Abstract
Background
Blacks and Hispanics in the United States (US) have the lowest survival rates of hepatocellular carcinoma (HCC), mainly associated to the presence of advanced disease at diagnosis when intervention is least beneficial. This study compared the survival distribution and relative survival of HCC in Puerto Rico (PR) during 1988-1992 and 1998-2002.
Methods
All HCC cases in the PR Central Cancer Registry database for 1988-1992 (n=306) and 1998-2002 (n=333) were identified. Patient characteristics and clinical variables were compared between study periods. Survival by age at diagnosis, sex, tumor stage and treatment was estimated using the Kaplan-Meier method, and survival curves were compared using the Wilcoxon test. A Cox proportional hazards model was employed to assess the effect of period of diagnosis on survival, after adjusting for confounders. One- and three-year survival rates were also calculated.
Results
Patients diagnosed during 1998-2002 (median: 3.08 months, 95% CI: 2.30-4.16) had a longer observed survival than those diagnosed from 1988-1992 (median: 1.80 months, 95% CI: 1.44-2.52). A significant interaction was observed between the variables age and period of diagnosis, where only among persons aged ≥ 60 years the risk of HCC death was lower (sex-adjusted HR=O.72; 95%CI: 0.59-0.88) in patients diagnosed during 1998-2002 as compared to those diagnosed during 1988-1992. The overall one- and three-year relative survival during 1998-2002 was approximately 6% (22.4% vs.16.6%) and 2% higher (9.0% vs. 6.7%) respectively, as compared to 1988-1992.
Conclusion
We observed a temporal improvement in the survival of HCC in PR during the last decade. However, this survival is inferior to the one observed in the US population. Further studies are needed to identify factors that explain these disparities.
Keywords: Hepatocellular carcinoma, Epidemiology, Survival
Primary liver cancer is the third most common cause of cancer-related mortality worldwide and the cancer with the fastest increasing trend in the United States (US) (1-2). The age-adjusted incidence rate of hepatocellular carcinoma (HCC) in the US increased two-fold from a yearly age-adjusted rate of 1.3/100,000 during 1978-1980 to 3.0/100,000 during 1996-1998 (3). In Puerto Rico (PR), the overall age-adjusted incidence rate of liver cancer was 6.1 per 100,000 from 1999-2003 (4). Meanwhile, the age-adjusted incidence rates increased an average of 2.4% per year in men (p<0.05) and 1.0% per year in women (p>0.05) from 1987-2003 (4). Most cases of HCC develop in persons with chronic liver disease, and an increase in the prevalence of cirrhosis, mostly secondary to chronic infection with hepatitis C virus (HCV), is the likely explanation for the rising incidence of HCC (2).
Chronic hepatitis C is estimated to affect 1.5% of the US population and leads to cirrhosis in 20% to 30% of individuals within 20 to 30 years, with a predicted annual rate of HCC in cirrhotics of 1% to 6% (5-6). A recent population-based study in the municipality of San Juan, Puerto Rico (PR) revealed a higher HCV seroprevalence (6.3%, 95% CI: 3.6%-10.9%) in comparison to estimates in other metropolitan areas of Spain, France and Northern Italy (7). Therefore, the incidence of HCC in PR is expected to increase further in the next decades as a result of the projected higher prevalence of HCV-related cirrhosis, making HCC an increasingly demanding health care problem.
Despite the improved survival observed in other cancers, the prognosis of HCC in the US population is still grim, with one and three-year relative survival rates of 36% and 17%, respectively, during 1998-2000 (2). This dismal prognosis of HCC patients is associated to the presence of advanced disease at the time of diagnosis, when intervention is least likely to be beneficial. Patients with early stage HCC (tumor size of 5 cm or less) treated with surgical resection, liver transplantation, chemoembolization or radiofrequency ablation have shown five-year survival rates between 60% and 70% (8-10). Up to 25%-30% of patients with HCC in US, Europe and Japan are in this group. Thus, effective treatment is available for patients with HCC, especially if detected early.
A recent US population-based study examining racial/ethnic differences in survival of HCC during 1987-2001 showed that Blacks and Hispanics had the lowest survival, independent of stage of disease at presentation (11). These variations in survival were partly explained by a lower likelihood of receiving therapy and more advanced HCC at diagnosis in Blacks and Hispanics. These findings suggest that an improvement in screening strategies and treatment access in these populations may improve survival.
Even though the epidemiology of HCC varies among different ethnic groups, information on HCC survival in the Puerto Rican population is scarce. We compared the overall and relative survival (one- and three-year) of HCC in PR during the periods of 1988-1992 and 1998-2002. An improvement in survival in the past decade may reflect an earlier detection of HCC, appropriate receipt of treatment or both. Alternatively, similar survival rates within the last decade would support the need of improving screening strategies and adequate treatment access in our population.
Methods
Data Source
The PR Central Cancer Registry (PRCCR) is one of the oldest population-based registries in the Americas (12). It was established in 1951 and collects data of newly diagnosed cancer cases in PR. The PRCCR maintains a continued search of information in hospitals, outpatient clinics (both public and private), pathology laboratories and radiotherapy/chemotherapy sites located throughout the island. For each case identified, the PRCCR collects demographic characteristics, date of cancer diagnosis, anatomic cancer site, histologic type, method of diagnosis, stage of disease at diagnosis, therapy and follow-up status. In addition, the PRCCR obtains information on vital status and cause of death from all incident cancer cases from the Division of Statistical Analysis, Auxiliary Secretariat for Planning and Development, Puerto Rico Deparment of Health. The International Classification of Diseases for Oncology (ICD-O) is used by the PRCCR database for codification of malignant tumors (13). In 1997, the PRCCR joined the National Program of Cancer Registries (NPCR) administered by the Centers for Disease Control and Prevention (CDC). In 2003, an audit performed by the CDC revealed that 95.3% of cases diagnosed or treated in Puerto Rican medical facilities were reported appropriately to the PRCCR (12).
Study Population
All incident cases from the PRCCR database with a diagnosis of primary HCC (ICD-02 codes 155.0 and 155.1 and ICD-03 codes C220 and C221) with histological confirmation (ICD-02, ICD-03 codes 8170 or 8180) diagnosed during the following two periods were included: January 1, 1988 to December 31, 1992 and January 1, 1998 to December 31, 2002. Only cases with diagnostic confirmation of HCC were included in our analysis. Diagnostic confirmation was defined as having positive histology (78.6%), positive cytology (2.4%), positive laboratory test/serum marker (0.2%), direct visualization (0.3%), positive radiology (14.1%) and clinical diagnosis (4.5%). Patients with unknown method of confirmation (n=146) and those with other malignancies (n=40) were excluded.
Statistical Analysis
Demographic characteristics (age and sex), stage of HCC at diagnosis and receipt of therapy were compared between the two periods of HCC diagnosis using Chi-square or Fisher’s exact test, when appropriate. Stage of disease at diagnosis was categorized using the Surveillance, Epidemiology, and End Results (SEER) staging defined as: localized (confined to the primary site), regional (spread to regional lymph nodes or by direct extension beyond the primary site), distant (metastatic spread) and unstaged. Depending on the year at diagnosis, PRCCR applied the stage at diagnosis according to SEER Summary Staging Guide 1977 (14) and SEER Summary Staging Manual 2000 (15). These staging rules are not compatible with each other, with discordance in stage of disease mainly involving regional and distant categories (15). For this reason, all HCC reports were examined and reviewed manually by the lead author to ensure accuracy in the staging variable, and cases diagnosed before December 31, 2000 were restaged to the latest staging rules (SEER Summary 2000). Receipt of therapy included surgery (resection or transplantation), chemotherapy or unknown.
One- and three-year observed and relative survivals were calculated using the incidence case file database of PR and estimated using the Kaplan Meier method. The observed survival rate is equivalent to the number of patients remaining alive for a specific time-span divided by the total number of patients at risk of death during that specified time period. The relative survival, which is an alternative to calculating cancer specific mortality, was calculated as the ratio of the observed survival to the expected survival for a group of people in a general population that is similar to that of the patient group with respect to race, sex, age, and calendar period of observation (16). Thus, the relative survival of PR was calculated using the expected survival of the Puerto Rican population.
Survival by period, age at diagnosis, sex, tumor stage and treatment were estimated using the Kaplan-Meier method and compared using the Wilcoxon test (17). The selection of the Wilcoxon test was based on the fact that this test statistic gives more weight to early survival times (when more cases were available for analysis given the high mortality of HCC patients since the beginning of the interval). Cases lost to follow up and those alive at the end of each follow-up period were considered censored observations. Cox regression models were used to compare HCC survival by period of diagnosis while adjusting for relevant covariates. The proportional hazards assumption was evaluated by plotting the graph of the log(-log(survival)) versus log of survival time graph (which results in parallel lines if the predictor is proportional) and by evaluating the significance of including time dependent covariates in the Cox model, by including interactions of the predictors and the log of the survival time. The significance of these time-dependent covariates was tested with the proportionality test (PROC PHREG in SAS) (SAS, 2008) (18). Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC) and SEER *Stat 6.4.4 (19). The Institutional Review Board of the University of Puerto Rico approved this study, and permission from the Director of the PRCCR to review their files was obtained prior to initiation.
Results
A total of 639 patients with HCC were identified for both study periods: 306 patients during 1988-1992 and 333 subjects during 1998-2002 (Table 1). Less than 2% of the study population had an age of diagnosis below 40 years (data not shown). In both study periods, the largest proportion of patients was diagnosed at the age of 60 years and older (71.2% in 1988-1992 and 66.5% in 1998-2002). No significant (p>0.05) difference in the proportion of HCC patients by age was observed between the two periods. A similar proportion of men and women were diagnosed with HCC in 1988-1992 and in 1998-2002 (p>0.05), with approximately 75% of all patients being men. In terms of stage of HCC at diagnosis, 51.6% of HCC cases diagnosed during 1988-1992 had localized disease, 13.1% had regional extent, 16.3% had distant involvement, and 19.0% had unknown staging. During the second study period, 34.8% of patients had localized disease, 11.7% had regional extent, 14.1% had distant metastases, and 39.3% were unknown. Although there were significant (p<0.0001) differences in the stage of HCC at diagnosis between the two periods, the large amount of unstaged for both study periods (19.0% and 39.3%, respectively) precluded reaching any conclusions with regard to this difference. Similar significant (p<0.0001) differences were seen in terms of receipt of therapy, yet again, 85.3% of cases diagnosed during 1988-1992 and 27.3% of those diagnosed during 1998-2002 had unknown status for receipt of therapy.
Table 1.
Demographic and tumor features of HCC patients by period of diagnosis (n=639).
| Variable | 1988-1992 (n = 306) |
1998-2002 (n = 333) |
P value |
|---|---|---|---|
| Age (yrs) * | |||
| < 60 | 88 (28.7%) | 111 (33.5%) | 0.19 |
| ≥ 60 | 218 (71.2%) | 220 (66.5%) | |
| Sex | |||
| Male | 226 (73.9%) | 255 (76.6%) | 0.43 |
| Female | 80 (26.1%) | 78 (23.4%) | |
| Stage at diagnosis | |||
| Localized | 158 (51.6%) | 116 (34.8%) | <0.0001 |
| Regional | 40 (13.1 %) | 39 (11.7%) | |
| Distant | 50 (16.3%) | 47 (14.1%) | |
| Unstaged | 58 (19.0%) | 131 (39.3%) | |
| Receipt of therapy | |||
| None | 1 (0.3%) | 193 (58.0%) | <0.0001 |
| Any** | 44 (14.4%) | 49 (14.7%) | |
| Unknown | 261 (85.3%) | 91 (27.3%) |
Missing: n=2
Surgery or Chemotherapy
Since individuals with localized disease are more likely to benefit from therapy, in subjects for whom information was available, we examined the proportion of patients with favorable (localized) disease who received therapy in both study periods. Among individuals diagnosed during 1988-1992, 25/44 (56.8%) patients who received any form of therapy had localized disease. All of these patients (25/25) received systemic chemotherapy and none received surgical therapy. Among patients diagnosed during the second study period, 24/49 (49.0%) patients who received any therapy had localized disease. Of these 24 patients, 13 (54.2%) received surgical therapy, 9 (37.5%) received systemic chemotherapy and 2 patients (8.3%) received both therapies (data not shown).
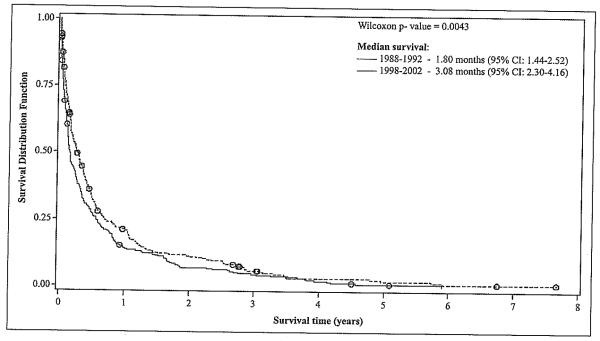
The cumulative observed survival of HCC patients by study period is presented in Figure 1. Patients diagnosed during the second study period (1998-2002) had significantly (p<0.01) longer observed survival (median= 3.08 months, 95% CI: 2.30-4.16) compared to those diagnosed in the first study period (median =1.8 months, 95% CI: 1.44-2.52). These differences were more pronounced during the first year after HCC diagnosis. The cumulative observed survival was not affected by age (p>0.05) or sex (p>0.05) (data not shown).
Figure 1.
HCC Survival after Diagnosis, by Study Period (n = 638)*.
*One person was eliminated from the models as he had an extreme value (outlier) for the survival time variable.
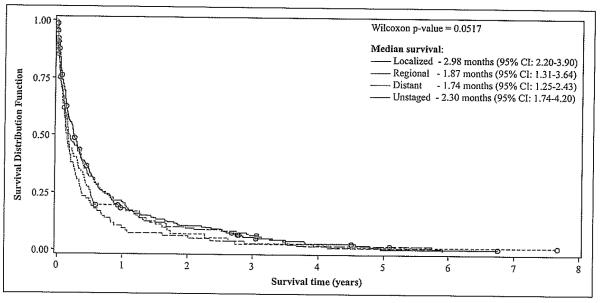
Patients with localized disease at the time of diagnosis had a significantly longer overall survival than those with regional or distant disease at diagnosis (p<0.05) (Figure 2). Patients with unknown stage at diagnosis showed a similar survival time as those with localized disease. Further analysis showed that the inclusion of persons with unknown staging in the localized groups decreased median survival of these patients by approximately 0.16 months (5 days) [from 2.98 months (95% CI: 2.20-3.90) to 2.82 months (95% CI: 2.0-3.57)]. Meanwhile, inclusion of patients with unknown staging in the distant stage group increased median survival time by approximately 0.23 months (7 days) [from 1.74 months (95% CI: 1.25-2.43) to 1.97 months (95% CI: 1.61-2.52)] (data not shown).
Figure 2.
HCC Survival after Diagnosis, by Tumor Stage (n = 638)*.
*One person was eliminated from the models as he had an extreme value (outlier) for the survival time variable.
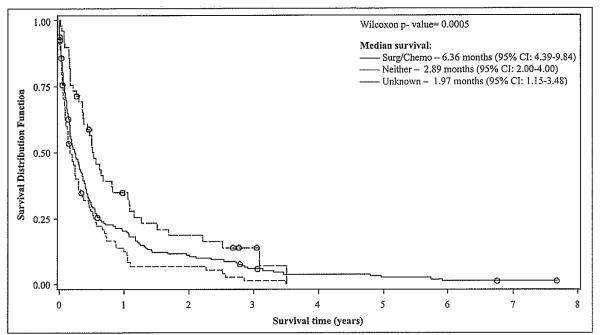
Since the majority of HCC patients diagnosed during the first period had missing information on receipt of therapy, the impact of therapy on survival was evaluated only in HCC patients diagnosed during the second study period. A significantly (p<0.05) higher survival was observed for subjects who received surgical management or chemotherapy (median=6.36 months, 95% CI: 4.39-9.84) during 1998-2002 in comparison to those that did not receive any therapy (median=2.89 months, 95% CI: 2.00-4.00) during that period (Figure 3).
Figure 3.
HCC Survival after Diagnosis, by Type of Therapy (1998-2002) (n = 638)*
*One person was elimmated from the models as he had an extreme value (outlier) for the survival time variable.
The large number of unknown status in the variables stage at diagnosis and receipt of therapy precluded their inclusion in the bivariate and multivariate Cox proportional hazards models. The unadjusted Cox proportional hazards model showed significant differences in mortality risk on the basis of time period of cancer diagnosis (Table 2). Compared with patients diagnosed in 1988-1992, those diagnosed during 1998-2002 had a 16% lower mortality risk (unadjusted HR=0.84; 95% CI: 0.72-0.99). Nonetheless, non-significant differences in mortality risk on the basis of age (p>0.05) or sex (p>0.05) were observed.
Table 2.
Hazard ratios with 95% confidence intervals for HCC mortality according to time period of diagnosis and other covariates (n = 638)*.
| Age at diagnosis (n = 636)* | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| < 60 years (n = 199) | ≥ 60 years (n = 437) | |||||
| Predictor Variable | HR crude | 95% CI | HR adjusted | 95% CI | HR adjusted | 95% CI |
| Period of diagnosis | ||||||
| 1988-1992 | 1.00 | --- | 1.00 | --- | 1.00 | --- |
| 1998-2002 | 0.84 | 0.72-0.99 | 1.11 | 0.83-1.50 | 0.72 | 0.59-0.88 |
| Sex | ||||||
| Male | 1.00 | --- | 1.00 | --- | 1.00 | --- |
| Female | 0.96 | 0.80-1.16 | 0.82 | 0.55-1.23 | 0.99 | 0.80-1.23 |
| Age at diagnosis** | ||||||
| ≥ 60 years | 1.00 | --- | --- | --- | --- | --- |
| < 60 years | 1.00 | 0.84-1.20 | ||||
One person was eliminated from the models as he had an extreme value (outlier) for the survival time variable.
Missing: n = 2.
For the multivariate analysis, a model was constructed which included the variables period, age, sex and their time-dependent interaction terms. No significant time-dependent interaction terms were identified in the model (Proportionality test: X2=1.72, p=0.6316), and thus we concluded that the proportional hazards assumption was met. After excluding time-dependent interaction terms, the likelihood ratio test statistic was used to assess the significance of interaction terms among the main predictor variables (17). This test showed a significant interaction between the main predictor variable (period of diagnosis) and the covariate age at diagnosis (X2=6.33, p=0.0119). Thus, the Cox proportional hazards model was stratified by age (Table 2). Among individuals aged < 60 years, no difference in the risk of death from HCC was observed between those diagnosed from 1998-2002 and those diagnosed from 1988-1992 after adjusting for sex. Nonetheless, among persons aged ≥ 60 years, an 18% lower mortality risk (adjusted HR=0.72; 95% CI: 0.59-0.88) in patients diagnosed during the second study period (1998-2002) was observed as compared to those diagnosed during the first study period (1988-1992), after adjusting for sex (Table 2).
The one- and three-year relative survivals of HCC for both study periods in the Puerto Rican population are presented in Figure 4. The one-year relative survival of cases diagnosed during 1998-2002 was approximately 6% higher (22.4%) as compared to those diagnosed in 1988-1992 (16.6%). Similarly, the three-year unadjusted relative survival for the second study period was 2% higher (9.0%) compared to the first study period (6.7%).
Figure 4.
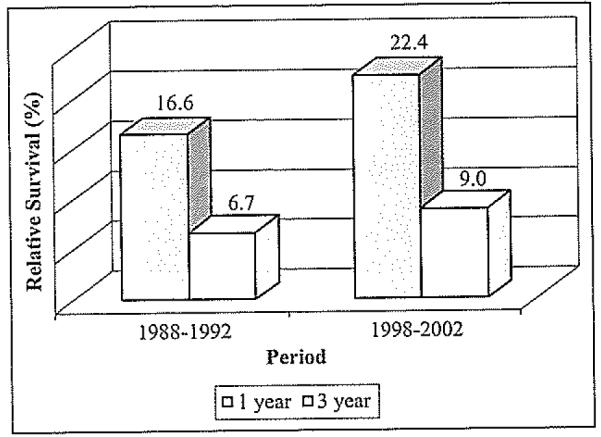
The One- and three-year relatlve survival of HCC in PR, by study period.
Discussion
Variations in survival of HCC patients in PR were examined during two different study periods (1988-1992 vs. 1998-2002). An interaction was observed between the variables period and age of diagnosis where only among persons aged ≥ 60 years a lower HCC mortality risk (adjusted HR=0.72; 95% CI: 0.59-0.88) was observed in patients diagnosed during the second study period (1998-2002) as compared to those diagnosed during the first study period (1988-1992). Whereas, among individuals aged < 60 years, no difference in the risk of death from HCC was observed between patients diagnosed in these study periods. Meanwhile, the one- and three-year relative survival of HCC in PR was approximately 6% and 2% higher respectively, during 1998-2002 in comparison to the first study period.
Improvement in survival observed in certain population groups may be partially explained by major changes that occurred over the past decade in the diagnosis and treatment of HCC. At the time of the first study period, HCC was a relatively rare malignancy, typically diagnosed at an advanced stage in which the patient was symptomatic, and there were no known effective palliative or therapeutic alternatives (20). During the past decade, screening high-risk cirrhotic patients with abdominal ultrasound and alpha-fetoprotein levels has been shown to be effective, and surveillance for HCC seems to be well accepted by physicians (21-22). In addition, adequate treatment for early stage asymptomatic HCC is now available. Surgical resection (when limited to tumors < 5 cm in diameter with normal liver synthetic function without portal hypertension), liver transplantation (when restricted to Milan criteria - a single nodule < 5 cm and three nodules or less each < 3 cm in diameter) and percutaneous ablative techniques (when restricted to the Milan criteria) have shown to provide 5-year survival rates between 50% and 70% in these carefully selected patients (23). Despite this wide array of therapy available in the US, less than half of candidates for potentially curative therapies receive them (24).
The reasons for an improved survival only among persons aged ≥ 60 years may reflect differences in health-seeking behavior between the two age groups. The impact of screening and surveillance of HCC may have been greater among older patients leading to an earlier diagnosis, possibly reflecting a higher health-seeking behavior among this age group. In addition, differences in risk factors for liver disease among age groups may be possible. A higher prevalence of chronic HCV infection is expected among younger age cohorts as seen in other populations, based on differences in risk factors. The aging of the HCV cohort of patients infected by intravenous drug use during the 1960’s-1980’s has been responsible for the increasing incidence trends of HCC in other populations (3). Unfortunately, the lack of information with regard to etiology of liver disease among our study population and the limited information with regards to stage of disease at diagnosis precludes reaching any further conclusions about these findings.
In our study, during 1998-2002, 15% of patients had documented receipt of therapy for HCC, and those treated with surgery or chemotherapy showed significant improvement in survival in comparison to those who did not receive any kind of therapy. In addition, subjects with localized cancer had better survival than patients with regional and distant disease. However, the amount of missing information in the database limited our ability to assess the impact of stage at diagnosis and of appropriate therapeutic interventions in the improvement survival observed during the study periods.
Comparison of our results with SEER data for the US shows that the relative survival during both study periods in PR was inferior to the one observed for the US general population. The one-year relative survival among the US population was higher (almost 3% over Puerto Ricans) in the first study period, yet this gap further increased to more than 10% (32.6% vs. 22.4%) between 1998-2002. Similarly, the three-year relative survival was approximately 1.3% higher (8.0% vs. 6.7%) during the first study period for the US population and increased to approximately 7% (15.7% vs. 9.0%) during the most recent study period (unpublished data, Romero et al.). Our findings coincide with previously described ethnic differences in survival of HCC in the US (11). A recent US population-based study using the SEER database found that Asians had the best one- and three-year overall survival, followed by non-Hispanic Whites, Blacks and Hispanics (11). In this study, the higher mortality risk in Hispanics was associated to a diagnosis made at more advanced stage and less likelihood of undergoing curative therapy.
The disparities in relative survival of HCC in the Puerto Rican and the US population may also be associated to differences in the stage of disease and treatment implementation between both populations. The worse prognosis of HCC in Puerto Ricans may be related to more advanced disease at the time of diagnosis, and decreased access to effective therapies, such as liver transplantation, surgical resection, chemoembolization and ablation. The absence of a liver transplant center in PR may further worsen the prognosis of HCC patients by delaying access to effective treatment alternatives, which are usually implemented in the setting of a transplant center. There is definitely a need of effective preventive, screening and therapeutic programs as an increased incidence of this cancer is expected in the next years.
A recent study from our group reported the incidence trends of liver cancer among island Puerto Ricans, US Hispanics and non-Hispanic Whites during 1998-2002 (unpublished data, Ortiz, et al.). This study found that the adjusted incidence rate of liver cancer was highest among US Hispanics (9.0 per 100,000), followed by Puerto Ricans (7.0 per 100,000), and non-Hispanic Whites (4.3 per 100,000). Another study using the SEER database reported that Hispanics were second only to Asians/Pacific Islanders in age-adjusted incidence rates of HCC (6.3 per 100,000 vs. 10.8 per 100,000) between 1992-2002, surpassing both Blacks (5.0 per 100,000) and non-Hispanic Whites (2.4 per 100,000) (24). Therefore, it seems that both US Hispanics and Puerto Ricans are among the groups more likely to benefit from screening, preventive and treatment strategies to improve survival of HCC.
Among the limitations of the study, the large proportion of cases missing information on stage at diagnosis and therapy administered limited our ability to adjust for the potential confounding influence of these variables on HCC survival. This fact stresses the importance of improving the mechanisms by which health-care professionals and institutions report cancer cases to the PRCCR. In addition, the PRCCR has limited information regarding other factors that might affect survival, including disease comorbidity, underlying risk factors, size of tumor, severity of liver disease and health-seeking behaviors of individuals. Lastly, the potential for lead time bias may be present giving the illusion of better survival only because of earlier detection of the cancer, in a setting in which better radiographic detection technology may have been available during the second study period. Also, the intensity and type of screening strategy may have been different during both study periods.
Nonetheless, this is the first population-based study to examine trends in survival of HCC in PR, and thus our results should be applicable to the Puerto Rican population. All cases of HCC included in this study were diagnostically confirmed and follow-up information regarding vital status was excellent. Our data suggests that survival among Puerto Rican patients with HCC has improved during the last decade, particularly among persons aged ≥ 60 years, although survival rates continue to be low. Even though our study cannot assess the impact of HCC screening and therapy in improving survival, our findings suggest that an important public health strategy to further improve the survival of patients with HCC is the application of adequate screening for early stage at diagnosis and appropriate treatment. Further studies designed to specifically evaluate the role of other biological, clinical and environmental factors that affect HCC survival in PR, such as disease comorbidity, underlying HCC risk factors, size of tumor, severity of liver disease and health-seeking behaviors of individuals, are warranted.
Resumen
Los hispanos y afro-americanos residentes en los Estados Unidos tienen las tasas más bajas de supervivencia de cáncer hepatocelular (CHC), asociado a un diagnóstico en etapa tardía cuando la terapia es de menor beneficio. Este estudio comparó la distribución de supervivencia y supervivencia relativa de CHC en Puerto Rico (PR) durante el 1988-1992 y 1998-2002. Todos los casos de CHC en el Registro Central de Cáncer de PR diagnosticados durante 1988-1992 (n=306) y 1998-2002 (n=333) fueron identificados. Se compararon las características individuales y variables clínicas entre ambos periodos. La supervivencia según la edad al diagnóstico, el sexo, el estadío del tumor y el tratamiento se estimó utilizando el método Kaplan-Meier, y las curvas de supervivencia se compararon mediante la prueba de Wilcoxon. Un modelo de regresión de Cox fue utilizado para determinar el efecto del periodo de diagnóstico luego de ajustar para variables de confusión. Las tasas de supervivencia relativa (a uno y tres años) también fueron calculadas. Los pacientes diagnosticados en 1998-2002 (mediana: 3.08 meses, 95% CI: 2.30-4.16) tuvieron una supervivencia significativamente mayor que aquellos diagnosticados en 1988-1992 (mediana: 1.80 meses, 95% CI: 1.44-2.52). Un término significativo de interacción se observó entre las variables edad y periodo de diagnóstico, donde solo entre las personas ≥ 60 años el riesgo de morir por CHC fue menor (HR ajustado por sexo=0.72; 95% CI: 0.59-0.88) en pacientes diagnosticados durante el segundo periodo (1998-2002) al ser comparados con aquellos diagnosticados en el primer periodo de estudio (1988-1992). La supervivencia relativa durante 1988-1992 fue aproximadamente 6% (22.4% vs. 16.6%) y 2% (9.0% vs. 6.7%) mayor a uno y tres años, respectivamente, en comparación con 1988-1992. Se observó una mejoría en la supervivencia de CHC en PR durante la pasada década. Sin embargo, esta supervivencia es inferior a la observada en la población estadounidense. Se necesitan estudios adicionales para identificar los factores que expliquen las disparidades observadas.
Acknowledgements
The authors thank Dr. Nayda Figueroa and Ms. Mariela Cintrón from the PRCCR for their support with the study database, and Dr. Erick Suárez from the Department of Biostatistics and Epidemiology, Graduate School of Public Health, UPR for his contributions to the statistical analysis. We also acknowledge Dr. Hernando Mattei Torres from the Department of Social Sciences, Graduate School of Public Health, UPR for calculating and providing us with the information of the expected life tables Puerto Rico. This publication was made possible by Grant R25 RR17589 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). In addition, this work was supported by the National Cancer Institute [Grant U54CA96297] for the Puerto Rico Cancer Center / The University of Texas M. D. Anderson Cancer Center, Partners for Excellence in Cancer Research; the Research Centers in Minority Institutions Program [Grant G12RR03051] from the University of Puerto Rico; and by the Centers for Disease Control and Prevention / National Program Cancer Registries [Grant U58DP000782-01] for the Puerto Rico Central Cancer Registry. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
The authors have nothing to disclose with regard to this article.
References
- 1.El-Serag HB. Global epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Figueroa NR, De la Torre T, Ortiz KJ, Pérez J, Torres M, editors. Cancer of the Liver and Intrahepatic Bile Duct Stat Fact Sheet, Puerto Rico Central Cancer Registry. PR; San Juan: 2008. Available at: URL: http://www.salud.gov.pr/RCancer/Reports/Pages/default.aspx, based on May 2007 Puerto Rico Central Cancer Registry data submission, posted on the Puerto Rico Department of Health web site. [Google Scholar]
- 5.E1-Serag HB, Dávila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: An update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 6.Goodgame B, Shaheen NJ, Galanko J, El-Serag HE. The risk of end stage liver disease among persons infected with hepatitis C virus: Publication bias? Am J Gastroenterol. 2003;98:2535–2542. doi: 10.1111/j.1572-0241.2003.07678.x. [DOI] [PubMed] [Google Scholar]
- 7.Pérez CM, Suárez E, Torres EA, Román K, Colón V. Seroprevalence of hepatitis C virus and associated risk behaviours: A population-based study in San Juan, Puerto Rico. Int J Epid. 2005;34:593–599. doi: 10.1093/ije/dyi059. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 9.Lencioni R, Allgaier HP, Cioni D, et al. Early stage hepatocellular carcinoma in cirrhosis: Long-term results of percutaneous image-guided percutaneous radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 10.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: Current surgical management. Gastroenterology. 2004;127:S248–60. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Dávila JA, El-Serag HB. Racial differences in survival of hepato-cellular carcinoma in the United States: A population-based study. Clin Gastroenter Hepatol. 2006;4:104–110. [PubMed] [Google Scholar]
- 12.Puerto Rico Central Cancer Registry Bulletin. 2008 Jan-Mar;1:1. [Google Scholar]
- 13.World Health Organization . International Classification of Diseases for Oncology. World Health Organization; Geneva, Switzerland: 1976. [Google Scholar]
- 14.Shambaugh EM, Weiss MA, editors. Summary Staging Guide: Cancer Surveillance Epidemiology and End-Results Reporting: SEER Program 1977. Public Health Service, NIH publication; Bethesda, MD: 1998. 98-2313. [Google Scholar]
- 15.Young JL, Roffers SD, Ries LAG, Fritz AG, Hurbu1t AA, editors. SEER Summary Staging Manual 2000: Codes and Coding Instruction. National Cancer Institute; Bethesda, MD: 2001. NIH Pub. No 01-4969. [Google Scholar]
- 16.National Center for Health Statistics, Centers for Disease Control. Available at: URL: www.cdc.gov/nchs/datawh/nchsdefs/
- 17.Collet D. Modeling survival data in medical research. Chapman & Hill; London: 1994. p. 44. [Google Scholar]
- 18.Academic Technology Services, Statistical Consulting Group Test of proportionality in SAS, STATA, Rand SPLUS. from http:// www.ats.ucla.edufsas/faq/testyroportionality.htm.
- 19.Surveillance Research Program. National Cancer Institute SEER *Stat software. (version6.4.4) www.seer.cancer.gov/seerstat.
- 20.El-Serag HB, Marrero JA, Rudolph L, Rajender-Reddy K. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 21.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 22.Marrero JA. Screening tests for hepatocellular carcinoma. Clin Liver Dis. 2005;9:235–251. doi: 10.1016/j.cld.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Sherman M. Management of hepatocellular carcinoma: AASLD Practice Guideline. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HE, Lan M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]