully acceptable, suggesting a need for improved formulations.
MTN-007
The study was designed to assess the safety, adherence, and acceptability of the reduced glycerin formulation of tenofovir 1% gel (RG-TFV 1% gel). An N9 arm was included as a positive control for the mucosal safety biomarker assays. Sixty-five participants (45 men and 20 women) aged 18–61 were recruited from Pittsburgh, PA; Boston, MA; and Birmingham, AL. Participants were randomized 1:1:1:1 to receive the RG-TFV 1% gel, a HEC placebo gel, an N9 gel, or to a no treatment arm. Participants were evaluated at Baseline, after a single dose, and after seven daily doses of study product. Systemic and mucosal safety, acceptability, and adherence were evaluated at all three visits. Comprehensive mucosal safety biomarker evaluation included histology, fecal calprotectin, epithelial sloughing, cytokine expression (mRNA and protein), microarray analysis, flow cytometry of mucosal T cell phenotype, and rectal microflora. Acceptability and adherence were determined by computer-administered questionnaires and interactive telephone response respectively. Product adherence was ≥ 94%. AEs were generally mild or moderate. There was no significant difference in the prevalence of AEs across the four arms of the study. Likelihood of future product use (acceptability) was 86.7% (RG-TFV 1% gel), 93.3% (HEC gel), and 62.5% (N9 gel). Fecal calprotectin and epithelial sloughing did not alter during the study. In contrast, significant changes were seen in mucosal cytokine/chemokine expression, T cell phenotype, and rectal microflora, which were mostly confined to the N9 gel arm. Overall the study suggested that the RG formulation of 1% tenofovir was safe and well tolerated and should be advanced to Phase 2 RM development.
A number of additional RM studies are ongoing or will start enrollment in 2012. The National Institutes of Health (NIH) has recently funded a project entitled “Microbicide safety and acceptability in young men” that attempts to evaluate RM safety, adherence, and acceptability in young ethnic minority MSM in Boston, MA; Pittsburgh, PA; and San Juan, PR. The study design has two stages: A clinical and behavioral evaluation (Stage 1A) with an acceptability and adherence trial (Stage 1B), followed by a Phase 1 randomized, double-blind, multi-site, placebo-controlled safety trial (Stage 2). The first 120 eligible participants who complete Stage 1A and report unprotected RAI in the previous 3 months will continue on to Stage 1B. During Stage 1B, participants will be given condoms and a placebo gel to use during receptive anal intercourse. Over a three month period they will report the frequency of product use and be interviewed about the acceptability of the product. The first 42 participants who complete Stage 1B with ≥ 80% adherence to product use will be eligible to participate in Stage 2 where they will be randomized to receive an actual microbicide (RG-TFV 1% gel) or matched placebo. It is hoped that data from this study will provide unique insights into the acceptability, safety, and adherence of rectal microbicides in young MSM.
The Combination HIV Antiretroviral Rectal Microbicide or CHARM Program will develop and evaluate a combination antiretroviral rectal specific product. Tenofovir and maraviroc are the two lead compounds and the ultimate goal is to develop a tenofovir/maraviroc combination product. Two Phase 1 studies, CHARM-01 and CHARM-02 will start in 2012. CHARM-01 will assess the safety, acceptability, and PK/PD profile of three tenofovir gel formulations; the original tenofovir 1% gel used in vaginal microbicide studies, the RG-TFV 1% gel, and a rectal specific TFV gel (Table 2). CHARM-02 will evaluate the safety, PK, and distribution of the same three gels. Similar techniques have been used to characterize the distribution of semen surrogates and microbicide products in the presence and absence of simulated receptive anal intercourse (54*;55**). Collectively, these studies will provide unique data on the influence of formulation characteristics, including osmolality, and product safety, PK/PD, and distribution. The final RM study to start in 2012 will be MTN-017, a Phase 2 expanded safety study of the RG-TFV 1% gel used in MTN-007. The study will enroll 186 MSM and transgendered women in the US, Peru, Thailand, and South Africa. Each participant will receive 8 weeks exposure to oral Truvada, daily RG-TFV 1% gel, and use of RG-TFV 1% gel before and after sex (analogous to the regimen used in the CAPRISA 004 vaginal microbicide study (56)). Apart from general safety and acceptability, a subset of approximately 30 participants in the US and Thailand will undergo more intensive mucosal sampling for evaluation of mucosal safety biomarkers and PK/PD. If successful, it is hoped that MTN-017 will set the stage for a Phase 2B/3 RM trial in 2015.
Table 2.
Characteristics of the currently available formulations of tenofovir 1% gel
| Chemical Name | Original VF % w/w | RGF % w/w (used in MTN-007) | RGF % w/w (to be used in CHARM-01) | RF % w/w |
|---|---|---|---|---|
| Glycerin | 20.00 | 5.00* | 5.00 | 2.50 |
| Hydroxyethyl cellulose | 2.50 | 2.75 | 3.00 | 0 |
| Carbopol 974 | 0 | 0 | 0 | 0.50 |
| Sodium carboxymethylcellulose | 0 | 0 | 0 | 1.00 |
| Methylparaben | 0.18 | 0.22 | 0.22 | 0.18 |
| Propylparaben | 0.02 | 0.024 | 0.05 | 0.02 |
| Purified water | 75.23 | 89.936 | 89.66 | 94.78 |
| Disodium edetate (EDTA) | 0.05 | 0.05 | 0.05 | 0.01 |
| Citric acid | 1.00 | 1.00 | 1.00 | 0 |
| PMPA | 1.00 | 1.00 | 1.00 | 1.00 |
| Sodium hydroxide | As needed | As needed | As needed | As needed |
| Diluted hydrochloric Acid | As needed | As needed | As needed | As needed |
| pH | 4.5 | 4.6 | 4.5 | 7 |
| Osmolality (mOsmol/kg) | 3111 | 836 | 846 | 479 |
VF: vaginal formulation, RGF: reduced glycerin formulation, RF: rectal specific formulation
Drug development does not occur in a vacuum and from the outset advocacy groups have played a critical role in RM development. The International Rectal Microbicide Advocates group (IRMA; http://www.rectalmicrobicides.org/) has helped focus attention on RM development including conducting community/internet based studies on lubricant usage (57). IRMA has also lead efforts to define the need for RM for men and women at risk of HIV infection associated with URAI in Sub Saharan Africa (http://www.rectalmicrobicides.org/ProjectARMreport2012.pdf).
Conclusions
Given the ongoing epidemic of HIV infection associated with URAI it is clear that there remains an urgent need to develop safe and effective methods of HIV prevention that the target population will be willing to use. Although the iPrEx study demonstrated the efficacy of PrEP in MSM (58), overall adherence in the participants receiving Truvada was estimated to only be about 50%. This suggests that an RM, possibly used in addition to oral PrEP, might increase overall protection from HIV infection. The design of Phase 1 RM studies has evolved such that key data on safety, acceptability, and PK/PD can generated at an early stage of product development. The development of rectal specific formulations and combination antiretroviral products also increases the likelihood of developing a safe and effective RM.
Key points.
URAI in both men and women continues to be a significant driver of the HIV-1 epidemic.
Proof of concept studies in non-human primates suggests that an antiretroviral RM might be effective in HIV prevention.
Complex Phase 1 RM studies allow collection of key data including PK/PD early in product development and provide an efficient mechanism for product selection.
An expanded safety Phase 2 RM domestic and international study of tenofovir 1% gel will start in 2012.
Acknowledgments
Funding: RM research has been extensively funded through the National Institute of Allergy and Infectious Diseases, Division of AIDS (5UM1AI068633 and 5U19AI082637) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development as well as the National Institute of Mental Health (5R01HD059533).
References
- 1.Stein ZA. HIV prevention: the need for methods women can use. Am J Public Health. 1990 Apr;80(4):460–2. doi: 10.2105/ajph.80.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings R, Clegg A. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in-vitro. J Antimicrob Chemother. 1993 Jul;32(1):71–82. doi: 10.1093/jac/32.1.71. [DOI] [PubMed] [Google Scholar]
- 3.Tabet SR, Surawicz C, Horton S, Paradise M, Coletti AS, Gross M, et al. Safety and toxicity of nonoxynol-9 gel as a rectal microbicide. Sex Transm Infect. 1999 Nov;26(10):564–71. doi: 10.1097/00007435-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Gross M, Celum CL, Tabet SR, Kelly CW, Coletti AS, Chesney MA. Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis. 1999 Nov;26(10):572–8. doi: 10.1097/00007435-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002 Sep 28;360(9338):971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 6.McGowan I. Microbicides: a new frontier in HIV prevention. Biologicals. 2006 Dec;34(4):241–55. doi: 10.1016/j.biologicals.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR., III Rectal microbicides for U.S. gay men. Are clinical trials needed? Are they feasible? HIVNET Vaccine Preparedness Study Protocol Team. Sex Transm Dis. 1998 Jul;25(6):296–302. doi: 10.1097/00007435-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Carballo-Dieguez A, O’sullivan LF, Lin P, Dolezal C, Pollack L, Catania J. Awareness and attitudes regarding microbicides and Nonoxynol-9 use in a probability sample of gay men. AIDS Behav. 2007 Mar;11(2):271–6. doi: 10.1007/s10461-006-9128-0. [DOI] [PubMed] [Google Scholar]
- 9.Carballo-Dieguez A, Stein Z, Saez H, Dolezal C, Nieves-Rosa L, Diaz F. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health. 2000 Jul;90(7):1117–21. doi: 10.2105/ajph.90.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carballo-Dieguez A, Bauermeister JA, Ventuneac A, Dolezal C, Balan I, Remien RH. The use of rectal douches among HIV-uninfected and infected men who have unprotected receptive anal intercourse: implications for rectal microbicides. AIDS Behav. 2008 Nov;12(6):860–6. doi: 10.1007/s10461-007-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carballo-Dieguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomised, crossover acceptability trial among men who have sex with men. Sex Transm Infect. 2008 Nov;84(6):483–7. doi: 10.1136/sti.2008.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carballo-Dieguez A, Exner T, Dolezal C, Pickard R, Lin P, Mayer KH. Rectal microbicide acceptability: results of a volume escalation trial. Sex Transm Dis. 2007 Apr;34(4):224–9. doi: 10.1097/01.olq.0000233715.59239.83. [DOI] [PubMed] [Google Scholar]
- 13.McGowan I, Elliott J, Cortina G, Tanner K, Siboliban C, Adler A, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056) J Acquir Immune Defic Syndr. 2007 Dec 1;46(4):417–25. doi: 10.1097/QAI.0b013e318156ef16. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011 May 19;364(20):1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009 Feb;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000 Aug 18;14(12):1761–5. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 17.Wolitski RJ, Fenton KA. Sexual health, HIV, and sexually transmitted infections among gay, bisexual, and other men who have sex with men in the United States. AIDS Behav. 2011 Apr;15( Suppl 1):S9–17. doi: 10.1007/s10461-011-9901-6. [DOI] [PubMed] [Google Scholar]
- 18.Hart GJ, Williamson LM. Increase in HIV sexual risk behaviour in homosexual men in Scotland, 1996–2002: prevention failure? Sex Transm Infect. 2005 Oct;81(5):367–72. doi: 10.1136/sti.2004.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baral S, Scheibe A, Sullivan P, Trapence G, Lambert A, Bekker LG, et al. Assessing Priorities for Combination HIV Prevention Research for Men Who have Sex with Men (MSM) in Africa. AIDS Behav. 2012 May 19; doi: 10.1007/s10461-012-0202-5. [DOI] [PubMed] [Google Scholar]
- 20.Gorbach PM, Manhart LE, Hess KL, Stoner BP, Martin DH, Holmes KK. Anal intercourse among young heterosexuals in three sexually transmitted disease clinics in the United States. Sex Transm Dis. 2009 Apr;36(4):193–8. doi: 10.1097/OLQ.0b013e3181901ccf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalichman SC, Simbayi LC, Cain D, Jooste S. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Transm Infect. 2009 Oct;85(6):411–5. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Anton PA, Saunders T, Elliott J, Khanukhova E, Dennis R, Adler A, et al. First Phase 1 Double-Blind, Placebo-Controlled, Randomized Rectal Microbicide Trial Using UC781 Gel with a Novel Index of Ex Vivo Efficacy. PLoS ONE. 2011;6(9):e23243. doi: 10.1371/journal.pone.0023243. A comprehensive assessment of the NNRTI UC781 gel. The article contains provides extensive details on Phase 1 RM clinical trial methodology and demonstrates the ex vivo/in vitro efficacy of UC781 gel in the colorectal explant system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anton P, Cranston R, Carballo-Dieguez AKA, Khanukhova E, Elliott J, Janocko LE, et al. RMP-02/MTN-006: A phase 1 placebo-controlled trial of rectally applied 1% vaginal TFV gel with comparison to oral TDF. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- 24.Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob Agents Chemother. 2009 May;53(5):1797–807. doi: 10.1128/AAC.01096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. Colorectal microbicide design: triple combinations of reverse transcriptase inhibitors are optimal against HIV-1 in tissue explants. AIDS. 2011 Oct 23;25(16):1971–9. doi: 10.1097/QAD.0b013e32834b3629. An in vitro assessment of combinations of tenofovir, UC781, emtricitabine, and TMC120 that demonstrates that double and triple antiretroviral combinations provide increased efficacy in the colorectal explant system compared to single agents and that quadruple combinations did not provide additional benefit. [DOI] [PubMed] [Google Scholar]
- 26*.Harman S, Herrera C, Armanasco N, Nuttall J, Shattock RJ. Preclinical evaluation of the HIV-1 fusion inhibitor L’644 as a potential candidate microbicide. Antimicrob Agents Chemother. 2012 May;56(5):2347–56. doi: 10.1128/AAC.06108-11. A preclinical evaluation of four fusion inhibitors: C34, T20, T1249, and L’644. L’644 a cholesterol-derivatized version of C34 was the most potent fusion inhibitor and was active against RTI-resistant virus and appeared and provided protection even when used after viral exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Herrera C, Shattock RJ. Potential use of protease inhibitors as vaginal and colorectal microbicides. Curr HIV Res. 2012 Jan 1;10(1):42–52. doi: 10.2174/157016212799304607. A interesting discussion on the potential use of protease inhibitors as candidate microbicides. Useful properties include low systemic absorption, a high genetic barrier to HIV-1 resistance, and potential activity against other pathogens including human papilloma virus (HPV) infection as well as HPV-associated dysplasia. [DOI] [PubMed] [Google Scholar]
- 28*.Stefanidou M, Herrera C, Armanasco N, Shattock RJ. Saquinavir Inhibits Early Events Associated with Establishment of HIV-1 Infection: Potential Role for Protease Inhibitors in Prevention. Antimicrob Agents Chemother. 2012 Jun 4; doi: 10.1128/AAC.00399-12. Stefanidou et al. present preliminary data on the protease inhibitor Saquinavir as a candidate microbicide. Intriguingly, Saquinavir was able to block infection in colorectal explants but was unable to do so in penile or cervical explants. Moreover, Saquinavir appeared to be active when added to colorectal explants after infection. However, virus released from penile or cervical explants was unable to infect indicator T cells. Although Saquinavir may be suitable as a single agent RM, it is likely that a combination would be needed for an effective protease inhibitor based vaginal microbicide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abner SR, Guenthner PC, Guarner J, Hancock KA, Cummins JE, Jr, Fink A, et al. A Human Colorectal Explant Culture to Evaluate Topical Microbicides for the Prevention of HIV Infection. J Infect Dis. 2005 Nov 1;192(9):1545–56. doi: 10.1086/462424. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher PS, Elliott J, Grivel JC, Margolis L, Anton P, McGowan I, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS. 2006 Jun 12;20(9):1237–45. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 31.Dezzutti CS, Rohan LC, Wang L, Uranker K, Shetler C, Cost M, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother. 2012 May 11; doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patton DL, Sweeney YT, Paul KJ. A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model. Sex Transm Dis. 2009 Jun;36(6):350–6. doi: 10.1097/OLQ.0b013e318195c31a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, et al. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003 Jul;19(7):535–41. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- 34.Cranage M, Sharpe S, Herrera C, Cope A, Dennis M, Berry N, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008 Aug 5;5(8):e157. doi: 10.1371/journal.pmed.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Singer R, Derby N, Rodriguez A, Kizima L, Kenney J, Aravantinou M, et al. The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J Virol. 2011 Jun;85(11):5504–12. doi: 10.1128/JVI.02422-10. A successful demonstration of efficacy of the MIV-150/Carageenan gel developed by the Population Council. The study was conducted in adult Chinese rhesus macques with a single high dose SHIV-RT challenge. All animals were protected when gel was applied 30 minutes of 4 hours before viral challenge. These data support initiation of Phase 1 human trials of MIV-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ventuneac A, Carballo-Dieguez A, McGowan I, Dennis R, Adler A, Khanukhova E, et al. Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav. 2010 Jun;14(3):618–28. doi: 10.1007/s10461-009-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGowan I, Hoesley C, Cranston RD, Andrews P, Janocko LE, Dai J, et al. MTN-007: A Phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel. 19th Conference of Retroviruses and Opportunistic Infections; Seattle, WA. 2012. [Google Scholar]
- 38.Fuchs EJ, Lee LA, Torbenson MS, Parsons TL, Bakshi RP, Guidos AM, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis. 2007 Mar 1;195(5):703–10. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 39*.Begay O, Jean-Pierre N, Abraham CJ, Chudolij A, Seidor S, Rodriguez A, et al. Identification of personal lubricants that can cause rectal epithelial cell damage and enhance HIV type 1 replication in vitro. AIDS Res Hum Retroviruses. 2011 Sep;27(9):1019–24. doi: 10.1089/aid.2010.0252. A comprehensive preclinical evaluation of 41 sexual lubricants that demonstrated that the majority of these products are hyperosmolar and none of them had activity against HIV-1. In contrast, 4 of the lubricants (Astroglide Liquid, Astroglide Warming Liquid, Astroglyde Glycerin & Paraben-Free Liquid, and Astroglide Silken Secret) appeared to increase in vitro HIV-1 replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Gorbach PM, Weiss RE, Fuchs E, Jeffries RA, Hezerah M, Brown S, et al. The slippery slope: lubricant use and rectal sexually transmitted infections: a newly identified risk. Sex Transm Dis. 2012 Jan;39(1):59–64. doi: 10.1097/OLQ.0b013e318235502b. An evaluation of 380 individuals with a history of rectal lubricant use. Consistent lubricant use was associated with increased risk of STIs (9.5%) compared to inconsistent use (2.9%). One limitation of the study was a lack of temporal association between lubricant use and STI acquisition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Wang L, Schnaare RL, Dezzutti C, Anton PA, Rohan LC. Rectal microbicides: clinically relevant approach to the design of rectal specific placebo formulations. AIDS Res Ther. 2011;8:12. doi: 10.1186/1742-6405-8-12. The authors describe the development and preclinical assessment of four placebo formulations (two lipid and two aqueous) designed for rectal use. The aqueous formulations were almost iso-osmolar with a neutral pH. These products have now moved into the clinic for safety, acceptability, and product distribution studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agashe H, Hu M, Rohan L. Formulation and delivery of microbicides. Curr HIV Res. 2012 Jan 1;10(1):88–96. doi: 10.2174/157016212799304599. [DOI] [PubMed] [Google Scholar]
- 43.Lard-Whiteford SL. Recommendations for the Nonclinical Development of Topical Microbicides for Prevention of HIV Transmission: An Update. J Acquir Immune Defic Syndr. 2004 Apr 20;36(1):541–52. doi: 10.1097/00126334-200405010-00001. [DOI] [PubMed] [Google Scholar]
- 44.Mauck C, Rosenberg Z, Van Damme L. Recommendations for the clinical development of topical microbicides: an update. AIDS. 2001 May 4;15(7):857–68. doi: 10.1097/00002030-200105040-00006. [DOI] [PubMed] [Google Scholar]
- 45.Buckheit RW, Jr, Buckheit KW. An algorithm for the preclinical development of anti-HIV topical microbicides. Curr HIV Res. 2012 Jan 1;10(1):97–104. doi: 10.2174/157016212799304698. [DOI] [PubMed] [Google Scholar]
- 46.Richardson-Harman N, Lackman-Smith C, Fletcher PS, Anton PA, Bremer JW, Dezzutti CS, et al. Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J Clin Microbiol. 2009 Nov;47(11):3530–9. doi: 10.1128/JCM.00673-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veazey RS. Microbicide safety/efficacy studies in animals: macaques and small animal models. Curr Opin HIV AIDS. 2008 Sep;3(5):567–73. doi: 10.1097/COH.0b013e32830891bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Romero JA, Abraham CJ, Rodriguez A, Kizima L, Jean-Pierre N, Menon R, et al. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob Agents Chemother. 2012 Jan;56(1):358–68. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denton PW, Garcia JV. Humanized mouse models of HIV infection. AIDS Rev. 2011 Jul;13(3):135–48. [PMC free article] [PubMed] [Google Scholar]
- 50.Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol. 2011 Aug;85(15):7582–93. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Garcia-Lerma JG, Aung W, Cong ME, Zheng Q, Youngpairoj AS, Mitchell J, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol. 2011 Jul;85(13):6610–7. doi: 10.1128/JVI.00311-11. An important non-human primate study in which animals were given oral GS7340, a potent tenofovir prodrug and then exposed to low dose repeated rectal viral challenge. Suprisingly, despite adequate PK levels, the animals were not protected from infection. The authors suggest that this finding was due to competition between endogenous and exogenous nucleotides which reduced the antiviral efficacy of GS7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz JL, Ballagh SA, Kwok C, Mauck CK, Weiner DH, Rencher WF, et al. Fourteen-day safety and acceptability study of the universal placebo gel. Contraception. 2007 Feb;75(2):136–41. doi: 10.1016/j.contraception.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Gross M, Celum CL, Tabet SR, Kelly CW, Coletti AS, Chesney MA. Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis. 1999 Nov;26(10):572–8. doi: 10.1097/00007435-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 54*.Louissaint NA, Nimmagadda S, Fuchs EJ, Bakshi RP, Cao YJ, Lee LA, et al. Distribution of cell-free and cell-associated HIV surrogates in the colon after simulated receptive anal intercourse in men who have sex with men. J Acquir Immune Defic Syndr. 2012 Jan 1;59(1):10–7. doi: 10.1097/QAI.0b013e3182373b5e. Radiolabeled simulated HIV-infected semen with cell-associated and cell free surrogates was introduced into the rectum following a period of simulated intercourse. Migration of semen surrogates was characterized using single photon emission computerized tomography (SPECT)/CT for up to 24 hours following intoduction of the semen into the rectum. In all subjects semen reamined localized within the rectosigmoid throughout the 24 hour period. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Cao YJ, Caffo BS, Fuchs EJ, Lee LA, Du Y, Li L, et al. Quantification of the Spatial Distribution of Rectally Applied Surrogates for Microbicide and Semen in Colon with SPECT and Magnetic Resonance Imaging. Br J Clin Pharmacol. 2012 Mar 9; doi: 10.1111/j.1365-2125.2012.04267.x. Distribution and concentration of rectally administered gels and semen surrogates were monitored in healthy volunteers using SPECT/CT, magnetic resonance imaging, and sigmoidoscopic collection and analysis of colorectal tissue samples. Gel distribution was limited to the rectosigmoid in 86% (26 of 31) of the imaging sessions. This type of approach has great potential in the assessment of microbicide formulations and will be used to characterize multiple formulations of tenofovir 1% gel in the CHARM-02 study discussed earlier in this article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdool KQ, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Javanbakht M, Murphy R, Gorbach P, LeBlanc MA, Pickett J. Preference and practices relating to lubricant use during anal intercourse: implications for rectal microbicides. Sex Health. 2010 Jun;7(2):193–8. doi: 10.1071/SH09062. [DOI] [PubMed] [Google Scholar]
- 58.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]