Abstract
This study describes biochemical and biological properties of Naja kaouthia (Indian monocled cobra) venom of North-East India. The LD50 of the crude venom was found to be 0.148mg/kg and neurotoxicitic symptoms like paralysis of lower limbs and heavy difficulty in breathing at sub-lethal dose in mice was observed. The venom exhibited PLA2, indirect hemolytic and myotoxic activities but showed weak proteolytic and low direct hemolytic activities. It did not exhibit any hemorrhage when injected intradermally to mice. Anticoagulant activity was prominent when recalcification, prothrombin and activated partial thrombinplastin time were tested on platelet poor plasma. Rotem analysis of whole citrated blood in presence of venom showed delay in coagulation time and clot formation time. Fibrinogen of whole citrated blood was depleted by venom when analyzed in Sonoclot. Crude venom at 10µg and after 16hr of incubation was found to degrade α chain of fibrinogen. Neutralization study showed that Indian polyvalent antivenom could neutralize some of the biochemical and biological activities as well as its fibrinogenolytic activity.
Keywords: Naja kaouthia, haemostasis, thromboelastometry, myotoxicity, polyvalent antivenom
INTRODUCTION
Snakebite envenoming is a neglected tropical disease (WHO), which requires immediate attention. It is estimated that globally 2.5 million people are bitten by snakes each year with ∼85,000 deaths (Gutierrez et al, 2010); in India, approximately 35,000 to 40,000 people die of snakebites annually (Chippaux, 1998; Kasturiratne et al, 2008). According to recent National Mortality Survey data, the incidence of snakebite cases is likely to be more than 50,000 per year in India (Mohapatra et al, 2011). However, these data may be far from the truth as most of the incidences happen in rural areas and these deaths mostly remain unreported. In India the “Big Four”, Naja naja, Bungarus caeruleus, Daboia russelii and Echis carinatus are considered to be medically important snakes and are responsible for most of the deaths. Recently, it has been reported that hump-nosed pit viper (Hypnale hypnale) from Kerala, is capable of causing lethal envenomation (Joseph et al, 2007). Hence, in addition to the “Big Four”, there might be other medically important snakes in specific geographical locations, which need attention. This is important for clinical diagnosis for treatment and for production of effective antivenoms. In India, polyvalent antivenom is raised against the “Big Four” venoms but these snakes may not be present throughout the country. Moreover, administration of this polyvalent antivenom has well documented limitations (Offerman et al, 2001; Lalloo and Theakston, 2003; Williams et al, 2007).
Naja kaouthia is recognized phenotypically with the presence of O-shaped or monocellate hood pattern. They are widely distributed in Nepal, North East India, Bangladesh, Myanmar, Thailand and Peninsular Malaysia (Whitaker, 1978; Viravan et al, 1992; Mukherjee and Maity, 2002). According to WHO, it belongs to Category 1 of venomous snakes. The symptoms of cobra bite are general neurotoxicity leading to flaccid paralysis and death by respiratory failure, and also severe hypertension (Agarwal et al, 2006; Halesha et al, 2013). Symptoms of coagulopathy have also been reported in victims of Naja kaouthia of Asian origin (Khandelwal et al, 2007). The Naja kaouthia venom of North-East India origin has not been explored though venom of West Bengal (India) origin have been studied extensively (Mukherjee and Maity, 2002; Lalloo and Theakston, 2003; Mukherjee, 2007; Debnath et al, 2010; Sekhar and Chakrabarty, 2011) . Hence, some work on biochemical and biological characterization of the Naja kaouthia venom and its in vitro neutralization by Indian polyvalent antivenom has been undertaken previously.
MATERIALS AND METHODS
Reagents and kits
sPLA2 assay kit was procured from Cayman Chemical Company (MI, USA). NEOPLASTINE® CL PLUS and APTT reagent were obtained from STAGO (France). AGAPEE kit for CK/LDH analysis was purchased from AGAPPE diagnostics (Switzerland), Glass beads gbACT+ kit was obtained from Sienco, Inc. (USA). Polyvalent antivenom manufactured by Bharat Serums and Vaccines Limited (India) was purchased locally. Bovine plasma fibrinogen was obtained from Sigma-Aldrich and all other reagents used were of analytical grade and were either from Merck or Sigma-Aldrich, (USA).
Animals
Male Swiss albino mice of 40±3gm were obtained from central animal facility, University of Mysore. All animal were housed in well ventilated cages and experiments were carried out according to the Animal Ethical Committee Protocol (University of Mysore, Mysore, India, Proposal no. UOM/IAEC/25/2011).
Collection of snake venom, preparation and storage
Adult Naja kaouthias were captured from Jamugurihat, district Sonitpur, Assam, North-East India in the, month of May from its natural habitat and venom was extracted by allowing the snake to bite into a sterile beaker covered with para-film. The crude venom was immediately desiccated using dehydrated silica gel and stored in -20°C until further use. The permission for milking of snakes was obtained from Principal Chief Conservator of Forest (Wild Life) and Chief Wild Life Warden of Assam, India (WL/FG.27/tissue Collection/09 dated 07/10/2011).
Determination of protein content
Total protein content of Naja kaouthia venom was determined according to Lowry’s method using BSA as standard (Lowry et al, 1951).
Phospholipase A2 (PLA2) activity
PLA2 activity was assayed using sPLA2 assay kit according to the manufacturer’s protocol (Cayman Chemical Company, MI, USA). Briefly, in a 96-well microtitre plate, 10µl of venom (0.1mg/ml), 10µl DTNB (5, 50-dithio-bis-(2-nitrobenzoic acid)) and 5µl assay buffer were added. The reaction was initiated by adding 200µl of substrate solution (diheptanoyl Thio-PC). After gentle shaking, the optical density was measured every minute at 405nm using MultiSkan GO multi plate reader (Thermo Scientific, USA) for 10min. Assay buffer was used as blank and bee venom PLA2 (0.01mg/ml) was used as a positive control. Tests were carried out in triplicate and mean values were taken. The activity was expressed as micromoles of diheptanoyl Thiol-PC hydrolyzed per min per mg of enzyme.
Caseinolytic assay
Digestion of casein was evaluated according to the method of Ouyang and Teng (Ouyang and Teng, 1976). Briefly, 1% (w/v) casein in 20mM Tris-Cl, pH 7.4, was incubated with various amounts of venom protein (1, 5, 10, 50 and 100µg) for 1hr at 37°C. Reaction was stopped by addition of ice cold 10% (v/v) TCA and centrifuged for 10min at 5000rpm (Thermo Scientific, USA, Heraeus Multifuge X1R). The digested protein in the supernatant was determined according to Lowry’s method (Lowry et al, 1951). Tyrosine curve was used to determine the protease activity and one unit of protease activity is defined as n mole equivalent of tyrosine formed per min per ml.
LD50 determination
Toxicity of the venom was analyzed according to the method of Meier and Theakston (Meier and Theakston, 1986). Briefly, various amount of freshly dissolved venom (0.05 to 1mg/kg) in saline was injected intraperitoneally to eight male Swiss albino mice in a final volume of 150µl and the controls were injected with saline alone. The animals were carefully monitored for 24hr and their survival time was recorded and LD50 was determined.
Edema inducing activity
The procedure of Yamakawa et al, (Yamakawa et al, 1976) as modified by Vishwanath et al, (Vishwanath et al, 1988) was followed. Mice weighing 20–30gm were injected with varying amount of venom (2–15µg) in a total volume of 20µl saline into intra plantar surface of right hind foot pad. Respective left foot pad received 20µl of saline and served as vehicle. Control mice were injected with 20µl saline into intra plantar surface of both hind foot pads. After 45min the mice were anesthetized (barbitone, 30mg/kg, i.p.) before sacrifice and hind limbs were removed at the ankle joint and weighed individually. The increase in weight due to edema is expressed as the ratio of the weight of edematous limb to the weight of vehicle (saline injected) limb x100. The amount of venom required to cause an edema ratio of 120% (20% above the basal level) is defined as minimum edema dose (MED).
Hemorrhagic activity
Hemorrhagic activity was assayed as described by Kondo et al, (Kondo et al, 1960). Various amount of venom (2–15µg) in 30µl saline were injected intradermally into mice and control mice received saline instead of venom sample. After 3hr, mice were sacrificed using anesthesia (barbitone, 30mg/kg, i.p.). The dorsal surface of the skin was removed and the inner surface was observed for hemorrhagic lesions. E. carinatus venom was used as positive control. The minimum hemorrhagic dose (MHD) is defined as the concentration of venom that induce a hemorrhagic spot of 1cm diameter from the spot of injection.
In-vivo myotoxicity
For myotoxicity, release of serum creatine kinase (CK) and lactate dehydrogenase (LDH) in the blood were determined using AGAPPE kit (AGAPPE diagnostics, Switzerland). Group of six male albino mice were injected (i.m) with 15µg crude venom (40µl) and control received 40µl of saline. After 3hr, mice were anesthetized and 0.5ml of blood samples was drawn using cardiac puncture. The serum obtained by centrifugation was diluted with saline at 1:20 ratio. The CK and LDH activity were measured in 10µl of plasma according to the manufacturer’s protocol and were expressed in Units/liter (U/l). The results are mean ±SD of three experiments.
Collection of Blood and Platelet Poor Plasma (PPP) preparation
Fresh goat blood was collected in citrated tube (0.11M tri sodium citrate) at 1:9 ratios (citrate: blood) from local butcher’s shop. Human blood was collected from healthy donors (27Yr) who had not taken any medication for last 48hr. 9ml of blood was drawn with 20 gauge 3/4" needle and immediately transferred to a plastic tube containing 1ml of 0.11M tri sodium citrate (Suntravat et al, 2010). The tubes were centrifuged at 3000rpm (Thermo Scientific, USA, Heraeus Multifuge X1R) for 15mins to separate the red blood cells (RBC) and platelet poor plasma (PPP) and used within 4hr of collection.
Direct and indirect hemolytic activity
The RBC pellet obtained from the blood (as described above) was washed 4–5 times and re-suspended in 0.9% (w/v) saline to a final concentration of 10% (v/v). Various amount of venom were incubated for 60min at 37°C with 150µl of 10% RBC to a final volume of 2ml with 0.9% (v/v) NaCl. The tubes were centrifugation at 5000rpm (Thermo Scientific, USA, Heraeus Multifuge X1R) for 10min and the absorbance of the supernatant was measured at 540nm in a MultiSkanGO, UV-Vis spectrophotometer (Thermo Scientific, USA). The hemolysis caused by dH2O was considered as 100%. For Indirect hemolytic, 20µl of egg yolk substrate solution was added to the reaction mixtures at the time of incubation and hemolysis was measured as described for direct hemolytic activity. The results are mean ±SD of three experiments.
Fibrinogenolytic activity
Fibrinogenolytic activity was assayed according to the method of Ouyang and Teng, using bovine fibrinogen (2mg/ml) dissolved in 50mM Tris HCl buffer, pH 7.5, 0.15M NaCl (Ouyang and Teng, 1976). To 300µl of dissolved fibrinogen, various amount of venom in 150µl of buffer was incubated for different time intervals at 37°C. The incubated mixtures were then run on a 12.5% (w/v) SDS-PAGE according to the method of Laemmeli (Laemmli, 1970). Staining was done with 0.25% (w/v) Coomassie brilliant blue R250 and de-stained till the protein bands were visible.
In-vitro coagulant assays
Recalcification time
Recalcification time of human PPP was measured using coagulation analyzer (STAGO, France). Various amount of venom in 50µl of PBS was pre-incubated with 50µl of human PPP at 37°C for 3min and 50µl of 25mM CaCl2 was added to initiate the clot formation. The clotting time with PBS was considered as normal clotting time. The results are as mean ±SD of three experiments.
Prothrombin time (PT) test
Prothrombin time was measured using PT reagent (NEOPLASTINE® CL PLUS) obtained from STAGO (France) according to the manufacturer’s protocol on a coagulation analyzer (STAGO, France). Various amount of venom in 50µl of PBS was pre-incubated with 50µl of human PPP at 37°C for 1min and 100µl of PT reagent was added to initiate the clot formation. The clotting time with PBS was considered as normal clotting time. The results are mean ±SD of three experiments.
Activated partial thrombin time (APTT) test
Activated partial thrombin time was determined using APTT reagent obtained from STAGO (France) according to the manufacturer’s protocol on a coagulation analyzer (STAGO, France). Various amount of venom in 50µl PBS was incubated with 50µl of human PPP and 50µl of APTT reagent for 3min at 37°C. The clot formation was initiated by adding 50µl of 25mM CaCl2. The clot formation time with PBS was considered as normal clotting time. The results are mean ±SD of three experiments.
Whole citrated blood analysis
Thromboelastometry analysis
To quantify the CT (clotting time, in seconds), CFT (clot formation time, in seconds) and MCF (maximum clot firmness, in mm) of the whole citrated blood, Rotem® Analyzer (ROTEM®Pentapharm GmbH Diagnostic Division; Munich, Germany) was used. For the analysis, blood samples from healthy volunteers were collected in 0.11M tri sodium citrate at 9:1 (blood: citrate) ratio. Various amount of venom in 20µl of PBS was mixed with 20µl of 200mM CaCl2, to this reaction mixture, 320µl of whole citrated blood was added and clot formation was observed over 30min. Clot formation function with only PBS was considered as control. The results are mean ±SD of three experiments.
Sonoclot analysis
A glass bead activated test tube (gbACT+ Kit obtained from Sienco, Inc, USA) was used to monitor clot detection, clot rate and platelet function (clot retraction) in a Sonoclot Coagulation and Platelet Function Analyzer (Sienco, Inc, USA). Various amount of venom in 20µl of PBS was added to 320µl of citrated human blood followed by 20µl 200mM CaCl2. The head assembly of the analyzer was closed 10s after the start button was pressed. Data were acquired and analyzed with Signature Viewer software (Sienco, Inc.). The results are mean ±SD of three experiments.
Neutralization studies
For neutralization studies, various amount of polyvalent antivenom was pre-incubated with 1µg of Naja kaouthia venom in a final volume of 20µl for 1hr at 37°C and assays were performed as described above. The percentage inhibition was calculated by considering the activity in absence of polyvalent antivenom as 100%. The results are mean ±SD of three experiments.
RESULTS
Biological characterization
The biochemical and biological activities of the crude venom are listed in Table 1. The median lethal dose (LD50) was found to be 0.148mg/kg when injected intraperitoneally to experimental mice. When sub-lethal dose of venom was injected to mice, neurotoxic symptoms like difficulty in movement; breathing and frequent drinking of water were observed followed by death after 40min. The amount of CK and LDH released after injection of 15µg of venom was found to be 6.605U/l and 26.38U/l respectively in the plasma. The CK was 10 times more than observed for the control mice (0.63U/l), however, the LDH was found to be only 3U more. The minimum edema dose (MED) of the venom was found to be 11.25µg. No direct hemolytic activity was observed up to 10µg of venom but when the amount was increased up to 100µg, it exhibited 1.4% RBC hemolysis. For indirect hemolytic activity, 23% hemolysis was observed for 1µg of venom. The venom showed weak proteolytic activity when tested on casein. The amount of tyrosine liberated was 0.14±0.02 moles by 100µg of venom in 1min. PLA2 activity of the venom was 7.584µmol/min/mg when assayed using sPLA2 assay kit. However no haemorraghic spot was observed when 3µg of venom was injected intradermally (Figure 1).
Table 1.
Some biochemical and biological activities of Naja kaouthia venom
| Parameters | Activity |
|---|---|
| LD50 | 0.148 mg/kg |
| PLA2 activity assay | 7.9±0.24Ψ |
| Direct hemolytic assay (100µg venom) | 1.4±0.51% |
| Indirect hemolytic assay ( 1µg venom) | 23.0 ±3% |
| Caseinolytic activity (100µg venom) | 0.14±0.02* |
| Creatine kinase (CK) ( 15µg i.m. injection) | 6.6±0.2 U/l |
| Lactate dehydrogenase (LDH) ( 15µg i.m. injection) | 26.3±2.3U/l |
| Minimum edema dose (MED) | 11.2±0.18 µg |
| Haemorraghic activity (up to 15µg) | NA |
Normal CK and LDH values are 0.63 U/l and 23.39 U/l respectively; Ψµmol of diheptanoyl Thiol-PC hydrolyzed/min/mg; *n moles of tyrosine formed/min; NA= No Activity. Results are mean ±SD (n=3)
Figure 1.
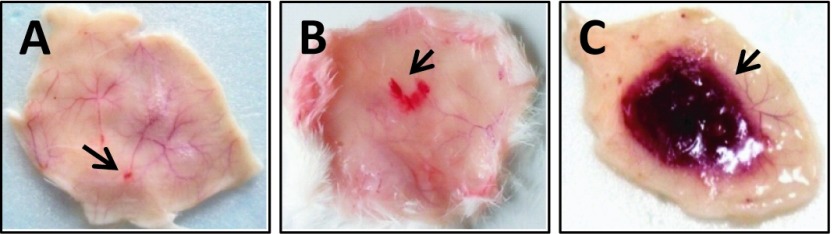
Haemorraghic activity of Naja kaouthia venom. A. Control (30µl of saline), B. Naja kaouthia venom (15µg), C. Saw scaled viper venom (3µg) (Positive control), the arrow indicates site of injection.
In-vitro coagulation activities
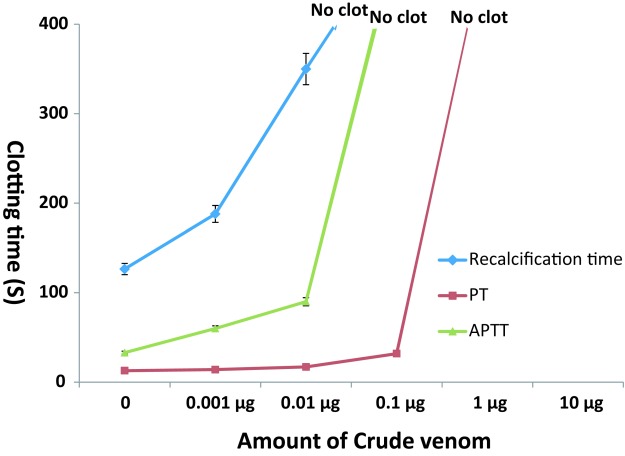
The venom showed anticoagulant activity in dose dependent manner. When recalcification time of human plasma was tested with 1µg venom, the plasma did not form clot up to 500s whereas the normal clotting time was 126.5sec (Figure 2). Prothrombin time increased dose dependently and at 0.1µg, clot formation was not observed up to 500sec. The APTT test on plasma did not increase significantly up to 0.1µg but when the amount was increased to 1µg venom the plasma did not form clot (Figure 2).
Figure 2.
Dose dependent anticoagulant activity of Naja kaouthia venom on human plasma. Effect of crude venom on Recalcification time, Prothrombin Time test (PT) and Activated Partial Thrombin Time test (APTT). The results are mean ±SD of three experiments.
In Rotem® Analyzer, coagulation time (CT) for 0.1µg venom was 634±15sec and for the control it was observed to be 503±10sec. When the amount of the venom was increased to 1 and 10µg, clot formation was not observed which is depicted by a straight line (Data not shown). The clot formation time (CFT) in presence of 0.1µg of venom was recorded to be 266±10sec, whereas the CFT for control plasma was only 87±3s (Table 2). Maximum clot firmness (MCF) value at 0.1µg of venom was 61±1.3mm, whereas for the control the value was 65±2mm. However, at higher concentration of venom the blood clot did not form. Hence, the values were not measurable in the Rotem® analyzer (Table 2).
Table 2.
Anticoagulant activity of Naja kaouthia venom on whole citrated blood. Results are expressed as mean ±SD of three experiments.
| Parameters | PBS | Crude venom (µg/ml) | ||
|---|---|---|---|---|
| 0.1 | 1.0 | 10 | ||
| Thromboelastometry analysis | ||||
| Coagulation time (CT) (s) | 503±10 | 634±15 | >1200 | >1200 |
| Clot formation time (CFT) (s) | 87±3 | 266±10 | NCF | NCF |
| Maximum clot firmness (MCF) (mm) | 65±2 | 61±1.3 | NCF | NCF |
| Sonoclot analysis | ||||
| Activated clotting time (ACT) (s) (range: 128–213) | 176±5.2 | 215±7.4 | 243±6.3 | 591±10 |
| Clot rate (CR)(range: 9.0–35) | 23±0.5 | 23±0.32 | 16±0.21 | 1.2±0.2 |
| Platelet function (range: 3–5) | 2.8±0.01 | 3.8±0.02 | 3.3±0.01 | 0 |
NCF: No clot formation, the results are expressed as mean ± SD
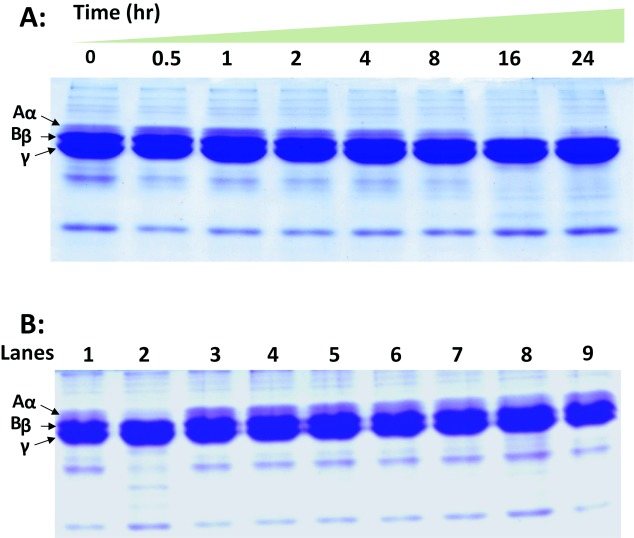
The activated clotting time (ACT) increases dose dependently and at 10µg of venom it was recorded to be 591±1.3sec in Sonoclot Coagulation and Platelet Function Analyzer. At 0.1µg venom the clot rate was similar to normal clot rate (normal range 9–35sec) but with increase in concentration, the clot rate decreased which might be due to depletion of fibrinogen. However, up to 1.0µg the platelet function was found to be normal but at 10µg the platelet function was not observed (Table 2). Lower amount of venom did not show any digestion of fibrinogen (data not shown). However, when the amount of venom was increased to 10µg, clear digestion of α chain of fibrinogen was observed after 16hr of incubation (Figure 3A).
Figure 3.
A. Fibrinogenolytic activity of Naja kaouthia venom. SDS-PAGE of bovine fibrinogen (reduced) after incubation with 10µg crude Naja kaouthia venom at various time intervals. B. Inhibition of fibrinogenolytic activity of Naja kaouthia by polyvalent antivenom. The venom:polyvalent antivenom (1:1, w/w) mixture was pre-incubated for 1hr at 37°C. This mixture was incubated with 300μl of fibrinogen (2mg/ml) for 24hr and aliquots were withdrawn at different time interval and fractionated in 12.5% (w/v) SDS-PAGE. Lane 1. Undigested fibrinogen (control). Lane 2. Fibrinogen incubated with only venom; Lane 3. After 0.5hr; Lane 4. 1hr; Lane 5. After 2hr; Lane 6. After 4hr; Lane 7. After 8hr; Lane 8. After 16hr; and Lane 9. After 24hr.
Neutralization studies
Effect of polyvalent antivenom on some of the biochemical and biological properties of Naja kaouthia venom are shown in Table 3. At 1:1 ratio, the polyvalent antivenom could not neutralize the PLA2 activity of the venom but at 1:100 ratios, 97.38 ± 4.8% inhibition was observed. Inhibition of the indirect hemolytic activity of venom was also observed similar to the PLA2 activity. When the concentration of the polyvalent antivenom was increased by 100 times, indirect hemolytic activity was completely neutralized. Recalcification time of the venom was neutralized up to 49.34% at 1:1 ratio and with 10 times increase in polyvalent antivenom, 92.03% neutralization was observed. Similarly, the APTT and PT was also brought to the normal clotting time when the polyvalent antivenom was 10 times excess of the venom concentration. Moreover, degradation of α chain of fibrinogen by venom was inhibited by polyvalent antivenom at 1:1 ratio (Figure 3B).
Table 3.
In vitro neutralization of whole venom activity by polyvalent antivenom
| Activity | % inhibition by polyvalent antivenom | ||
|---|---|---|---|
| 1:1 | 1:10 | 1:100 | |
| PLA2 activity | 0 | 40.0±5.0 | 97.38 ± 4.8 |
| Indirect hemolytic | 11.96±2.12 | 68.15±0.15 | 100 |
| Recalcification time | 49.34±5.01 | 92.03±3.0 | 96.52±2.81 |
| PT | 36.44±5.8 | 78.19±3.86 | 99±1.76 |
| APTT | 32.33±6.44 | 92.1±5.83 | 100 |
| Fibrinogenolytic | α chain present | α chain present | α chain present |
The results are expressed as mean ± SD (n=3)
Values indicate % inhibition at each venom:antivenom (µg:µg) ratio
DISCUSSION
The patho-physiological effect post-snakebite envenomation varies greatly among the various species and even within species due to variation in the venom proteins and biological activities (Glenn et al, 1983; Minton and Weinstein, 1986; Daltry et al, 1996; Saravia et al, 2002; Menezes et al, 2006). These variations affect the clinical manifestation of envenomation and require specific consideration for treatment. Hence understanding the biochemical and biological properties of snake venom from a particular geographic location is important.
The LD50 of the Naja kaouthia venom was found to be 0.148mg/kg, whereas those for cobra venoms of Thailand and Kolkata origin were reported to be 0.23mg/kg and 0.7mg/kg, respectively (Mukherjee and Maity, 2002; Leong et al, 2012). Though the route of injection was different (Kolkata origin venom given via tail vein injection) in these experiments, the lethal dose of North East origin venom was less than that of the other geographical locations suggesting it might be more lethal. However, the comparative study with indistinguishable experimental conditions would be necessary to differentiate these venoms. In mice the venom did not induce haemorraghic activity and venom of Kolkata origin is reported to be devoid of such activities. The haemorraghic is mainly caused by metalloproteases, which are abundantly found in viper venom (Kamiguti et al, 1996; Chakrabarty et al, 2000; Mukherjee, 2008). Moreover, the edema inducing activity was not found to be significant. Hence this venom might not induce inflammation and tissue damage at the site of bite. Interestingly, the venom at 100µg showed only 1.4% hemolysis of RBC, whereas at the same amount Kolkata venom activity is reported to be 39.0% (Mukherjee and Maity, 2002). The membrane damaging activity is mainly contributed by the low molecular weight proteins which might be absent in this venom. The indirect hemolytic activity of the venom in presence of the egg yolk is due to PLA2 enzymes. The lysophospholipids and free fatty acids formed during the catalysis of phospholipids by PLA2 enzyme exhibits this activity as they are lytic in nature (Condrea et al, 1964). The presence of various PLA2 isoenzymes and neurotoxins in Naja kaouthia venom have been reported by various workers (Joubert and Taljaard, 1980; Meng et al, 2002; Qiumin et al, 2002; Doley et al, 2004). When the crude venom was analyzed for the PLA2 activity using diheptanoyl Thiol-PC as substrate, the amount of substrate hydrolyzed product was 7.9±0.24µmol/min/mg suggesting the presence of enzymatically active PLA2 in the venom. PLA2 is one of the major constituent in the elapid venom, which confers multiple toxicity to the prey or victim such as membrane damaging, neurotoxicity, edema and prolongation of coagulation time (Kini and Evans, 1989; Doley et al, 2004). Hence the myotoxicity, neurotoxicity and edema induced by this venom are due to the presence of large amount of PLA2 enzyme in the venom. The observed differences in the biochemical and biological activities in the venoms of Indian origin might be due to variation in the venom composition and content due to difference in geographical locations. Both venoms were collected during summers; however, in the present study, the ages of the snakes were unknown as they were captured from the wild. Detailed analysis of Naja kaouthia venoms from different locations of India need to be carried out to decipher the differences in the venom composition as well as the presence of unique toxins.
Snake venom proteins affect the haemostasis process of victim/prey either by prolonging or shortening the clotting time. Elapid venoms are anticoagulant in nature due to the presence of large amount of strong and weak anticoagulant PLA2 enzymes. Moreover, non-enzymatic protein from elapid venom like Cardiotoxins from Naja nigricollis crawshawii and Hemextin A and hemextin AB complex from Hemachatus haemachatus venom are also reported to be anticoagulant in nature (Kini et al, 1988; Banerjee et al, 2005). The venom significantly delayed the recalcificaion time, PT and APTT of plasma under in vitro condition, which is due to strong anticoagulant proteins present in the venom. The plasma did not form clot at 0.01, 0.1 and 1µg concentration of venom when tested for recalcificaion time, PT and APTT, respectively. This suggests that the anticoagulant activity of the venom is most likely to affect all the pathways. Venom PLA2 enzymes inhibit activation of FX to FXa which leads to disruption in the formation of prothrombinase complex, which is required for blood coagulation (Stefansson et al, 1990; Kerns et al, 1999; Kini, 2005). The higher amount of venom required in case of PT and APTT for non-coagulation of blood might be due to the addition of extra phospholipids during these tests; however, this needs to be verified. The venom proteins, especially the PLA2 enzymes, hydrolyze the phospholipids which are required for the prothrombinase complex formation. The Sonoclot and Rotem analysis also demonstrated that the Naja kaouthia venom is anticoagulant in nature. The whole citrated blood analysis by sonoclot clearly indicated the depletion of fibrinogen in the reaction when pre-incubated with venom. The lower value of MCF by Rotem analysis indicates decreased platelet number or function, decreased fibrinogen level or fibrin polymerization disorders, or low activity of factor XIII. Recently, Nk a mettaloprotease, which cleaves the α- chain, as well as a low molecular protein with fibrin(ogen)olytic activity have been reported (Wijeyewickrema et al, 2007; Debnath et al, 2010). The weak proteolytic activity towards casein and higher amount of venom and time required for complete degradation of α chain of bovine serum fibrinogen might be due to presence of these proteins in lower amount. Hence anticoagulant activity of Naja kaouthia might not be only due to degradation of phospholipids or α chain of fibrinogen but action of different venom proteins which might be acting enzymatically or non-enzymatically on coagulation factors and complexes.
Polyvalent antivenom is currently used by the medical practitioners for the treatment of snakebite patients in India. The Indian polyvalent antivenom is prepared using the venoms of four major poisonous snake species viz: Naja naja, Daboia russelii, Echis carinatus and Bungarus caeruleus. In most of the cases, it has been observed that the efficacy is highly reduced when antivenoms raised against venom from a particular geographic region is used to treat victims from another region (Shashidharamurthy et al, 2002; Shashidharamurthy and Kemparaju, 2007). The polyvalent antivenom could neutralize some of the biochemical and biological activity partially at 1:10 ratio (venom: polyvalent antivenom) and complete neutralization was observed when the dose of the polyvalent antivenom was increased to 10 fold. The partial inhibition might be due to the antibodies of Naja naja proteins present in the polyvalent antivenom, which recognizes the Naja kaouthia venom proteins. Present study documents that the polyvalent antivenom can neutralize some of tested biochemical and biological activities of Naja kaouthia venom under in vitro condition.
ACKNOWLEDGEMENTS
We acknowledge DBT, Govt of India, for grant under twinning programme for NER India, Tezpur University for the start-up grant and DBT, UGC and DST, New Delhi, for various grants to the department. We also thank the anonymous reviewers for their valuable comments and suggestions that have improved the manuscript.
LIST OF ABBREVIATIONS
- CFT
clot formation time
- CT
Coagulation time
- MCF
Maximum clot firmness
- ACT
Activated clotting time
COMPETING INTERESTS
None declared.
REFERENCES
- Agarwal R, Aggarwal AN, Gupta D. Elapid snakebite as a cause of severe hypertension. J Emerg Med. 2006;30:319–320. doi: 10.1016/j.jemermed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Banerjee Y, Mizuguchi J, Iwanaga S, Kini RM. Hemextin AB complex--a snake venom anticoagulant protein complex that inhibits factor VIIa activity. Pathophysiol Haemost Thromb. 2005;34:184–187. doi: 10.1159/000092420. [DOI] [PubMed] [Google Scholar]
- Chakrabarty D, Datta K, Gomes A, Bhattacharyya D. Haemorrhagic protein of Russell’s viper venom with fibrinolytic and esterolytic activities. Toxicon. 2000;38:1475–1490. doi: 10.1016/s0041-0101(99)00243-3. [DOI] [PubMed] [Google Scholar]
- Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76:515–524. [PMC free article] [PubMed] [Google Scholar]
- Condrea E, Mammon Z, Aloof S, Devries A. Susceptibility of erythrocytes of various animal species to the hemolytic and phospholipid spitting action of snake venom. Biochim Biophys Acta. 1964;84:365–375. doi: 10.1016/0926-6542(64)90001-0. [DOI] [PubMed] [Google Scholar]
- Daltry JC, Wuster W, Thorpe RS. Diet and snake venom evolution. Nature. 1996;379:537–540. doi: 10.1038/379537a0. [DOI] [PubMed] [Google Scholar]
- Debnath A, Saha A, Gomes A, et al. A lethal cardiotoxic-cytotoxic protein from the Indian monocellate cobra (Naja kaouthia) venom. Toxicon. 2010;56:569–579. doi: 10.1016/j.toxicon.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Doley R, King GF, Mukherjee AK. Differential hydrolysis of erythrocyte and mitochondrial membrane phospholipids by two phospholipase A2 isoenzymes (NK-PLA2-I and NK-PLA2-II) from the venom of the Indian monocled cobra Naja kaouthia. Arch Biochem Biophys. 2004;425:1–13. doi: 10.1016/j.abb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Glenn JL, Straight RC, Wolfe MC, Hardy DL. Geographical variation in Crotalus scutulatus scutulatus (Mojave rattlesnake) venom properties. Toxicon. 1983;21:119–130. doi: 10.1016/0041-0101(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Gutierrez JM, Williams D, Fan HW, Warrell DA. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon. 2010;56:1223–1235. doi: 10.1016/j.toxicon.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Halesha BR, Harshavardhan L, Lokesh AJ, Channaveerappa PK, Venkatesh KB. A study on the clinico-epidemiological profile and the outcome of snake bite victims in a tertiary care centre in southern India. J Clin Diagn Res. 2013;7:122–126. doi: 10.7860/JCDR/2012/4842.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JK, Simpson ID, Menon NC, et al. First authenticated cases of life-threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans R Soc Trop Med Hyg. 2007;101:85–90. doi: 10.1016/j.trstmh.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Joubert FJ, Taljaard N. Snake venoms. The amino acid sequences of two Melanoleuca-type toxins. Hoppe Seylers Z Physiol Chem. 1980;361:425–436. doi: 10.1515/bchm2.1980.361.1.425. [DOI] [PubMed] [Google Scholar]
- Kamiguti AS, Hay CR, Theakston RD, Zuzel M. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon. 1996;34:627–642. doi: 10.1016/0041-0101(96)00017-7. [DOI] [PubMed] [Google Scholar]
- Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns RT, Kini RM, Stefansson S, Evans HJ. Targeting of venom phospholipases: the strongly anticoagulant phospholipase A(2) from Naja nigricollis venom binds to coagulation factor Xa to inhibit the prothrombinase complex. Arch Biochem Biophys. 1999;369:107–113. doi: 10.1006/abbi.1999.1345. [DOI] [PubMed] [Google Scholar]
- Khandelwal G, Katz KD, Brooks DE, Gonzalez SM, Ulishney CD. Naja Kaouthia: two cases of Asiatic cobra envenomations. J Emerg Med. 2007;32:171–174. doi: 10.1016/j.jemermed.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Kini RM. Structure-function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon. 2005;45:1147–1161. doi: 10.1016/j.toxicon.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Kini RM, Evans HJ. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 1989;27:613–635. doi: 10.1016/0041-0101(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Kini RM, Haar NC, Evans HJ. Non-enzymatic inhibitors of coagulation and platelet aggregation from Naja nigricollis venom are cardiotoxins. Biochem Biophys Res Commun. 1988;150:1012–1016. doi: 10.1016/0006-291x(88)90729-2. [DOI] [PubMed] [Google Scholar]
- Kondo H, Kondo S, Ikezawa H, Murata R. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn J Med Sci Biol. 1960;13:43–52. doi: 10.7883/yoken1952.13.43. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalloo DG, Theakston RD. Snake antivenoms. J Toxicol Clin Toxicol. 2003;41:277–290. doi: 10.1081/clt-120021113. [DOI] [PubMed] [Google Scholar]
- Leong PK, Sim SM, Fung SY, et al. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro Polyvalent Snake Antivenom) PLoS Negl Trop Dis. 2012;6:e1672. doi: 10.1371/journal.pntd.0001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Meier J, Theakston RD. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon. 1986;24:395–401. doi: 10.1016/0041-0101(86)90199-6. [DOI] [PubMed] [Google Scholar]
- Menezes MC, Furtado MF, Travaglia-Cardoso SR, Camargo AC, Serrano SM. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon. 2006;47:304–312. doi: 10.1016/j.toxicon.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Meng QX, Wang WY, Lu QM, et al. A novel short neurotoxin, cobrotoxin c, from monocellate cobra (Naja kaouthia) venom: isolation and purification, primary and secondary structure determination, and tertiary structure modeling. Comp Biochem Physiol C Toxicol Pharmacol. 2002;132:113–121. doi: 10.1016/s1532-0456(02)00049-2. [DOI] [PubMed] [Google Scholar]
- Minton SA, Weinstein SA. Geographic and ontogenic variation in venom of the western diamondback rattlesnake (Crotalus atrox) Toxicon. 1986;24:71–80. doi: 10.1016/0041-0101(86)90167-4. [DOI] [PubMed] [Google Scholar]
- Mohapatra B, Warrell DA, Suraweera W, et al. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5:e1018. doi: 10.1371/journal.pntd.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee AK. Correlation between the phospholipids domains of the target cell membrane and the extent of Naja kaouthia PLA(2)-induced membrane damage: evidence of distinct catalytic and cytotoxic sites in PLA(2) molecules. Biochim Biophys Acta. 2007;1770:187–195. doi: 10.1016/j.bbagen.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Mukherjee AK. Characterization of a novel pro-coagulant metalloprotease (RVBCMP) possessing alpha-fibrinogenase and tissue haemorrhagic activity from venom of Daboia russelli russelli (Russell’s viper): evidence of distinct coagulant and haemorrhagic sites in RVBCMP. Toxicon. 2008;51:923–933. doi: 10.1016/j.toxicon.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Mukherjee AK, Maity CR. Biochemical composition, lethality and pathophysiology of venom from two cobras-- Naja naja and N. kaouthia. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:125–132. doi: 10.1016/s1096-4959(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Offerman SR, Smith TS, Derlet RW. Does the aggressive use of polyvalent antivenin for rattlesnake bites result in serious acute side effects? West J Med. 2001;175:88–91. doi: 10.1136/ewjm.175.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang C, Teng CM. Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim Biophys Acta. 1976;420:298–308. doi: 10.1016/0005-2795(76)90321-4. [DOI] [PubMed] [Google Scholar]
- Qiumin L, Qingxiong M, Dongsheng L, et al. Comparative study of three short-chain neurotoxins from the venom of Naja kaouthia (Yunnan, China) J Nat Toxins. 2002;11:221–229. [PubMed] [Google Scholar]
- Saravia P, Rojas E, Arce V, et al. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: pathophysiological and therapeutic implications. Rev Biol Trop. 2002;50:337–346. [PubMed] [Google Scholar]
- Sekhar CC, Chakrabarty D. Fibrinogenolytic toxin from Indian monocled cobra (Naja kaouthia) venom. J Biosci. 2011;36:355–361. doi: 10.1007/s12038-011-9068-3. [DOI] [PubMed] [Google Scholar]
- Shashidharamurthy R, Jagadeesha DK, Girish KS, Kemparaju K. Variations in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol Cell Biochem. 2002;229:93–101. doi: 10.1023/a:1017972511272. [DOI] [PubMed] [Google Scholar]
- Shashidharamurthy R, Kemparaju K. Region-specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern regional venom: A comparative study of the venoms from three different geographical distributions. Int Immunopharmacol. 2007;7:61–69. doi: 10.1016/j.intimp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Stefansson S, Kini RM, Evans HJ. The basic phospholipase A2 from Naja nigricollis venom inhibits the prothrombinase complex by a novel nonenzymatic mechanism. Biochemistry. 1990;29:7742–7746. doi: 10.1021/bi00485a024. [DOI] [PubMed] [Google Scholar]
- Suntravat M, Nuchprayoon I, Perez JC. Comparative study of anticoagulant and procoagulant properties of 28 snake venoms from families Elapidae, Viperidae, and purified Russell’s viper venom-factor X activator (RVV-X) Toxicon. 2010;56:544–553. doi: 10.1016/j.toxicon.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viravan C, Looareesuwan S, Kosakarn W, et al. A national hospital-based survey of snakes responsible for bites in Thailand. Trans R Soc Trop Med Hyg. 1992;86:100–106. doi: 10.1016/0035-9203(92)90463-m. [DOI] [PubMed] [Google Scholar]
- Vishwanath BS, Kini RM, Gowda TV. Purification and partial biochemical characterization of an edema inducing phospholipase A2 from Vipera russelli (Russell’s viper) snake venom. Toxicon. 1988;26:713–720. doi: 10.1016/0041-0101(88)90278-4. [DOI] [PubMed] [Google Scholar]
- Whitaker R. The Macmillan Co.; (India): 1978. The Venomous Snakes. Common Indian Snakes. [Google Scholar]
- Wijeyewickrema LC, Gardiner EE, Shen Y, Berndt MC, Andrews RK. Fractionation of snake venom metalloproteinases by metal ion affinity: a purified cobra metalloproteinase, Nk, from Naja kaouthia binds Ni2+-agarose. Toxicon. 2007;50:1064–1072. doi: 10.1016/j.toxicon.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Williams DJ, Jensen SD, Nimorakiotakis B, Muller R, Winkel KD. Antivenom use, premedication and early adverse reactions in the management of snake bites in rural Papua New Guinea. Toxicon. 2007;49:780–792. doi: 10.1016/j.toxicon.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Nozaki M, Hokoma Z. Fractionation of Sakishima habu (Trimeresurus elegans) venom and lethal hemorrhagic and edema forming activity of the fraction. In: Ohsaka A, Hayashi K, Sawai Y, editors. Animal Plant and Microbial Toxins. New York: Plenum Press; 1976. pp. 97–109. (Eds) [Google Scholar]