Abstract
Background
Plasma apolipoprotein (apo) C-III strongly predicts myocardial infarction (MI) and directly activates atherogenic processes invascularcells.Geneticvariationintheinsulinresponseelementofthe APOC3 promoter is associated with an increased risk of MI.
Objective
The objective was to determine whether the APOC3 promoter variation affects plasma apo C-III concentrations and MI only when insulin sensitivity is normal.
Design
TheAPOC3*222haplotype,definedbytheminorallelesofthe single nucleotide polymorphisms 3238C→G, –455T→C, and –482C→T, was studied in 1703 matched nonfatal case-control pairs with MI in the Central Valley of Costa Rica. We used fasting hyper-glycemia and abdominal obesity as surrogates for insulin sensitivity.
Results
The APOC3*222 haplotype was associated with higher apo C-III concentrations only in those with the lowest waist circumference or fasting glucose concentration. The association between the APOC3*222 haplotype and nonfatal MI, previously reported in this population, was strongly influenced by fasting hyperglycemia and abdominal obesity. The odds ratios for MI for the APOC3*222 haplotype were 1.72 (95% CI: 1.16, 2.54) and 1.84 (1.31, 2.59) in subjects in the lowest quintiles of abdominal obesity and fasting hyperglycemia, respectively, and were 0.75 (0.54, 1.05) and 1.16 (0.85, 1.59) in subjects in the highest quintiles, respectively (P for interaction <0.05).
Conclusion
The results support the concept that mutations in the APOC3 promoter inhibit the down-regulation of APOC3 expression by insulin. This cardioprotective system becomes dysfunctional in abdominal obesity and hyperglycemia.
INTRODUCTION
The plasma concentration of apolipoprotein (apo) C-III is a strong predictor of the risk of coronary heart disease (CHD) (1, 2). Apo C-III impairs the clearance of apo B lipoproteins from plasma, which results in an increase in plasma triacylglycerol concentrations (3–7) and directly activates atherosclerotic and inflammatory pathways in vascular cells (8, 9). Therefore, genetic variation affecting the expression of the APOC3 gene may alter apo C-III metabolism and influence CHD. Minor alleles of the 3238C→G (SstI site), –482C→T, and –455T→C polymorphisms are associated with higher plasma triacylglycerol (10–13) and apo C-III (14) concentrations and with an increased risk of CHD (13, 15–17).
The association between genetic variation in the APOC3 gene and CHD risk has been linked to the action of insulin. Both the –482 and –455 sites are located in an insulin response element (IRE), and both minor alleles (–482T and –455C) are associated with higher in vitro transcription of the APOC3 gene in the presence of insulin in cultured hepatocytes (12). In humans, one small study using liver biopsy samples found that the minor –482T allele, but not the –455C allele was associated with higher APOC3 mRNA expression (18). Thus, it has been hypothesized that the minor alleles in the –482 and –455 sites cause the loss of insulin down-regulation of APOC3 expression, an increase in apo C-III synthesis, and an increase in plasma concentrations of apo C-III and atherogenic lipoprotein remnants containing apo C-III (12). We hypothesize that the effect of APOC3 promoter polymorphisms on CHD is modified by conditions associated with insulin sensitivity, specifically abdominal obesity and fasting hyperglycemia. If insulin were indeed regulating APOC3 expression through the IRE of the APOC3 gene, the IRE would be highly responsive to insulin in persons with good insulin sensitivity, such as lean persons. However, in persons with insulin resistance, the IRE of the APOC3 gene would be unresponsive to insulin action because of suppression of upstream signal transduction pathways that regulate APOC3 expression (19–21).
We examined whether the common APOC3*222 haplotype, defined by the minor alleles of the 3238C3G (SstI site), –482C→T, and –455T→C polymorphisms and which is associated with an increased risk of nonfatal MI (17), interacts with abdominal obesity and fasting hyperglycemia to affect the risk of nonfatal MI in the Costa Rican population.
SUBJECTS AND METHODS
Study population
The study population was described previously (17). Briefly, the case participants were adult patients who were survivors of a first acute MI as diagnosed by a cardiologist at any of the recruiting hospitals in the Central Valley of Costa Rica between 1994 and 2004. All cases met the World Health Organization criteria for MI, which requires typical symptoms plus either an elevation in cardiac enzyme concentrations or diagnostic changes in the electrocardiogram. For each case, one population-based control subject, matched for age (±5 y), sex, and area of residence (county), was recruited. The controls were randomly selected by using data from the National Census and Statistics Bureau of Costa Rica. Participation was 98% for cases and 88% for controls. All subjects gave informed consent on documents approved by the Human Subjects Committee of both the Harvard School of Public Health and the University of Costa Rica.
Trained personnel visited all study participants at their homes to collect data. All measurements were performed in duplicate, and the average was used for analyses. Biological specimens were collected from all subjects in the morning after an overnight fast. Plasma triacylglycerol was assayed by using enzymatic reagents (Boehringer-Mannheim Diagnostics, Indianapolis, IN). Blood glucose was analyzed by using an Accu-Check II Blood Glucose Monitor with Chemstrip bG Test Strips (Boehringer-Mannheim Diagnostics) as previously described (22). Impaired fasting glucose was indicated by a fasting glucose concentration ≥110 mg/dL (23). This study includes 1703 case-control pairs with genotype information and complete data on all the descriptive variables and potential confounders. Apo C-III concentrations were measured in a subgroup of 300 controls, with known haplotypic phase, by a sandwich enzyme-linked immunosorbent assay (ELISA) using affinity-purified antibodies (Academy-Biomedical, Houston, TX). The intraassay CV for apo C-III measurements was 5%, and the interassay CV was 8%. Selection of these 300 controls was based on them having a known haplotypic phase for the wild-type APOC3*111 haplotype and the variant APOC3*222 haplotype. This group of controls included 131 persons homozygous and 68 persons heterozygous for the APOC3*111 haplotype and 20 persons homozygous and 81 persons heterozygous for the APOC3*222 haplotype.
GENETIC POLYMORPHIMS
We selected 3 single nucleotide polymorphisms in the APOC3 gene—3238C→G (rs5128), –455T→C (rs2854116), and –482C→T (rs2854117)—because they have been found to be associated with CHD risk (13, 15, 17). In particular, the common APOC3*222 haplotype confers a higher risk of nonfatal MI in the Costa Rican population (17).
Genotyping
Genotyping was carried out by using a variation of the allele specific assay (ASA). The entire procedure of single nucleotide polymorphism (SNP) genotyping consisted of 3 steps. In step 1, DNA fragments were obtained by using polymerase chain reaction primers designed according to each SNP's vicinity sequence. The reverse primers contained an artificially introduced sequence (derived from the bacteriophage M13 at the 5′-end) that was identical across all SNPs. In step 2, SNPs were genotyped by ASA in 3 different multiplex reactions, with allele-specific forward primers and a reverse primer the sequence of which is universal for all the SNPs. Universal primers were labeled at the 5′-end with 1 of 3 fluorescent dyes (FAM, HEX, and TET). In step 3, ASA products were separated by capillary electrophoresis with the ABI Prism 310 genetic analyzer (Applied Biosystems, Perkin-Elmer, Norwalk, CT) and were analyzed using the Genotyper software (Applied Biosystems Perkin-Elmer). To confirm the accuracy of genotyping, 10 DNA samples for each SNP were subjected to direct DNA sequencing of the polymerase chain reaction products. There was 100% agreement between the 2 procedures. Eight control samples were genotyped for each plate throughout the study to assess the genotyping reproducibility. Reproducibility was 99.9% and <1% of the control, and unknown samples had missing values. All samples, cases and controls, were double-blinded.
Statistical analysis
All data were analyzed with Statistical Analysis Systems software version 9 (SAS Institute Inc, Cary, NC). Differences in health characteristics and potential confounders between cases and controls were assessed by Wilcoxon's rank-sum tests for continuous variables and with chi-square tests for categorical variables. Allele frequencies were estimated by the gene-counting method, and an exact test was performed to identify departures from Hardy-Weinberg proportions. The PROC HAPLOTYPE in the SAS/Genetics Software was used to estimate maximum-likelihood haplotype relative frequencies.
Haplotypic odds ratios (ORs) were estimated by using an expectation substitution approach (24, 25) that estimated the probabilities of all possible haplotype configurations of each individual in the sample, based on their genotype and case-control status. Conditional models matched by sex, age (±5 y), and county of residence were used in all analyses. Models were adjusted in the analysis for waist and hip circumference (quintiles based on the distribution in controls), physical activity measured in metabolic equivalents (METS; quintiles based on the distribution in controls), income (quintiles based on the distribution in controls), smoking (never smoker, past smoker, or current smoker of <10 cigarettes/d, ≥10 to <20 cigarettes/d, or ≥20 cigarettes/d), alcohol consumption (never, past, and 3 tertiles of current drinkers), and history of hypertension (yes or no). We used likelihood ratio tests to assess the interaction between the APOC3*222 haplotype and obesity and between the APOC3*222 haplotype and fasting glucose on nonfatal MI risk.
RESULTS
The general characteristics of the study participants are shown in Table 1. Compared with the controls, the cases had a lower income, lower physical activity, higher fasting triacylglycerol concentration, and higher prevalence of smoking, diabetes, and hypertension. The mean (±SD) apo C-III concentration measured in controls was 18.6 ± 7.2 mg/dL. The triple variant APOC3*222 haplotype was more frequent in cases than in controls (17.4% compared with 13.7%; P < 0.001). The OR for the risk of nonfatal MI for the APOC3*222 haplotype was 1.27 (95% CI: 1.09, 1.48).
TABLE 1.
General characteristics in cases of myocardial infarction and population-based controls in the Central Valley of Costa Rica
| Characteristics | Controls (n = 1703) | Cases (n = 1703) |
|---|---|---|
| Age (y) | 58 ± 111 | 58 ± 11 |
| Sex (% female) | 26 | 26 |
| Residence (% rural) | 25 | 25 |
| Monthly income (US$) | 574 ± 437 | 491 ± 3962 |
| Waist circumference (cm) | ||
| Men | 92.2 ± 9.6 | 92.0 ± 8.4 |
| Women | 86.4 ± 10.1 | 87.2 ± 10.2 |
| Physical activity (METS)3 | 1.55 ± 0.72 | 1.50 ± 0.722 |
| Smoking status (%) | ||
| Never smoked | 39 | 302 |
| Past smokers | 40 | 302 |
| Current smokers, <10 cigarettes/d | 9 | 82 |
| Current smokers, ≥10 to <20 cigarettes/d | 5 | 82 |
| Current smokers, ≥20 cigarettes/d | 7 | 242 |
| History of diabetes (%) | 15 | 242 |
| History of hypertension (%) | 29 | 382 |
| Alcohol status (%) | ||
| Never drank | 21 | 202 |
| Past drinkers | 26 | 302 |
| Current drinkers | 53 | 502 |
| APOC3 haplotypes4 | ||
| 111 | 0.487 ± 0.008 | 0.489 ± 0.008 |
| 112 | 0.034 ± 0.003 | 0.034 ± 0.003 |
| 121 | 0.074 ± 0.004 | 0.061 ± 0.004 |
| 122 | 0.205 ± 0.007 | 0.187 ± 0.007 |
| 211 | 0.036 ± 0.003 | 0.031 ± 0.003 |
| 212 | 0.020 ± 0.002 | 0.014 ± 0.002 |
| 222 | 0.137 ± 0.006 | 0.174 ± 0.006 |
| Plasma triglyceride (mg/dL) | 210 ± 116 | 223 ± 1112 |
| Plasma cholesterol (mg/dL) | 223 ± 47 | 202 ± 452 |
| Plasma fasting glucose (mg/dL) | 83 ± 34 | 85 ± 37 |
| Plasma apo C-III (mg/dL)5 | 18.6 ± 7.2 | — |
x̄ ± SD for continuous variables (all such values).
Significantly different from controls (P < 0.05) by Wilcoxon's rank-sum test for continuous variables and chi-square test for categorical variables.
METS, metabolic equivalent tasks.
The order of the single nucleotide polymorphisms within each haplotype is APOC3 3238G → C, APOC3 –455C → T, and APOC3 –482T → C; 1 codes for the wild-type allele, and 2 codes for the variant-type allele. The overall difference in haplotypic frequencies between cases and controls was assessed with a permutation test. P values are in parentheses. P for global test = 0.001.
Apo C-III measurements were conducted in a subgroup of 300 control individuals.
The ORs for the risk of nonfatal MI for the APOC3*222 haplotype compared with the wild-type APOC3*111 haplotype within each quintile of waist circumference are shown in Figure 1A. The APOC3*222 haplotype was associated with risk of nonfatal MI in individuals with a smaller waist circumference, and the strength of the association was attenuated in those with larger waist circumferences (P for interaction = 0.01). The ORs (95% CI) for risk of nonfatal MI for the APOC3*222 haplotype compared with the wild-type APOC3*111 within each quintile of waist circumference were as follows: 1.72 (1.16, 2.54) for the first, 1.52 (1.05, 2.18) for the second, 1.36 (0.97, 1.89) for the third, 1.70 (1.18, 2.45) for the fourth, and 0.75 (0.54, 1.05) for the fifth quintile. The association between the APOC3*222 haplotype and risk of nonfatal MI was reduced by 13.3% (95% CI: 4.7%, 21.2%) with each 5-cm increase in waist circumference. Using as reference the group consisting of the wild-type APOC3*111 haplotype within the first quintile of waist circumference, the ORs (95% CI) for risk of nonfatal MI were 1.72 (1.16, 2.54) for the APOC3*222 haplotype in the first quintile; 1.95 (1.46, 2.62) and 2.96 (2.00, 4.38) for the APOC3*111 and APOC3*222 haplotypes in the second quintile, respectively; 2.50 (1.80, 3.46) and 3.39 (2.29, 5.01) for the APOC3*111 and APOC3*222 haplotypes in the third quintile, respectively; 2.24 (1.56, 3.22) and 3.81 (2.48, 5.85) for the APOC3*111 and APOC3*222 haplotypes in the fourth quintile, respectively; and 4.38 (2.94, 6.52) and 3.29 (2.12, 5.12) for the APOC3*111 and APOC3*222 haplotypes in the fifth quintile, respectively.
FIGURE 1.
Odds ratios (ORs) and 95% CIs for the risk of nonfatal myocardial infarction (MI) for the APOC3*222 haplotype within quintiles of waist circumference (A) and quintiles of fasting glucose concentrations (B). ORs for the risk of nonfatal MI for the APOC3*222 haplotype were estimated by using the wild-type APOC3*111 within each quintile of waist circumference or fasting glucose concentration as the reference (n = 1703 case-control pairs). ORs and 95% CIs were estimated by using conditional logistic regression models adjusted for hip circumference, physical activity, smoking, income, alcohol consumption, and history of hypertension. Linear regression of the ORs on the median values of waist circumference and fasting glucose is shown. Interactions were assessed with a likelihood ratio test that compared logistic models with and without interaction terms.
Abdominal obesity is a major determinant of insulin resistance. We therefore examined whether increasing concentrations of fasting plasma glucose, as occurs in insulin resistance, would have similar effects to those found for waist circumference on the association between the APOC3*222 haplotype and risk of non-fatal MI. The ORs for the risk of nonfatal MI for the APOC3*222 haplotype, compared with the APOC3*111 haplotype, within quintiles of fasting glucose concentration are shown in Figure 1B. Consistent with the results for waist circumference, the APOC3*222 haplotype was associated with an increased risk of nonfatal MI only in individuals with lower fasting glucose concentrations. The ORs (95% CI) for the risk of nonfatal MI for the APOC3*222 haplotype compared with the wild-type APOC3*111 haplotype within each quintile of fasting glucose concentration were as follows: 1.84 (1.31, 2.59) for the first, 1.50 (1.04, 2.18) for the second, 1.34 (0.94, 1.91) for the third, 1.35 (0.94, 1.94) for the fourth, and 1.16 (0.85, 1.59) for the fifth quintile. The association between the APOC3*222 haplotype and the risk of nonfatal MI was reduced by 4.6% (95% CI: 0.3%, 8.7%) with each 10-mg/dL increase in fasting glucose concentration (P for interaction = 0.03). Using as reference the group consisting of the wild-type APOC3*111 haplotype within the first quintile of fasting glucose concentration, the ORs (95% CI) for risk of nonfatal MI were 1.84 (1.31, 2.59) for the APOC3*222 haplotype in the first quintile; 0.86 (0.64, 1.15) and 1.29 (0.90, 1.86) for the APOC3*111 and APOC3*222 haplotypes, in the second quintile, respectively; 0.87 (0.65, 1.15) and 1.16 (0.81, 1.65) for the APOC3*111 and APOC3*222 haplotypes, in the third quintile, respectively; 0.93 (0.69, 1.24) and 1.26 (0.87, 1.80) for the APOC3*111 and APOC3*222 haplotypes, in the fourth quintile, respectively; and 1.34 (1.02, 1.77) and 1.57 (1.13, 2.17) for the APOC3*111 and APOC3*222 haplotypes, in the fifth quintile, respectively. No significant interactions were found between the individual SNPs and either abdominal obesity or fasting plasma glucose on the risk of nonfatal MI (see Table S1 under “Supplemental data” in the online issue).
The highest quintiles of abdominal obesity were 104 cm in men and 97.7 cm in women compared with the lowest quintiles of 80.4 and 74.2 cm, respectively. Compared with subjects in the lowest quintile, those in the highest quintile were more likely to be diabetic (23.1% compared with 6.1%) and to be taking glucose-lowering medications (26.1% compared with 11.8%). The highest quintile of fasting glucose was 111 mg/dL. Forty-seven percent of the subjects in this top category were diabetic, and 46.2% were taking glucose-lowering medications.
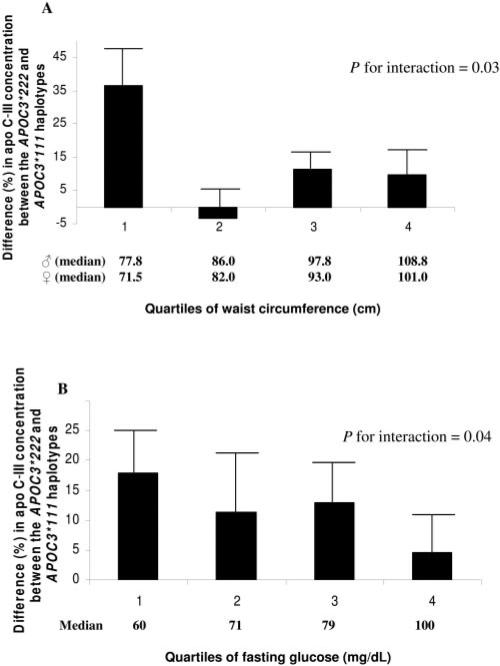
To test whether the interaction between waist circumference or fasting glucose level and the APOC3*222 haplotype on the risk of nonfatal MI could be explained by the action of this haplotype on APOC3 gene expression, we measured the plasma concentration of apo C-III in a group of 300 control subjects. Overall, the apo C-III concentration was higher in individuals with the APOC3*222 haplotype than in homozygotes for the wild-type APOC3*111 haplotype (20.5 ± 0.8 compared with 17.7 ± 0.6 mg/dL, respectively; P = 0.01). These concentrations corresponded to an overall 15% (95% CI: 2%, 29%) higher apo C-III concentration in individuals with the APOC3*222 haplotype than in those homozygous for the wild-type APOC3*111 haplotype. The percentage differences in apo C-III concentrations for each one-copy increase in the APOC3*222 haplotype compared with the APOC3*111 haplotype, within each quartile of waist circumference, are shown in Figure 2. The percentage difference in apo C-III concentrations was larger in individuals with a smaller waist circumference than in those with a larger waist circumference: 37% for the first, –3% for the second, 11% for the third, and 10% for the fourth quartile (P for interaction = 0.03); the corresponding differences in apo C-III concentrations were 5.8 ± 1.8 mg/dL for the first, –0.6 ± 1.5 mg/dL for the second, 2.0 ± 1.0 mg/dL for the third, and 1.8 ± 1.4 mg/dL for the fourth quartile. Least-squares mean apo C-III concentrations in the first quartile of waist circumference for homozygotes of the APOC3*111 haplotype and carriers of the APOC3*222 haplo-type, respectively, were 16.6 ± 1.5 and 21.3 ± 2.1 mg/dL; in the second quartile were 17.6 ± 1.4 and 17.4 ± 1.3 mg/dL, respectively; in the third quartile were 17.5 ± 1.6 and 22.9 ± 1.3 mg/dL, respectively; and in the fourth quartile were 18.7 ± 1.7 and 21.3 ± 2.1 mg/dL, respectively.
FIGURE 2.
Percentage difference in plasma apolipoprotein (apo) C-III concentrations between the APOC3*222 haplotype and the APOC3*111 haplotype by quartiles of waist circumference (A) and quartiles of fasting glucose concentrations (B). Percentage differences (+SEM) for apo C-III concentrations were estimated in 300 control subjects using as reference the APOC3*111 haplotype within each quartile of waist circumference or the fasting glucose concentration. Mean plasma apo C-III concentrations were adjusted for age, sex, area of residence, physical activity, and hip circumference by using general linear models. Interactions were assessed with a likelihood ratio test that compared models with and without interaction terms.
As shown in Figure 2B, a similar pattern was observed when the analysis was conduced using quartiles of fasting glucose. Compared with the APOC3*111 haplotype, each one-copy increase in the APOC3*222 haplotype was associated with an 18% (or 3.3 mg/dL) higher apo C-III concentration in individuals in the lowest quartile of fasting glucose. In contrast, this difference was only 5% (or 0.8 mg/dL) in the highest quartile of fasting glucose (P for interaction = 0.04).
DISCUSSION
Genetic variation in the APOC3 gene, particularly in the promoter region, is associated with an increased risk of CHD (13, 15–17, 26). However, the mechanisms underlying this association are unknown. One hypothesis is that mutations in an IRE of the APOC3 promoter cause a decrease in insulin down-regulation of APOC3 expression (12). This increases the secretion into plasma of VLDL that contains apo C-III, an atherogenic lipoprotein. If true, the status of insulin resistance could modify the effect of variation in the APOC3 promoter on CHD risk. We found that abdominal obesity and fasting hyperglycemia, surrogates for insulin resistance in this population, do indeed interact with the common APOC3*222 haplotype, which confers a higher risk of nonfatal MI in the Costa Rican population (17). The APOC3*222 haplotype is associated with the risk of nonfatal MI in leaner individuals and in those with a low fasting glucose concentration, but not in those with high abdominal obesity or a high fasting glucose concentration. The APOC3*222 haplotype was shown to be associated with a higher plasma apo C-III concentration only in leaner individuals or in those with a lower fasting glucose concentration and not in those with abdominal obesity or with a high fasting glucose concentration. These observations suggest that the effect of APOC3*222 haplotype on plasma apo C-III concentrations and on the risk of nonfatal MI is dependent on normal insulin sensitivity in hepatocytes that produce apo C-III.
The association between APOC3 genetic variation and risk of CHD has not been consistently replicated (13, 15, 27, 28). Although some studies have found that persons homozygous for the minor allele of the –455 site have a higher risk of CHD than do those homozygous for the major allele (13, 15), others have found no such association with the highly linked –482 and SstI sites (27, 28). If the effect of APOC3 promoter polymorphisms on CHD risk is mediated through overexpression of the APOC3 gene, the present results suggest that this effect is mostly present in situations of adequate insulin sensitivity and disappear in conditions of insulin resistance. For example, it has been found that APOC3 promoter variation does not affect triacylglycerol concentrations in diabetic subjects (11).
The APOC3*222 haplotype was not associated with the risk of nonfatal MI in the highest quintile of abdominal obesity. The highest quintile of abdominal obesity (104 cm in men and 97.7 in women) in this study was higher than the cutoff used to define the presence of the metabolic syndrome in men (>102 cm) and women (>88 cm) (29). Thus, a considerable level of insulin resistance should be present in these individuals, as suggested by the higher fasting glucose concentration and prevalence of self-reported type 2 diabetes. This genetic interaction led us to propose a molecular mechanism by which insulin affects APOC3 expression.
We hypothesize that the regulation of APOC3 expression by insulin involves a repressor, and that it is similar to the regulation of the glucose-6-phosphatase (GP6ase) catalytic subunit gene. Nuclear factors that act as repressors are the endogenous mediators of GP6ase gene expression by insulin (30–32). For example, deletion of the GP6ase promoter IREs results in overexpression of a GP6ase fusion gene in HepG2 cells (31). The GP6ase and APOC3 genes are both down-regulated in response to insulin (12, 33). These 2 genes also have mutations in the IREs that result in their overexpression (12, 31). The alternate hypothesis would be that an activator such as the nuclear transcription factor Fork-head box O1 (FoxO1) would be involved (34). FoxO1, a protein that is bound to DNA in the absence of insulin, can stimulate APOC3 expression in hepatocytes. Transgenic mice overex-pressing FoxO1 have higher plasma apo C-III and triacylglycerol concentrations (34). However, in a more recent study, overexpression of FoxO1 was not associated with increased APOC3 expression in the liver of transgenic mice or in isolated hepatocytes (35). Also, variants of the APOC3-IRE that are associated with higher APOC3 gene expression (12) and plasma apo C-III concentrations (the present study and that of Tilly et al; 14) reduce the affinity of the IRE to DNA-binding proteins (12, 18). If an activator such as FoxO1 were responsible for the expression of APOC3 in response to insulin, mutations in the IRE that reduce the binding affinity of FoxO1 would reduce APOC3 expression. Thus, it is more likely and consistent with our finding that APOC3*222 affects the binding of a repressor.
We propose a model in which the major factor mediating the effect of insulin on APOC3 expression is a repressor (Figure 3). According to this model, in the absence of insulin, the transcriptional machinery is bound to the APOC3 promoter, and APOC3-mRNA is synthesized. Insulin in lean individuals who have good insulin sensitivity will activate the repressor that binds to the wild-type IRE, displaces the transcriptional machinery, and suppresses the transcription of the APOC3 gene (Figure 3; pathway 1). In contrast, the repressor would be unable to bind to the mutant IRE, APOC3 transcription would not be suppressed, and the plasma concentration of apo C-III and the CHD risk will increase (pathway 2). It is possible that the phosphatidylinositol 3′-kinase pathway, which mediates the insulin inhibition of GP6ase transcription (36), is involved in the repression of APOC3 expression by insulin. A different scenario would be observed in individuals with abdominal obesity or insulin resistance. In this setting, proinflammatory cytokines such as tumor necrosis factor-α block the phosphatidylinositol 3′-kinase pathway through the inhibition of the upstream protein insulin-receptor substrate 1 (19, 20). As a result, downstream insulin action would be blocked and APOC3 gene expression, the plasma apo C-III concentration, and the risk of CHD would increase regardless of the presence of mutations in the IRE (pathways 3 and 4).
FIGURE 3.
Model of inhibition by insulin of APOC3 transcription and modulation by abdominal obesity. A repressor rather than an activator mediates the action of insulin over APOC3 gene expression (pathway 1). In the absence of insulin, the transcriptional machinery (TM) is bound to the APOC3-promoter, which results in the transcription of APOC3-mRNA. In conditions of good insulin sensitivity, for example in lean individuals, the activated repressor is unable to bind to the mutant-type insulin response element (IRE), and the TM remains bound to the APOC3-promoter and results in overexpression of the APOC3 gene (pathway 2). In conditions of insulin resistance, for example in obese individuals, the repressor fails to be activated, and the TM remains bound to the APOC3-promoter and results in overexpression of the APOC3 gene regardless of the presence of mutations in the APOC3-IRE (pathways 3 and 4). IL, interleukin; TNF-α, tumor necrosis factor-α; TNFR, tumor necrosis factor receptor; JNK, jun N-terminal kinase; IRS-1, insulin receptor substrate-1; PI3K, phosphatidylinositol 3′-kinase.
In summary, the effect of the APOC3*222 haplotype on the risk of nonfatal MI was only evident in lean normoglycemic subjects. Our results support the hypothesis that genetic variation in the APOC3 promoter leads to overexpression of the APOC3 gene because of the loss of insulin down-regulation. An important conclusion from the present analysis is that the effect of APOC3 promoter variation on nonfatal MI risk is larger in conditions of adequate insulin sensitivity. In a state of insulin resistance, for example, because of the presence of abdominal obesity, the APOC3 gene would be overexpressed regardless of the presence of mutations in the promoter. In this last case, the APOC3*222 haplotype would not confer additional nonfatal MI risk.
Supplementary Material
Acknowledgments
We are grateful to Xinia Siles for data collection and study management in Costa Rica, to the study participants, and to the staff of Proyecto Salud Coronaria, San José, Costa Rica. We also thank Chunyu Zheng for his help with the ELISA analysis.
The authors’ responsibilities were as follows—EAR-N: designed and conducted the data analysis, interpreted the main aspects of the data, conducted the genotyping and the ELISA analysis, and wrote the manuscript; and HC and FMS: designed the study, contributed to the data analyses, and proofread and edited the manuscript.
Footnotes
Supported by grants HL49086 and HL60692 from the National Institutes of Health. EAR-N was supported by grants T90 DK070078 and R90 DK071507 from the National Institutes of Health as a Roadmap Fellow at the Harvard School of Public Health.
The authors had no conflicts of interest.
REFERENCES
- 1.Alaupovic P, Mack WJ, Knight-Gibson C, Hodis HN. The role of triglyceride-rich lipoprotein families in the progression of atherosclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler Thromb Vasc Biol. 1997;17:715–22. doi: 10.1161/01.atv.17.4.715. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Alaupovic P, Moye LA, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102:1886–92. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 3.Windler E, Chao Y, Havel RJ. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem. 1980;255:5475–80. [PubMed] [Google Scholar]
- 4.Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem. 1991;266:18259–67. [PubMed] [Google Scholar]
- 5.Aalto-Setala K, Fisher EA, Chen X, et al. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest. 1992;90:1889–900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Silva HV, Lauer SJ, Wang J, et al. Overexpression of human apolipoprotein C-III in transgenic mice results in an accumulation of apolipoprotein B48 remnants that is corrected by excess apolipoprotein E. J Biol Chem. 1994;269:2324–35. [PubMed] [Google Scholar]
- 7.Zheng C, Khoo C, Ikewaki K, Sacks FM. Rapid turnover of apolipoprotein CIII containing triglyceride-rich lipoproteins contributing to formation of LDL subfractions. J Lipid Res. 2007 doi: 10.1194/jlr.P600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113:691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2007;27:219–25. doi: 10.1161/01.ATV.0000249620.68705.0d. [DOI] [PubMed] [Google Scholar]
- 10.Ordovas JM, Civeira F, Genest J, Jr, et al. Restriction fragment length polymorphisms of the apolipoprotein A-I, C-III, A-IV gene locus relationships with lipids, apolipoproteins, and premature coronary artery disease. Atherosclerosis. 1991;87:75–86. doi: 10.1016/0021-9150(91)90234-t. [DOI] [PubMed] [Google Scholar]
- 11.Surguchov AP, Page GP, Smith L, Patsch W, Boerwinkle E. Polymorphic markers in apolipoprotein C-III gene flanking regions and hyper-triglyceridemia. Arterioscler Thromb Vasc Biol. 1996;16:941–7. doi: 10.1161/01.atv.16.8.941. [DOI] [PubMed] [Google Scholar]
- 12.Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest. 1995;96:2601–5. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivieri O, Stranieri C, Bassi A, et al. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res. 2002;43:1450–7. doi: 10.1194/jlr.m200145-jlr200. [DOI] [PubMed] [Google Scholar]
- 14.Tilly P, Sass C, Vincent-Viry M, Aguillon D, Siest G, Visvikis S. Biological and genetic determinants of serum apoC-III concentration: reference limits from the Stanislas Cohort. J Lipid Res. 2003;44:430–6. doi: 10.1194/jlr.M200006-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Olivieri O, Bassi A, Stranieri C, et al. Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J Lipid Res. 2003;44:2374–81. doi: 10.1194/jlr.M300253-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Ferns GA, Stocks J, Ritchie C, Galton DJ. Genetic polymorphisms of apolipoprotein C-III and insulin in survivors of myocardial infarction. Lancet. 1985;2:300–3. doi: 10.1016/s0140-6736(85)90350-2. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Narvaez EA, Yang Y, Nakanishi Y, Kirchdorfer J, Campos H. APOC3/A5 haplotypes, lipid levels, and risk of myocardial infarction in the Central Valley of Costa Rica. J Lipid Res. 2005;46:2605–13. doi: 10.1194/jlr.M500040-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Esterbauer H, Hell E, Krempler F, Patsch W. Allele-specific differences in apolipoprotein C-III mRNA expression in human liver. Clin Chem. 1999;45:331–9. [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–8. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 20.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 21.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119(suppl):S10–6. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos H, Bailey SM, Gussak LS, Siles X, Ordovas JM, Schaefer EJ. Relations of body habitus, fitness level, and cardiovascular risk factors including lipoproteins and apolipoproteins in a rural and urban Costa Rican population. Arterioscler Thromb. 1991;11:1077–88. doi: 10.1161/01.atv.11.4.1077. [DOI] [PubMed] [Google Scholar]
- 23.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 24.Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum Hered. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
- 25.Stram DO, Leigh Pearce C, Bretsky P, et al. Modeling and E-M estimation of haplotype-specific relative risks from genotype data for a case-control study of unrelated individuals. Hum Hered. 2003;55:179–90. doi: 10.1159/000073202. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Izawa H, Ichihara S, et al. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916–23. doi: 10.1056/NEJMoa021445. [DOI] [PubMed] [Google Scholar]
- 27.Russo GT, Meigs JB, Cupples LA, et al. Association of the Sst-I polymorphism at the APOC3 gene locus with variations in lipid levels, lipoprotein subclass profiles and coronary heart disease risk: the Framingham offspring study. Atherosclerosis. 2001;158:173–81. doi: 10.1016/s0021-9150(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 28.Wong WM, Hawe E, Li LK, et al. Apolipoprotein AIV gene variant S347 is associated with increased risk of coronary heart disease and lower plasma apolipoprotein AIV levels. Circ Res. 2003;92:969–75. doi: 10.1161/01.RES.0000069688.94567.7A. [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith S,J, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 30.Onuma H, Vander Kooi BT, Boustead JN, Oeser JK, O'Brien RM. Correlation between FOXO1a (FKHR) and FOXO3a (FKHRL1) binding and the inhibition of basal glucose-6-phosphatase catalytic subunit gene transcription by insulin. Mol Endocrinol. 2006;20:2831–47. doi: 10.1210/me.2006-0085. [DOI] [PubMed] [Google Scholar]
- 31.Vander Kooi BT, Streeper RS, Svitek CA, Oeser JK, Powell DR, O'Brien RM. The three insulin response sequences in the glucose-6-phosphatase catalytic subunit gene promoter are functionally distinct. J Biol Chem. 2003;278:11782–93. doi: 10.1074/jbc.M212570200. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA. Insulin-regulated gene expression. Biochem Soc Trans. 2001;29:552–8. doi: 10.1042/bst0290552. [DOI] [PubMed] [Google Scholar]
- 33.Streeper RS, Svitek CA, Chapman S, Greenbaum LE, Taub R, O'Brien RM. A multicomponent insulin response sequence mediates a strong repression of mouse glucose-6-phosphatase gene transcription by insulin. J Biol Chem. 1997;272:11698–701. doi: 10.1074/jbc.272.18.11698. [DOI] [PubMed] [Google Scholar]
- 34.Altomonte J, Cong L, Harbaran S, et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. 2004;114:1493–503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Patil S, Chauhan B, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–17. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 36.Dickens M, Svitek CA, Culbert AA, O'Brien RM, Tavare JM. Central role for phosphatidylinositide 3-kinase in the repression of glucose-6-phosphatase gene transcription by insulin. J Biol Chem. 1998;273:20144–9. doi: 10.1074/jbc.273.32.20144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.