Abstract
In Experiment 1, water deprived rats were given 5 min access to saccharin followed by either the active or the yoked delivery of saline or cocaine (0.33 mg/infusion) via an intravenous catheter. Both cocaine groups avoided intake of the saccharin cue following saccharin-cocaine pairings, however, the rats in the yoked condition exhibited greater avoidance of the taste cue than did those that actively self-administered the same dose of the drug. Experiment 2 evaluated subsequent self-administration behavior on fixed and progressive ratio schedules of reinforcement. The results showed that prior yoked exposure to cocaine reduced subsequent drug taking behavior on a progressive ratio but not on a fixed ratio schedule. Finally, Experiment 3 used a choice test to determine the impact of yoked drug delivery on the relative preference for cocaine vs. water. The results showed that rats with a history of self-administering cocaine preferred to perform operant behaviors on the side of the chamber previously paired with cocaine, whereas the rats with a history of yoked delivery of cocaine avoided this side and, instead, preferred to perform operant behaviors on the opposite side of the chamber. These data show that, in most rats, the unpredictable, uncontrollable delivery of cocaine protects against the subsequent motivation for cocaine through an aversive mechanism.
Keywords: addiction, abuse, craving, stimulant, tolerance, taste aversion, devaluation, motivation, reward, compensatory
Introduction
Rats avoid intake of a saccharin conditioned stimulus (CS) after repeated daily pairings with either a rewarding or punishing unconditioned stimulus (US). For example, when access to a palatable saccharin CS precedes the administration of an illness-inducing lithium chloride (LiCl) injection or exposure to X-radiation, rats will learn to avoid intake of saccharin in the future (Carroll & Smith, 1974; Garcia, Kimmeldorf, & Koelling, 1955; Nachman & Ashe, 1973; Nachman, Lester, & Le Magnen, 1970; Riley & Tuck, 1985). This phenomenon is an archetypal example of Pavlovian conditioning referred to as conditioned taste aversion (CTA). In a seemingly counterintuitive manner, rats also suppress intake of a palatable saccharin CS when it reliably comes to predict access to an even more palatable, highly concentrated sucrose solution (Flaherty, 1996; Flaherty & Checke, 1982). This phenomenon, referred to as an anticipatory contrast effect (ACE), is thought to occur because the saccharin CS pales in comparison to the impending availability of the preferred sucrose US. Therefore, the same behavioral outcome, avoidance of a saccharin CS, can be conditioned by opposing means, one aversive, the other appetitive.
The mere avoidance of a taste cue, therefore, reveals little about the subjective effects of a US (i.e., whether it is perceived as rewarding or aversive), particularly when the US has mixed effects and when it is delivered by the experimenter. Indeed, all drugs of abuse tested support robust taste avoidance learning (Cappell & Le Blanc, 1973; Cappell & LeBlanc, 1971; Cappell, LeBlanc, & Endrenyi, 1973; Glowa, Shaw, & Riley, 1994; Grigson, Twining, & Carelli, 2000; Vogel & Nathan, 1975) and many have been shown to exert both rewarding and aversive effects (Blanchard & Blanchard, 1999; Ettenberg, 2004; van der Kooy, Swerdlow, & Koob, 1983; Walker & Ettenberg, 2005). Even so, the results of prior investigations suggested that rewarding drug properties could account for these effects. For example, while rats decrease ingestion of a novel food or sapid stimulus that is paired with a drug of abuse, they simultaneously exhibit a conditioned place preference for a context associated with the drug (Reicher & Holman, 1977), increased speed in a runway to get to the drug (White, Sklar, & Amit, 1977), and even increased self-administration of the drug (Grigson & Twining, 2002; Wise, Yokel, & DeWit, 1976). The results of a more recent investigation, however, suggest that avoidance of a taste paired with cocaine self-administration could arise, more indirectly, from the development of an opposing conditioned compensatory response (CCR) (Wheeler, Twining, Jones, Slater, Grigson, & Carelli, 2008). Conditioned Compensatory Responses to drugs of abuse are by nature aversive as they oppose the drug’s primary rewarding effects, promote tolerance, and are physiologically similar to withdrawal (Hinson & Siegel, 1982, 1986; Krank, Hinson, & Siegel, 1984; Mutschler & Miczek, 1998a, 1998b; Siegel, 1982; Weise-Kelly & Siegel, 2001). The rats in this experiment exhibited pronounced aversive taste reactivity (i.e., gapes) following the intraoral delivery of a taste cue that predicted the opportunity to self-administer cocaine. Similar to our prior investigation where greater avoidance of the saccharin cue was associated with greater drug taking (Grigson & Twining, 2002), this more recent study showed that more vigorous rejection of the taste cue (i.e., more gapes) predicted shorter latencies to self-administer the first infusion and greater drug loading (i.e., more rapid cocaine intake in the first 10 minutes of the session). Taken together, these findings suggest that avoidance of a drug-associated saccharin CS cannot be due solely to an immediate aversive effect of the drug. Alternatively, avoidance of the CS more likely reflects the magnitude of the conditioned compensatory response which develops over time and contributes to the drive to vigorously self-administer cocaine in an attempt to ease escalating negative affect (i.e., negative reinforcement).
Relevant to the present work, the above interpretation begs the question as to what would happen if one were to remove the self-administration component from the equation by implementing a yoked control. For example, in the case of self-administration, the CCR promotes more rapid drug intake because the rat learns how to alleviate the ‘anticipatory withdrawal’ by rapidly self-administering cocaine when given the opportunity. When rats are yoked to a self-administering counterpart, on the other hand, approach behavior is not reinforced nor is it possible for the rats to accurately predict when or if they will receive a cocaine infusion. Removing voluntary control of the drug administration and preventing the accurate prediction of when the cocaine infusion will occur would theoretically lead to larger drug effects (Siegel & Ramos, 2002) and, conceivably, to a qualitatively different drug experience. It is important to note that the yoked procedure is traditionally intended to serve as an ideal control to separate the biological effects of the drug from the effects that may arise from self-administration behavior or from the impact of the drug on self-administration behavior. This tradition, however, discounts the possibility that the biological effects of the drug interact with self-administration behavior to contribute to the reinforcing efficacy of the drug. Indeed, while it often is assumed that there are no real differences between with these conditions, there is growing evidence that contingent and noncontingent cocaine administration have very different effects both on neurophysiology (Kuntz, Patel, Grigson, Freeman, & Vrana, 2008; Lecca, Cacciapaqlia, Valentini, Acquas, & Di Chiara, 2007; Mark, Hajnal, Kinney, & Keys, 1999; Sizemore, Co, & Smith, 2000; Smith, Koves, & Co, 2003; Smith, Vaughn, & Co, 2004) and on behavior (Kuntz, Twining, Baldwin, Vrana, & Grigson, 2008; Lecca et al., 2007; Mutschler & Miczek, 1998a; Siegel, 1978; Siegel & Ramos, 2002). Interestingly, some evidence suggests that the yoked delivery of drug even may be aversive (Dworkin, Mirkis, & Smith, 1995; Mutschler & Miczek, 1998a, 1998b). Taken together, these findings lead to a prediction that, relative to rats that self-administer cocaine, yoked controls will exhibit a reduced motivation to work for cocaine and, perhaps, increased avoidance of stimuli associated with drug delivery.
Indeed, we predict that the yoked exposure to cocaine will have powerfully disruptive effects on drug reinforcement and possibly will be aversive. As mentioned above, saccharin avoidance is difficult to interpret without the use of additional instrumental tasks designed to evaluate drug motivation. Therefore, evaluation of saccharin avoidance in cocaine self-administering vs. yoked rats will be followed by an assessment of their relative performance on instrumental tasks designed to measure drug motivation. The pharmacological yoked control group will experience the same dose and pattern of cocaine administration as the actively administering rats, but non-contingent upon their own behavior. Experiment 1 compares the reduction in CS intake in rats that actively administered the drug of abuse vs. yoked controls that received the same drug infusions, but irrespective of their behavior. Experiment 2 tests whether the purported aversive effects of non-contingently administered cocaine are sufficient to affect the subsequent motivation to self-administer cocaine under low fixed ratio and higher progressive ratio work requirements. Finally, Experiment 3 employs an alternating side choice procedure to test whether a history of yoked delivery would shift the relative preference for cocaine over water in thirsty rats.
General Methods
Subjects
The subjects were 111 naïve, male, Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) weighing between 350-500 g at start of the experiment. They were housed individually in standard wire mesh cages, in a colony room with temperature, humidity, and ventilation automatically controlled. The rats were maintained on a 12-hr light-dark cycle with the lights on at 0700. All experimental manipulations were conducted over 10-hrs, starting 1.5 hrs into the light phase of the cycle. Except where noted otherwise, the rats were maintained on water and food (Teklad) ad libitum.
Catheter Construction
The catheters are custom made in our laboratory using a modified procedure described by Koob and colleagues (1987). The catheter consists of two pieces of Silastic tubing (0.012 in I.D., 0.025 in O.D., 14 cm long, and 0.025 in I.D., 0.047 in O.D., and 2.5 cm long: Baxter Scientific) attached to a stainless steel guide cannula bent at one end at a 90 degree angle (Plastic One, Item #C3136). The cannula/tubing assembly is molded into a permanent dental cement base using a custom-designed mold. A 2.5 × 2.5 cm mesh (Small Parts) is permanently fixed to the base via dental cement and functions as a backplate for the catheter assembly. A small silicon rubber ball is placed appropriately 3.5 cm from the end of the small tubing. The 3.5 cm length of tubing is placed in the jugular vein and anchored to the vein and muscle with silk suture. The entire catheter is flushed with and then soaked in 200 proof alcohol for 24 h prior to implantation.
Catheter Implantation and Maintenance
The rat is anesthetized with the im administration of a Ketamine (80 mg/kg)/Xylazine (16 mg/kg). The hair is shaven on the rat in two places: 1) on the back of the rat between the shoulder blades and 2) directly on top of the jugular vein on the neck. One incision is made above the jugular vein at the neck (approximately 10 mm in length), at about a 30 degree angle away from midline. Another incision is made on the back of the rat (approximately 1″ in length), horizontally positioned between the shoulder blades. The skin is separated from the muscle in both locations using hemostats. A cannula is then subcutaneously pushed from the incision at the back, over the right foreleg, and through the incision on the ventrum of the rat. The catheter is inserted through the cannula, and then the cannula is removed. The rat is placed supine and the jugular vein is exposed by gently separating the muscle surrounding the vein using blunt microforceps. Once the jugular vein is located and cleared from surrounding tissue, a stainless steel rod (3 mm diameter) is moistened with saline and gently placed under the jugular vein. Once the rod is in place it is used to lift the vein to enable the experimenter to make a small incision (approximately 0.5 mm) in the vein. The catheter (0.025 in O.D. side) is then inserted into the vein through the incision. Verification that the catheter is in the jugular vein is completed by attaching a syringe filled with saline to the other end of the catheter (coming out the back of the rat) and drawing back blood through the syringe. Following verification of proper placement, the catheter is secured into position by tying silk suture gently around both the tubing and the jugular vein immediately anterior and posterior to the silicon ball. Once anchored, the muscle layer and superficial skin layers are sutured closed and Betadine antibiotic ointment (Baxter Co.) is applied over it. The rats are treated prophylactically with an intramuscular injection of G-Penicillin (300,000 units). Patency is verified, when necessary, using 0.25 cc of the short acting anesthetic (1% Diprivan; Propofol) administered iv.
Apparatus
Coupling Assembly
Prior to the start of each self-administration session, a coupling assembly is anchored to the back of the rat to provide protected passage of the catheter tubing from the animal. The coupling assembly (a metal spring attached to a metal spacer with Tygon tubing inserted down the center) is attached to the catheter assembly. The catheter tubing is attached to a counterbalanced swivel device (Instech, Inc.) that in turn is attached to a fluid injection assembly (syringe pump) in the experimental chambers. The fluid injection assembly enables iv infusion of cocaine during self-administration sessions. In the animal’s home cage, the catheter is sealed with a piece of Tygon tubing and a metal spacer is placed over the catheter assembly. General maintenance of catheter patency involves daily examination, cleaning of the coupling assembly, and flushing of the catheter with heparinized saline (0.2 ml of 13 IU/ml heparin).
Operant Chambers
The rats were trained in one of twelve identical modular operant chambers (MED Associates, Inc., St. Albans, VT) measuring 30.5 × 24.0 × 29.0 cm (length × width × height) and housed in a light and sound attenuated cubicle. All chambers have a clear Plexiglas top, front, and back wall. Side walls are made of aluminum and Plexiglas. The grid floors consist of nineteen 4.8-mm stainless steel rods spaced 1.6-cm apart (center to center). Each chamber is equipped with three retractable sipper tubes that can enter the chamber through 1.3-cm diameter holes spaced 16.4-cm apart (center to center). A stimulus light is located 6 cm above each. In the extended position, the tip of the sipper tube is aligned in the center of the hole, flush with the right end wall. A lickometer circuit is used to monitor licking. Each chamber is also equipped with a house light (25 W), a tone (Solalert Time Generator, 2,900 Hz), and a speaker for white noise (75 dB). Cocaine reinforcement is controlled by an electronic circuit that operates a syringe pump (Razel Scientific Inst., Model A). Control of events in the chamber and collection of the data are carried out on-line using a 1-GHz computer. Programs are written in the Medstate notation language (MED Associates, Inc.).
Water-deprivation
The 111 rats were run in two squads. Approximately two-weeks after surgery, all rats were placed on a water-deprivation regimen where they were given 1 h access to distilled water (dH2O) each afternoon, no sooner than 45 minutes after daily testing. If a rat failed to drink >5 ml of water during the rehydration period, they were given 10 ml overnight. These conditions were maintained throughout testing.
Habituation and matching
All rats were habituated to the operant chambers for 20 minutes for 5 days prior to testing. During the first 5 min of each 20 min session, rats were given 5 min access to water via the left spout. Rats were then matched on the basis of body weight and 5 min water intake and placed into one of 4 groups: COC-ACTIVE (CA; n=32), COC-YOKED (CY; n=32); SAL-ACTIVE (SA; n=15), SAL-YOKED (SY; n=32). Half of the sal-yoked rats were yoked to the rats that self-administered saline and half to the rats that self-administered cocaine.
Experiment 1: Conditioning
During conditioning, all rats were placed in the operant chambers and given 5 min access to a 0.15% saccharin CS from the leftmost spout located on the left wall of the chamber. This CS tube was then retracted while the empty US tube (right) and inactive tube (center) were advanced. The house light remained on at all times and the stimulus light was illuminated above the US tube. For the next 1 h of testing, completion of 10 licks on the US tube (i.e., Fixed Ratio 10 schedule of reinforcement; FR10) by an actively administering rat lead to an iv infusion of either saline or 0.33 mg cocaine (0.2 ml) over a 6 sec period. Drug or saline delivery was signaled by retraction of the US tube, offset of the US cue light, and initiation of a 20 sec time-out period signaled by the onset of a tone. Responding during the time-out period, or at anytime on the inactive spout, was not reinforced. Simultaneously, the computer initiated the very same event sequence for both the saline-yoked and cocaine-yoked controls. Supplemental water was provided for 1 h, no sooner than 45 min after being returned to the home cage. CS-US pairings occurred once daily for a total of 18 pairings.
Experiment 2: Fixed ratio and progressive ratio
Approximately two-weeks following conditioning in Experiment 1, all rats, regardless of their history in Experiment 1, were given the opportunity to self-administer cocaine for 1 h/day on an FR10 schedule of reinforcement for 2 days (no saccharin was delivered). This was followed 24-72 h later by testing on a progressive ratio schedule of reinforcement where the lick contingency on the empty US tube was incremented progressively by 20 licks per reinforcement (PR10+20/inf). This PR session ended when the rat failed to receive a cocaine infusion in a 30 min time period. The last completed ratio is termed the “break point”.
Experiment 3: Water vs. cocaine choice
Approximately two-weeks following Experiment 2, all rats were given 12 opportunities/h to choose between water vs. cocaine in an alternating-side choice procedure by performing 10 licks on either a right or left empty-spout operant. At the beginning of each session both a right and left empty spout were advanced. The cue light was illuminated over the empty spout on which 10 licks would lead to an i.v. infusion of cocaine (0.33 mg/inf). No cue light was illuminated over the opposite empty spout, upon which completion of 10 licks would lead to 20 sec access to the center spout filled with dH2O (the 20 sec access period did not begin until the rats first contact with the water spout). The right/left location of the water and the cocaine operandi was counterbalanced across trials and between rats (again, the cocaine operandum, whether on the left or the right, was always indicated by illumination of the cue light). After completion of the FR10 lick requirement for either water or cocaine, both empty spouts were retracted, the respective reward was delivered, and a 5 minute time-out was implemented. After the time-out period, when the right and left empty spout operandi returned, the location of the operandum for the two rewards was alternated. There was a single 1 h session, allowing for a total of 12 choices maximally.
Results
Experiment 1
A preliminary statistical analysis was conducted on the saline control groups (i.e., on those yoked to the Coc-Active rats vs. those yoked to the Sal-Active rats). No differences were found. Therefore, these saline groups were collapsed into one group and then similarly compared with the actively administering saline controls. Again, no differences were found. As a result these groups were collapsed and will be, hereafter, referred to as group saline (SAL). As stated above, the entire study was conducted in two replications across different months. A 3 × 18 × 2 mixed factorial analysis of variance (ANOVA) varying Group (Coc-Active, Coc-Yoked, and SAL) × Trials (1-18) × Replication (1, 2) indicated that the replication factor did not contribute meaningfully to any interactions. As such, the replication factor was dropped from further analyses.
CS Intake (Licks/5 min)
A mixed factorial ANOVA varying Group (Coc-Active, Coc-Yoked, SAL) × Trials (1-18) indicated that rats that received non-contingent cocaine infusions exhibited greater avoidance of the saccharin cue relative to their actively administering counterparts, despite receiving the exact same dose of cocaine over the same time course (see Figure 1).
Figure 1.
Mean (+/− SEM) intake (licks/5 min) of 0.15% saccharin following 18 pairings with either active or yoked cocaine (0.33 mg/infusion) for 1 h. * = significantly different from saline controls, # = significantly different from active cocaine and saline controls.
Newman-Keuls post-hoc tests of a significant 2-way interaction, F(34, 1836)=6.80, p<0.0001, revealed that while both the Coc-Active and Coc-Yoked rats consumed significantly less of the saccharin cue than the saline controls on trials 2-18, the Coc-Yoked rats consumed significantly less than the Coc-Active rats on trials 3-5, and 7-18, ps<0.05. Post-hoc tests of the significant main effect of Group, F(2, 108)=25.58, p<.0001, further supported this observation. Both the active and yoked cocaine rats consumed less saccharin overall than the saline controls, ps<0.05, and the Coc-Yoked rats consumed even less than their active counterparts, p<0.05.
CS Intake and individual differences
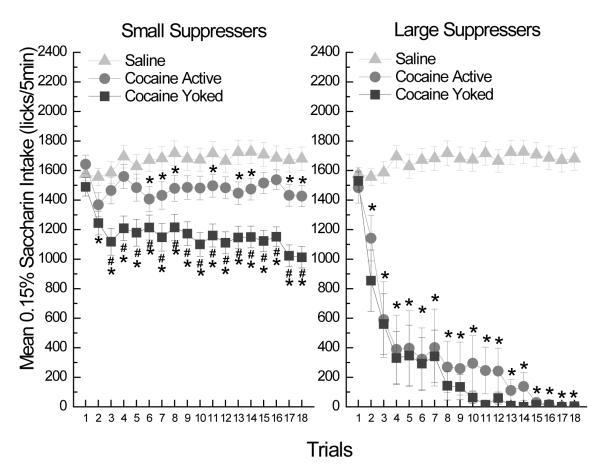
Upon closer inspection of the data it became evident that two distinct response profiles exist within the actively administering group. The two groups consisted of rats that suppressed their intake of the saccharin CS to a large degree (large suppressers) and rats that did not (small suppressers). These individual differences in intake are consistent with our previous report (Grigson & Twining, 2002), where CS intake was found to negatively correlate with cocaine self-administration (i.e., those rats that made the least licks for the saccharin cue, took the most drug). The use of yoked controls in the current report affords the opportunity to compare these subgroups as a function of contingency. Therefore, a criterion was established whereby rats in the Coc-Active group that made < 50 licks of the saccharin CS by the last trial were considered “large suppressers” (Coc-ActiveLg; n=8) and their data were redrawn and reanalyzed as described below with the remaining rats referred to as the “small suppressers” (Coc-ActiveSm; n=24). Similarly, the data of the Coc-Yoked rats were redrawn and reanalyzed, based on the particular Coc-Active rat to whom they were yoked throughout the acquisition phase of the experiment (Cocaine Yoked: “large suppressers” = Coc-YokedLg , n=8; Cocaine Yoked: “small suppressers” = Coc-YokedSm; n=24). The large and small suppressers were not included within the same mixed factorial ANOVA to avoid potential confounds within the statistical analysis that are inherent to dichotomizing a continuous variable and then analyzing those same data. Instead, the large and small subgroups for the active and yoked rats were analyzed separately with the saline group across trials. Thus, two separate mixed factorial ANOVAs were employed, each focusing on either the large or the small suppressers (see below).
The results of a mixed factorial ANOVA varying Group (Coc-ActiveLg, Coc-YokedLg, SAL) × Trials (1-18) showed that, relative to saline controls, the large suppressers (see Figure 2, right panel) exhibited a nearly identical pattern of robust saccharin avoidance irrespective of the cocaine contingency. This observation was supported by Newman-Keuls post hoc tests of the significant Group × Trials interaction, F(34, 1020)=30.99, p<0.0001, showing that both the active large suppressers and their yoked controls significantly reduced saccharin consumption relative to the saline group beginning on trial 2 (ps<.05). In addition, the yoked large suppressers consumed less saccharin than their active counterparts on only Trial 2, p<.05. In contrast to the large suppressers, the results of a similar mixed factorial ANOVA conducted on the small suppressers varying Group (Coc-ActiveSm, Coc-YokedSm, SAL) × Trials (1-18), revealed that the saccharin intake of the small suppressers was significantly more suppressed in the yoked controls than in their actively administering counterparts (see Figure 2, left panel). Post-hoc tests of the 2-way interaction, F(34, 1564)=3.14, p=0.0001, confirmed this finding. Specifically, the actively administering small suppressers (Coc-ActiveSm) mildly suppressed their intake of the saccharin CS relative to their own trial 1 intake on only 3 trials (2, 6, 18) and transiently suppressed their saccharin intake relative to the saline controls on trials 6-8, 11, 13, 14, 17, 18, (ps<.05). The yoked small suppressers (Coc-YokedSm), however, exhibited a more consistent and robust suppression of saccharin intake. Specifically, the yoked small suppressers significantly suppressed their intake of the saccharin CS relative to their own trial 1 intake, the intake of the saline controls, and even that of the active small suppressers on all subsequent trials (2-18, ps<.05).
Figure 2.
A depiction of the same data as in figure 5-1 but divided into small (left panel) and large (right panel) suppressers. Mean (±SEM) intake (licks/5 min) of 0.15% saccharin following 18 pairings with either saline or active vs yoked cocaine (0.33 mg/infusion) for 1 h. * = significantly different from saline controls, # = significantly different from active cocaine and saline controls.
Cocaine Intake and individual differences
As stated above, we reported previously that greater avoidance of the saccharin cue is associated with greater cocaine self-administration (Grigson & Twining, 2002). A similar pattern with the active rats was evident here. Of course, the active and yoked cocaine groups received identical amounts of cocaine at the discretion of the particular active rat controlling the cocaine infusions (Figure 3; left panel). As a consequence, the following analysis on cocaine infusions was conducted only on the actively administering rats.
Figure 3.
Left Panel: Mean (+/− SEM) number of infusions/h of either saline or cocaine (0.33 mg/inf) for both the active and yoked groups following 18 saccharin-cocaine pairings. The data are depicted with the rats in the cocaine groups divided into small and large suppressers on the basis of the saccharin intake of the active cocaine rats. * = significantly different from saline controls and large suppressers; # = large suppressers significantly > saline and small suppressers. Middle and Right Panels: A correlational analysis of each rat’s average intake (i.e., licks/5 min) across trials 2-18 of the saccharin conditioned stimulus as a function of the number of infusions of cocaine administered/h on these same trials. The results revealed a strong negative relationship where low saccharin intake was highly correlated with high drug self-administration behavior (middle panel) or high administration of yoked cocaine (right panel).
The results of a mixed factorial ANOVA varying Group (Coc-ActiveSm, Coc-ActiveLg, SAL) × Trials (1-18) indicated that the active large suppressers self-administered significantly more cocaine than did the active small suppressers. Newman-Keuls post hoc tests of this significant 2-way interaction, F(34, 731)=4.64, p<.0001, confirmed that the large suppressers self-administered significantly more cocaine than the small suppressers on every trial (ps<.05). Furthermore, the small suppressers, averaging between 2-6 infusions per trial, never obtained significantly more infusions of cocaine than the saline controls obtained of saline (ps>.05). These results were mirrored by the post hoc tests of the highly significant main effect of group, F(2, 43)=70.21, p<.0001, confirming that the large suppressers self-administered significantly more cocaine infusions overall than did the small suppressers and the number of infusions obtained by this latter group did not differ from the number initiated by the saline controls. Taken together the data indicate that these rats differ not only in their consumption of saccharin but also in the propensity to self-administer cocaine.
While the yoked controls received the same pattern of cocaine infusions as the active rats, Figure 2 shows that they consumed less saccharin overall. This is particularly true for the rats that were yoked to the active small suppressers. Separate correlational analyses, then, were conducted on the active (n=32) and yoked (n=32) cocaine rats to assess the strength of the relationship between saccharin intake and cocaine exposure across trials 2-18. The results of the analyses on collapsed trials 2-18 revealed a strong negative correlation for both cocaine contingencies, whereby low saccharin intake (i.e., avoidance of the saccharin cue) was associated with high drug intake by self-administering rats (r= −0.83; p<.0001; r2=.69) or high drug exposure by yoked rats (r= −0.76; p<.0001; r2=.58; see Figure 3, middle and right panels respectively).
Despite the similar significant negative correlation between saccharin intake and cocaine ‘intake’, the Coc-Yoked group exhibited greater avoidance of the saccharin cue then did the Coc-Active group and this effect seems to have been carried by the Coc-YokedSm subgroup. Therefore, further correlations were conducted on the small suppressers only for both the active and the yoked groups to assess the relative strength of the relationship between saccharin intake and this modest (relative to the large suppressers) cocaine intake collapsed across trials 2-18. Interestingly, even at this very modest level of cocaine intake there was a significant negative correlation in both the active, r = −0.46; p<0.02; r2=0.22, and yoked, r = −0.48; p<0.02; r2=0.24, small suppressers whereby lesser saccharin intake predicted greater cocaine intake when collapsed across trials 2-18. In contrast, when the analysis was restricted to only the final 2 acquisition trials of the experiment this predictive relationship disappeared for the active small suppressers, r = −0.30; p=0.15; r2=0.09. It remained highly significant, however, for their yoked controls, r = −0.56; p<0.005; r2=0.30. Rats with a history of yoked delivery of drug, then, avoid intake of a drug-associated cue and greater avoidance of this cue occurs (whether yoked to a small or a large drug-taker) as a function of the number of infusions of drug received/session.
Experiment 2
Fixed and Progressive Ratio Responding
As stated above, contingent and noncontingent drug administration are not considered by many to have distinct behavioral consequences. In Experiment 1, however, avoidance of a saccharin cue that reliably predicted i.v. cocaine infusions was greater in rats when the cocaine was noncontingently delivered. It is unclear, however, why the same dose of the drug, delivered over the same time course, results in greater behavioral avoidance of the sweet cue. The primary purpose of Experiment 2 was to compare the lasting impact of a history of either self-administered or yoked cocaine on the subsequent self-administration of cocaine. Therefore, water deprivation was maintained and no saccharin cue was presented prior to the self-administration sessions in Experiment 2 – all rats were equally thirsty. As described, the rats (i.e., Coc-Active, Coc-Yoked, SAL) now had the opportunity to self-administer cocaine for 1 h/day for 2 days on the FR10 schedule of reinforcement. Thereafter, the motivation to work for cocaine was probed by use of a PR test. Obtaining a higher number of cocaine infusions on a PR schedule is thought to reflect a greater motivation to work for cocaine (Arnold & Roberts, 1997; Smith, Ward, & Roberts, 2008).
Fixed ratio
For the cocaine treated rats, the history of contingency (yoked vs. active) during Experiment 1 also had no effect on the subsequent self-administration of cocaine during the 2 days on the FR10 schedule of reinforcement. This observation was supported by the null results of a mixed factorial ANOVA varying Group (Coc-Active, Coc-Yoked) × Day (1-2). Neither the main effect of Group, Day, nor the 2-way interaction, were even close to significant, Fs < 1 (see Figure 4, left panel, left of the dashed line).
Figure 4.
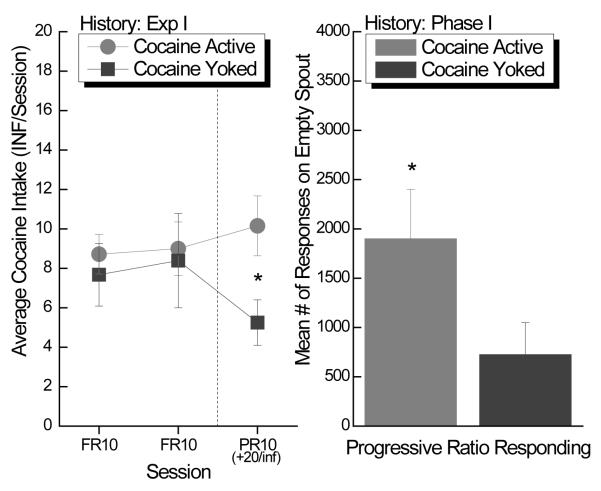
Left Panel: Mean (± SEM) active cocaine intake (0.33 mg/inf) on a fixed (left of dashed line) and progressive (right of dashed line) schedule of reinforcement for rats with a history of either active or yoked cocaine in Experiment 1. Right Panel: Mean (± SEM) number of total contacts with the active empty spout on the progressive ratio day for the rats with a history of either active or yoked cocaine in Experiment 1.
As mentioned in the methods section, prior experience with the delivery of saline infusions in an active or yoked manner in Experiment 1 had no impact on the data. Thus, prior establishment of a contingency between the operant behavior and the delivery of saline and the cue complex or not (as in the yoked saline controls), did not differentially affect subsequent responding on either the fixed or progressive ratio schedules when self-administering cocaine for the first time in Experiment 2 (data not shown). This result was surprising because the yoked saline controls during Experiment 1 were expected to exhibit retarded acquisition of cocaine self-administration and, thus, to require an extended training period on the FR 10 schedule of reinforcement, however, they did not. In fact, cocaine self-administration was quite similar for both groups on both day 1 and day 2 on the FR 10 schedule of reinforcement (history of saline active = Day 1: 16.7 ± 3.76; Day 2: 18.45 ± 3.48; vs. history of saline yoked Day 1: 18.04 ± 4.41; Day 2: 16.55 ± 4.96).
Progressive ratio
When performing on a progressive ratio schedule, however, where the work requirement to obtain a cocaine infusion increased after each earned infusion, the rats previously yoked for cocaine delivery self-administered significantly less cocaine than did their active counterparts t(1, 58)=6.28; p<0.015 (see Figure 4, left panel, right of dotted line). This difference does not reflect a generalized lack of responding on the part of the previously yoked controls as they averaged a respectable 726.1 ± 323.2 responses on the operant (Figure 4, right panel).
Fixed and Progressive Ratio Responding and individual differences
The previous section demonstrated that yoked exposure to i.v. cocaine does not significantly retard acquisition of cocaine self-administration when it is relatively easy to obtain (i.e., on an FR10). In contrast, a history of yoked cocaine experience reduced the motivation to work on the PR schedule for cocaine relative to rats with a history of cocaine self-administration. This result is particularly interesting since prior exposure to, or self-administration of, cocaine or other psychostimulants is typically thought to sensitize, not desensitize, rats to the reinforcing properties of the drug (Horger, Shelton, & Schenk, 1990; Liu, Morgan, & Roberts, 2007). The discovery of the individual differences in Experiment 1 calls for a similar analysis of these data. To this end, the data from Experiment 2 were reanalyzed with rats separated into their large and small subgroups based on their saccharin intake in Experiment 1.
Individual differences - Fixed Ratio Days
The results of a mixed factorial ANOVA conducted on only the fixed ratio days varying History (Coc-Active, Coc-Yoked) × Group (Large, Small) × Day (1-2) revealed that the cocaine intake of the large, but not the small, suppressers was differentially affected by their prior cocaine contingency (Figure 5, both panels, left of dashed line).
Figure 5.
A depiction of the same data that appears in Figure 4 separated on the basis of saccharin intake in Experiment 1. Mean (± SEM) active cocaine intake (0.33 mg/inf) on a fixed (left of dashed line) and progressive (right of dashed line) schedule of reinforcement for large (left panel) and small (right panel) suppressers with a history of either active or yoked cocaine in Experiment 1.
This effect was supported by post hoc tests of a significant History × Group interaction, F(1, 57)=8.36; p<0.005, which indicated that Coc-YokedLg rats that were yoked to Coc-ActiveLg, and thus received comparatively more yoked delivery of cocaine than the Coc-YokedSm rats, self-administered significantly less cocaine than their Coc-ActiveLg counterparts, p<.05 (see Figure 5, left panel). This reduction in responding for cocaine on the FR10 schedule was not evident in the Coc-YokedSm rats relative to the Coc-ActiveSm rats (see Figure 5, right panel). If anything, post-hoc tests indicated a tendency for this relatively small amount of prior yoked cocaine history to augment responding for cocaine relative to the Coc-ActiveSm rats, p=0.15. Comparisons within the contingencies indicated that the active large suppressers self-administered more cocaine than did the active small suppressers, p<.05, while there was a strong tendency for the reverse pattern (i.e., Coc-YokedLg < Coc-YokedSm) in the yoked controls, p=.06.
Individual Differences - Progressive Ratio Challenge
The results of a factorial ANOVA varying History (Coc-Active, Coc-Yoked) × Group (Large, Small) revealed that relative responding for cocaine on the progressive ratio challenge resembled that obtained on the fixed ratio days (Figure 5, both panels, right of the dashed line). Post hoc tests of this significant 2-way interaction, F(1, 57) = 5.21; p<0.026, showed that the Coc-YokedLg rats obtained significantly fewer cocaine infusions than did the Coc-ActiveLg rats on the progressive ratio test day, despite having received the same dose of the drug delivered over the same time course throughout the 18 trials of Experiment 1. There was no such difference on the progressive ratio test in the small suppresser groups that received a relatively small amount of cocaine (yoked or active) during Experiment 1. In fact, these two groups self-administered practically identical amounts of cocaine during the progressive ratio test (Coc-ActiveSm = 8.0 vs. Coc-YokedSm = 7.9 infusions).
Experiment 3
Alternating-Side Choice Test
In Experiment 2, it is clear that prior exposure to yoked cocaine reduced the subsequent motivation to work for cocaine on a progressive ratio test. This effect was exhibited by the rats that were yoked to large suppressers (i.e., large drug takers). Although unlikely due to their stable rates of responding on both the FR and the PR schedules, one potential concern is that these rats simply did not adequately learn the contingency between their behavior and the drug consequence. Furthermore, given adequate learning, it remains unclear how yoked exposure to cocaine reduced subsequent operant responding for cocaine. The following experiment was designed to evaluate whether the rats with a history of yoked cocaine exhibit retarded operant learning, reduced motivation for operant responding in general, reduced motivation for operant responding for cocaine specifically, and/or whether cocaine has been rendered aversive by its yoked administration.
An important component to this experiment is the saline controls. As mentioned above, the saline controls participated in each of the experiments and, consistently, the manner in which they received saline (active vs. yoked) was of no detectable consequence. For the purposes of Experiment 3, however, their unique unpaired experience with saccharin in Experiment 1 and with cocaine in Experiment 2 renders them important controls. In Experiment 1, they had access to the saccharin cue on the left side of the chamber followed by i.v. saline infusions. Saccharin, therefore, was never directly paired with cocaine and, thus, could not be directly devalued by cocaine in this group. In Experiment 2, no rats had access to saccharin and instead all rats had access to cocaine on the right side of the chamber.
Cocaine vs. water preference
First and foremost, a one-way ANOVA conducted between the SAL, Coc-Active, and Coc-Yoked groups revealed that there were no differences between any groups whatsoever in the number of total choices made. Thus, of the 12 possible, each group, on average, made about 10 choices between cocaine and water, F(2, 92)=1.13, p=0.33. This null finding demonstrates that none of the groups had impaired operant performance – all rats performed on the task and they were able to complete the same number of choice tests. In addition, a mixed factorial ANOVA varying Reward (water, cocaine) and Group (SAL, Coc-Active, Coc-Yoked) found no preferences between water and cocaine for any of the groups, as evidenced by the null 2-way interaction, F(2, 90)=1.66, p=0.20. Instead, the groups varied significantly in their preference to perform on either the left or the right side of the operant chamber. The left side was previously paired with saccharin (for all rats) and the right side was previously paired with either the yoked or the active delivery of cocaine and the associated compound cues. These side preferences emerged in spite of the fact that the presentation of the rewards in this task (water vs. cocaine) was alternated between sides, with the cue light tracking cocaine availability.
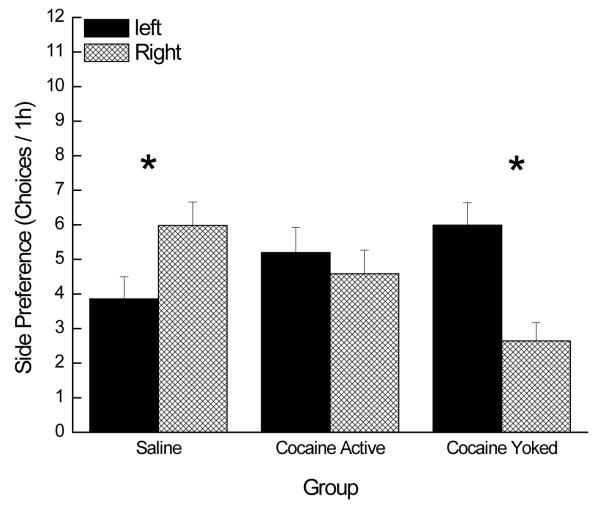
A mixed factorial ANOVA varying Group (Coc-Active, Coc-Yoked, SAL) × Side (Left, Right) revealed that the rats respective histories in Exp 1 and Exp 2 substantially influenced the side of the chamber on which the rats chose to respond during Experiment 3 (see Figure 6). Newman-Keuls post hoc tests of this significant 2-way interaction, F(2, 91)=5.26; p<0.007, showed that the SAL group responded more on the right than on the left side of the chamber. This SAL group had access to saccharin in Exp 1 on the left side of the chamber and then, as with all groups, the saccharin cue was removed and they had the opportunity to self-administer cocaine on the right side of the chamber across three trials in Exp 2. Thus, for the SAL group, three days experience with cocaine self-administration on the right side of the chamber in Exp2 was sufficient to offset the preference for the side associated with access to saccharin in Exp 1. The Coc-Active group, on the other hand, exhibited no preference for either the left side or the right side of the chamber, perhaps because of the opposing preferences by the large and small suppressers (see analysis below). Finally, the Coc-Yoked group was the only group that, as a whole, preferred to perform on the left (previously saccharin-associated) side of the chamber. This last finding was most likely due to avoidance of the side paired with the yoked administration of cocaine and its associated compound cues (i.e., bottle retraction, cue light offset, tone).
Figure 6.
Mean (±SEM) choices on the left or right empty spout operant to gain access to either water for 20 sec or a cocaine infusion (0.33 mg/inf) in the 1 hour choice tests for the rats separated by their history of saline or active vs. yoked cocaine in Exp 1.
Alternating-Side Choice Test and Individual Differences
Upon closer inspection of the data, it was clear that the responses in the choice test were complex and varied within each of the groups. Therefore, as was done for Exp 1 and Exp 2, the data were reanalyzed as a function of the large and small suppressers as identified in Exp 1. A mixed factorial ANOVA varying Group (Coc-ActiveLg, Coc-ActiveSm, Coc-YokedLg, Coc-ActiveSm, SAL) × Side (Left, Right) further clarified the differential effect of their respective histories on choice behavior (see Figure 7). Post-hoc tests of the significant 2-way interaction, F(4,89)=7.78; p<0.0001, revealed that the Coc-ActiveLg rats, like the SAL rats, preferred the side that was previously associated with cocaine self-administration. In fact, the Coc-ActiveLg rats exhibited an even stronger preference for the right side than the saline rats, p<0.01. This effect was most likely due to avoidance of the saccharin paired side by devaluation of the saccharin cue in Exp 1 plus a preference for the cocaine paired side with which they had a great deal of experience. In contrast, for the saline group, the saccharin cue was never directly devalued by cocaine so the saccharin paired side still retained value. Even so, the cocaine paired side was experienced for 3 trials during Exp 2 which was sufficient to shift the preference to the briefly cocaine-paired side for rats in the SAL group. The Coc-ActiveSm rats, on the other hand, significantly preferred the side previously associated with the saccharin cue. This occurred even though these rats took cocaine on the right side of the chamber during 18 days of training in Exp 1 and during fixed and progressive ratio testing in Exp 2. This observation confirms that these rats not only prefer the natural reward over cocaine-self administration (as in Exp 1), but they prefer to perform operant behaviors in the location previously associated with the saccharin cue. Taken together, these data explain why the Coc-Active group average “washed out”; the large suppressers preferred the drug-associated side of the chamber, while the small suppressers preferred the saccharin-associated side.
Figure 7.
A depiction of the same data as in Figure 6 but divided into large and small suppressers on the basis of saccharin intake in Exp 1. Mean (±SEM) choices on the left or right empty spout operant to gain access to either water for 20 sec or a cocaine infusion (0.33 mg/inf) in the 1 hour choice tests for the rats separated by their history of saline or active vs. yoked cocaine in Exp 1.
In contrast to the groups with a history of active cocaine self-administration, the Coc-YokedLg and Coc-YokedSm rats exhibited a relatively uniform left-side (i.e., saccharin or non-cocaine side) preference. This observation, however, was not perfect. Of the 8 Coc-YokedLg rats, 1 heavily preferred the right side. Omission of this one outlier resulted in significant left side preferences for both of the yoked subgroups, ps<.05. Together, these data demonstrate that, for nearly all rats tested, the inescapable, unpredictable delivery of cocaine is aversive leading to avoidance of the drug-associated side of the chamber.
General Discussion
When a saccharin cue predicts access to cocaine, the taste cue is devalued and subsequent intake suppressed or avoided and greater avoidance of the taste cue is correlated with greater cocaine self-administration (Grigson & Twining, 2002). This correlative relationship emerges between intake of the taste cue and intake of the drug largely because of the development of two very distinct groups of rats: Large suppressers that consume very little saccharin and self-administer a large amount of cocaine and small suppressers that consume a lot of saccharin and self-administer comparatively little cocaine. The results of the present study confirmed this earlier finding. Unlike the earlier study, however, a separate group of rats were yoked to these experimental subjects in the present study such that the yoked rats received a cocaine infusion at the same dose, time, and rate. Relative to active delivery of drug, yoked delivery of cocaine led to even greater avoidance of the saccharin cue. This effect was carried nearly exclusively by the rats that were yoked to the small suppressers (i.e., to those that infused ~2-6 infusions/trial on average) and for these rats more suppressed intake of the saccharin cue was associated with greater drug infusions. The rats yoked to large suppressers, on the other hand, failed to exhibit enhanced saccharin avoidance, perhaps because of a floor effect. Yoked delivery of drug, then, augmented cocaine-induced suppression of CS intake and this effect was most evident in those rats that were yoked to the small suppressers.
As stated above, conditioned avoidance of the saccharin CS by association with a rewarding drug US is difficult to interpret when the US is passively delivered and has complex or mixed effects. The interpretation is made more clear, however, if saccharin avoidance is evaluated within the context of instrumental behavior aimed at examining drug motivation. This was our reason for implementing self-administration in the reward comparison model (Grigson & Twining, 2002) and was the purpose of both Experiments 2 and 3. Experiment 2 established that yoked administration of cocaine results in reduced motivation to work for cocaine on a progressive ratio schedule of reinforcement. Moreover, when evaluated based on saccharin intake in Exp 1, it was found that the Coc-YokedLg subgroup, which received a comparatively large amount of yoked cocaine, exhibited greatly reduced cocaine self-administration on both the FR and the PR schedule. For the small suppressers, however, the drug contingency in Exp 1 did not differentially affect the subsequent motivation to self-administer cocaine on either the FR or the PR schedule of reinforcement. Therefore, the factors that led to an exaggerated reduction in CS intake in the Coc-YokedSm subgroup in Exp 1, did not have any lasting impact on the motivational properties of cocaine as assessed in Exp 2. Small suppressers, whether active or yoked in Exp 1, took less infusions of drug than did the active large suppressers on both the FR and the PR schedule in Exp 2. For the large suppressers, the effect of contingency was not evident in Exp 1 as both the active and the yoked large suppressers greatly avoided intake of the saccharin CS. The impact of contingency, however, was revealed for these subjects in Exp 2 when the Coc-YokedLg subgroup failed to work for cocaine even when the work requirements were low. A history of having received the uncontrollable, unpredictable delivery of relatively large quantities of cocaine, then, greatly disrupted subsequent drug-taking behavior. The question is why.
Experiment 3 was designed to address this question. Three explanations were considered: 1) Yoked delivery of cocaine retards instrumental learning; 2) Yoked delivery of cocaine is not as rewarding as is self-administered cocaine, but it is not aversive; and 3) Yoked delivery of cocaine is aversive and thereby reduces the motivational properties of cocaine. The results of Experiment 3 support the third explanation. The alternating-side choice procedure was originally intended to pit brief access to water in thirsty rats against the opportunity to self-administer cocaine. This particular choice, however, was not distinguished by any of the rats. All rats, regardless of prior contingencies, learned equally well to perform on the empty spouts that produced either of these rewards. Indeed, none of the groups differed in the total number of choices made (~10/12). This finding reduces our concern regarding the first explanation. That is, the decrease in responding for cocaine by the rats yoked to the large suppressers is not likely due to a simple learning/performance deficit because these same rats readily learned to respond on the operant for a reinforcer (either water or cocaine).
Despite the ability of all rats to learn to respond on the operant in the choice test, there was a surprising lack of preference for either reward. A review of the data showed that the expression of a clear choice for water vs. cocaine was prevented for all rats by an extremely strong preference for a given side of the chamber. As such, rats made a choice, but it was for the reward (water or cocaine) that was available on their preferred side of the chamber and the preferred side was dictated by the rat’s training history. Specifically, the active large suppressers and the saline rats preferred to perform operant behaviors on the right side of the chamber where the rats actively sought and self-administered cocaine. In contrast, the active small suppressers, and all of the yoked rats, avoided the right side in favor of performing on the left side of the chamber. For these small suppressers (75% of the actively administering rats) this may indicate a preference for saccharin over cocaine as has been reported to occur under certain parameters in a discrete trials choice procedure (Lenoir, Serre, Cantin, & Ahmed, 2007). Similarly, for the yoked controls this choice avoids the place and environmental cues associated with the inescapable, unpredictable delivery of cocaine in Exp 1. On the other hand, for the Coc-ActiveLg rats who clearly preferred cocaine over saccharin, this behavior is particularly striking because the choice to respond on the drug-associated side of the chamber prevented the rats from obtaining drug on half of the trials. Therefore, unlike the Lenoir et al. (2007) report, the behavior of the rats in the current report appears to be under stimulus control as their choices are determined by the cues that either predicted or were associated with the delivery of cocaine. This inflexible form of stimulus bound learning is reminiscent of sign-tracking (i.e., “autoshaping”) where the approach behavior is so locked into a conditioned stimulus that it can cause the animal to lose the opportunity to gain access to the goal (Krank, O’Neill, Squarey, & Jacob, 2008). As a whole, both the active and yoked rats appear to be ‘tracking’ contextual cues within the chamber and ignoring the available cue light, perhaps because of a blocking effect (Wheeler & Miller, 2007). As a result, the previously established contextual side cues retained their acquired value and blocked the cue light from attaining sufficient associative strength to trigger either approach behavior in the drug preferring rats or avoidance behavior in the non-drug preferring rats.
One conclusion made quite clear by these data is that the non-contingent delivery of cocaine is a poor control procedure for drug self-administration. Elimination of the cues (exteroceptive or interoceptive) that predict drug self-administration by yoked delivery causes more severe withdrawal from cocaine (Mutschler & Miczek, 1998a), reduces tolerance to morphine’s analgesic and hypothermic effects (Siegel, 1978, 1982; Siegel, Hinson, & Krank, 1978), and renders cocaine (Dworkin et al., 1995), pentobarbital (Vila, 1989), alcohol (Melchior, 1990) and heroin (Siegel, Hinson, Krank, & McCully, 1982) more lethal. Yoked delivery of cocaine also leads to markedly different turnover rates of virtually every neurotransmitter across several brain regions including those that mediate the rewarding effects of the drug (Smith et al., 2003; Smith et al., 2004). For example, relative to yoked controls, self-administration of cocaine yields significantly higher DA turnover rates in the nucleus accumbens, ventral pallidum, and lateral hypothalamus while glutamate and GABA turnover rates are lower in the NAc and ventral pallidum (Smith et al., 2003). These data are consistent with microdialysis experiments that report increases of DA in the ventral pallidum and increases in both ACh and DA in the NAc of self-administering rats compared with yoked rats (Mark et al., 1999; Sizemore et al., 2000). Furthermore, yoked delivery of cocaine increases the core/shell ratios of NAc DA release, sensitizes the emergence of stereotyped, “head bobbing”, “sniffing down”, and “gnawing” behaviors (Lecca et al., 2007) and reduces cocaine-induced reinstatement of drug-seeking behavior (Kippin, Fuchs, & See, 2006). The present report adds to this body of literature by providing evidence that yoked cocaine enhances avoidance of a cocaine-associated taste cue, reduces the motivation to work for cocaine, and causes rats to avoid the drug-associated context.
Why do self-administering rats avoid a taste that predicts cocaine access? Why does yoked administration of cocaine enhance that effect at low doses and retard acquisition of drug taking at higher doses? We stated in the Introduction that the evidence, so far, has pointed toward the reinforcing properties of drugs of abuse as causing the devaluation of the saccharin cue. This may be true in a direct sense. If the saccharin cue signals the availability of a more preferred reward such as sucrose or cocaine then it can be devalued as in anticipatory contrast. The reinforcing properties of the drug, however, also likely initiate a chain of conditioned physiological events that ultimately contribute to the reduction in CS intake and in the resulting devaluation of the saccharin cue. For example, as described, we recently found that rats gape to a flavored saccharin cue (CS+) when it is directly infused into the oral cavity with an intra oral cannulae 30 minutes prior to cocaine access (Wheeler et al., 2008). The emergence of aversive taste reactivity (i.e., “gaping” or “rejection responses”) to the CS+, and not to a control CS-solution that predicts only saline, indicates that the taste elicits a negative affective state and, therefore, is aversive when it predicts the opportunity to self-administer cocaine; this gaping is not predicted by the anticipatory contrast model. Nevertheless, the magnitude of the conditioned negative affect, as reflected by the number of gapes elicited, predicted a shorter latency to the first self-administered cocaine infusion, faster acquisition of stable cocaine self-administration behavior, and greater “load up” behavior (i.e., more rapid infusion rates in the first 10 minutes of the subsequent drug session). Therefore, upon gaining access to the drug, the negative affect may directly or indirectly drive the rat to self-administer cocaine very quickly and in a manner directly proportional to the negative affect (for a discussion see, Grigson, in press; Grigson, Twining, Freet, Wheeler, & Geddes, 2008; Wheeler et al., 2008).
The mechanism behind these complex behavioral relationships can be understood by examining the associative framework within which the rats learn about the cues that are available to them. In the previous report, the CS+ was forced into the oral cavity once per minute for ~ 3.2 seconds and is the only relevant predictor of both the onset of a 30 minute wait before cocaine becomes available and cocaine itself. In the present experiment, the saccharin cue was voluntarily approached and consumed and was one of many cues (e.g., removal from home cage, context) that predicted daily cocaine administration. Actively self-administering rats, but not their yoked controls, also have the foreknowledge of precisely when and if an infusion of cocaine is coming. These interoceptive self-administration cues and contextual cues have been shown by Siegel and colleagues to elicit conditioned compensatory responses (CCRs) that oppose the action of drugs of abuse and, therefore, are critical for the expression of normal drug tolerance (MacRae, Scoles, & Siegel, 1987; Poulos, Hinson, & Siegel, 1981; Ramos, Siegel, & Bueno, 2002; Siegel, 1975, 1978; Siegel, Baptista, Kim, McDonald, & Weise-Kelly, 2000; Siegel et al., 1982). In the absence of drug, cues associated with drug delivery elicit an aversive drug withdrawal and/or negative affect in both human smokers and in rats self-administering cocaine (Baker, Brandon, & Chassin, 2004; Sayette, Wertz, Martin, Cohn, Perrott, & Hobel, 2003; Wheeler et al., 2008). In rats, this negative affect decreases brain reward sensitivity as measured by increased ICSS thresholds and invigorates drug self-administration (Carlezon & Chartoff, 2007; Kenny, Chen, Kitamura, Markou, & Koob, 2006; Kenny & Markou, 2005). In human smokers, the negative affect is associated with a decrease in brain reward sensitivity as measured by a decrease in activation of the caudate nucleus to monetary gain and loss of reward (Wilson, Sayette, Delgado, & Fiez, 2008). Taken together, these data suggest that the taste cue is avoided because it elicits drug-opposing CCRs in anticipation of impending cocaine availability that are physiologically similar to withdrawal, and therefore, aversive. To escape this negative affective state, an actively self-administering rat learns to approach the drug context, and vigorously engage the drug operanda in a manner proportional to the severity of the negative affect (Wheeler et al., 2008). The yoked rats, on the other hand, never learned that controlling cocaine intake was an effective means to reduce negative affect brought on by the cues that predict cocaine. Instead, they attempt escape by avoiding the drug context altogether.
As a whole, both the active and yoked rats suppressed their intake of the saccharin cue that predicted cocaine administration. For the active rats, only the large suppressers avoided the saccharin cue and self-administered large amounts of cocaine. Not surprisingly, they worked hard for cocaine on a PR schedule and greatly preferred the side of the chamber that was associated, historically, with cocaine access. Together, these findings support the CCR/cued-withdrawal interpretation elaborated above in terms of the suppression of saccharin intake. That is, for the Coc-ActiveLg rats, the saccharin could elicit a negative affective state that devalues the taste and is proportional to their drive to self-administer cocaine. The active small suppressers, on the other hand, did not consistently avoid the saccharin cue and self-administered a modest amount of cocaine daily (~2-6 infusions). They did not work hard for cocaine on the PR schedule and they preferred the saccharin paired side of the chamber. Both of these groups behaved in accordance with the conditioned withdrawal interpretation. The large suppressers avoid the taste because it elicits a cued withdrawal and elevates drug motivation while the Coc-ActiveSm rats never administer enough cocaine to develop a drug dependence, do not avoid the saccharin cue and may, in fact, prefer the sweet over cocaine.
Furthermore, the saccharin avoidance exhibited by the yoked rats is best explained by the CCR/cued-drug withdrawal interpretation. The Coc-YokedSm rats, for example, fully carried the enhanced avoidance of the saccharin cue and this may be due to decreased tolerance to the drug via decreased efficacy of the CCRs to oppose drug action (Weise-Kelly & Siegel, 2001). Like the Coc-ActiveSm rats, the low dose of cocaine administered is probably too small to render the animals drug dependent and so the CCRs would be quite a bit smaller so as not to generate a negative affective state. In contrast, the higher doses of cocaine experienced by large suppresser sub groups are sufficient to render both active and yoked rats “dependent” (i.e., they experience withdrawal, and cued withdrawal or craving). For these groups the CCRs are strong enough to induce a profound conditioned negative affective state that results in rapid avoidance of the saccharin cue. If the negative affective state is part of the same mechanism that underlies the withdrawal experienced following cessation of drug use, then it may be even stronger in the yoked condition than in the active condition (Mutschler & Miczek, 1998a). The Coc-YokedLg rats, then, may be even more “dependent” on the cocaine but, strikingly, never exhibit anything resembling approach, drug-seeking, or drug-taking behaviors. In fact, they show a strong conditioned avoidance of both the taste and the place that is associated with the inescapable, uncontrollable, and unpredictable delivery of cocaine. Their avoidance of the taste is anything but paradoxical. The Coc-ActiveLg rats, on the other hand, are steadily rehearsing a habit of approach and operant behaviors that not only bring them the rewarding cocaine infusion that got them started, but now bring them relief from the negative affect. Indeed, it may be the practice of this habit in response to the cues that leads the actively administering rats and many addicted humans toward compulsive drug use (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Everitt & Robbins, 2005).
Finally, the implications of these findings and others point toward an unusual and previously unexplored adjuvant for the treatment of addiction to cocaine and, possibly, other addictive substances as well. Indeed, yoked administration also can alter the motivational properties of cocaine even when rats have a history of self-administered cocaine. For instance, rats that self-administered cocaine for 1 hr followed by 5 hrs of yoked administration exhibited reduced reinstatement to drug seeking following a cocaine priming injection but not to the presentation of the conditioned cues associated with drug delivery (Kippin et al., 2006). Interestingly, in humans there is evidence that attention hyperactivity disordered patients, a population at elevated risk for developing substance abuse disorders, are protected from developing substance abuse only if their oral psychostimulant medication (e.g., often methylphenidate) is taken regularly under the strict guidelines of the physician. If, however, psychostimulant treatment is initiated during adolescence and the treatment regimen is not adhered to then the risk for future substance abuse is not abated (Kollins, 2008). Taken together, these data indicate that the abuse liability of psychostimulants is, at least in part, mediated by the manner in which the drugs are consumed. Furthermore, the rodent data clearly demonstrate that the motivational properties of cocaine are reduced by noncontingent administration of the drug, the effect may be mediated by an aversive mechanism, and this experience may not only slow acquisition of drug self-administration behavior, but also may reduce drug seeking upon reinstatement in rats with a history of drug taking. Therefore, if cocaine is delivered in a quasi-random, uncontrollable, and inescapable manner, it reduces the incentive properties of cocaine and, in most cases, renders it aversive. This finding suggests that drug agonist replacement therapies may benefit by altering the method of drug delivery from steady state to random.
Acknowledgments
The authors would like to thank the National Institute on Drug Abuse for supplying the cocaine HCl used in this study. This work was supported by grants DA009815 and DA016512 from the National Institutes of Health.
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57(3):441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23(7):981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Cappell H, Le Blanc AE. Punishment of saccharin drinking by amphetamine in rats and its reversal by chlordiazepoxide. Journal of Comparative and Physiological Psychology. 1973;85(1):97–104. doi: 10.1037/h0034868. [DOI] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia. 1971;22(4):352–356. doi: 10.1007/BF00406873. [DOI] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE, Endrenyi L. Aversive conditioning by psychoactive drugs: effects of morphine, alcohol and chlordiazepoxide. Psychopharmacologia. 1973;29(3):239–246. doi: 10.1007/BF00414038. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2(11):2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Smith JC. Tme course of radiation-induced taste aversion conditioning. Physiol Behav. 1974;13(6):809–812. doi: 10.1016/0031-9384(74)90266-2. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117(3):262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27(8):721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Incentive Relativity. Cambridge University Press; New York: 1996. [Google Scholar]
- Flaherty CF, Checke S. Anticipation of incentive gain. Animal Learning and Behavior. 1982;10:177–182. [Google Scholar]
- Garcia J, Kimmeldorf KJ, Koelling RA. Conditioned aversions to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology (Berl) 1994;114(2):229–232. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Reward Comparison: The Achilles’ heal and hope for addiction. Drug Discovery Today: Disease Models. doi: 10.1016/j.ddmod.2009.03.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behavioral Neuroscience. 2002;116(2):321–333. [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Carelli RM. Heroin-induced suppression of saccharin intake in water-deprived and water-replete rats. Pharmacol Biochem Behav. 2000;66(3):603–608. doi: 10.1016/s0091-3057(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Freet CS, Wheeler RA, Geddes RI. Drug-induced suppression of CS intake: Reward, aversion, and addiction. In: Reilly S, Schachtman T, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2008. [Google Scholar]
- Hinson RE, Siegel S. Nonpharmacological bases of drug tolerance and dependence. J Psychosom Res. 1982;26(5):495–503. doi: 10.1016/0022-3999(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Siegel S. Pavlovian inhibitory conditioning and tolerance to pentobarbital-induced hypothermia in rats. J Exp Psychol Anim Behav Process. 1986;12(4):363–370. [PubMed] [Google Scholar]
- Horger BA, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav. 1990;37(4):707–711. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. Journal of Neuroscience. 2006;26(22):5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. Journal of Neuroscience. 2005;25(26):6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187(1):60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J Atten Disord. 2008;12(2):115–125. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- Koob GF, Vaccarino FJ, Amalric M, Swerdlow NR. Neural substrates for cocaine and opiate reinforcement. In: Fischer S, Raskin A, Uhlenhuth EH, editors. Cocaine: Clinical and biobehavioral aspects. Oxford University Press; New York: 1987. pp. 80–108. [Google Scholar]
- Krank MD, Hinson RE, Siegel S. Effect of partial reinforcement on tolerance to morphine-induced analgesia and weight loss in the rat. Behavioral Neuroscience. 1984;98(1):72–78. doi: 10.1037//0735-7044.98.1.72. [DOI] [PubMed] [Google Scholar]
- Krank MD, O’Neill S, Squarey K, Jacob J. Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 2008;196(3):397–405. doi: 10.1007/s00213-007-0971-0. [DOI] [PubMed] [Google Scholar]
- Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS. Heroin self-administration: I. Incubation of goal-directed behavior in rats. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaqlia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology. 2007;191(3):653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2(1):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Morgan D, Roberts DC. Cross-sensitization of the reinforcing effects of cocaine and amphetamine in rats. Psychopharmacology (Berl) 2007;195(3):369–375. doi: 10.1007/s00213-007-0909-6. [DOI] [PubMed] [Google Scholar]
- MacRae JR, Scoles MT, Siegel S. The contribution of Pavlovian conditioning to drug tolerance and dependence. Br J Addict. 1987;82(4):371–380. doi: 10.1111/j.1360-0443.1987.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143(1):47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Melchior CL. Conditioned tolerance provides protection against ethanol lethality. Pharmacol Biochem Behav. 1990;37(1):205–206. doi: 10.1016/0091-3057(90)90063-n. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 1998a;136(4):402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from i.v. cocaine “binges” in rats: ultrasonic distress calls and startle. Psychopharmacology (Berl) 1998b;135(2):161–168. doi: 10.1007/s002130050497. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav. 1973;10(1):73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- Nachman M, Lester D, Le Magnen J. Alcohol aversion in the rat: behavioral assessment of noxious drug effects. Science. 1970;168(936):1244–1246. doi: 10.1126/science.168.3936.1244. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Hinson RE, Siegel S. The role of Pavlovian processes in drug tolerance and dependence: implications for treatment. Addict Behav. 1981;6(3):205–211. doi: 10.1016/0306-4603(81)90018-6. [DOI] [PubMed] [Google Scholar]
- Ramos BM, Siegel S, Bueno JL. Occasion setting and drug tolerance. Integr Physiol Behav Sci. 2002;37(3):165–177. doi: 10.1007/BF02734179. [DOI] [PubMed] [Google Scholar]
- Reicher MA, Holman EW. Location preference and flavor aversion reinforced by amphetamine in rats. Animal Learning & Behavior. 1977;5:343–346. [Google Scholar]
- Riley AL, Tuck DL. Conditioned taste aversions: a behavioral index of toxicity. Ann N Y Acad Sci. 1985;443:272–292. doi: 10.1111/j.1749-6632.1985.tb27079.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Exp Clin Psychopharmacol. 2003;11(3):218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol. 1975;89(5):498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Siegel S. Tolerance to the hyperthermic effect of morphine in the rat is a learned response. J Comp Physiol Psychol. 1978;92(6):1137–1149. doi: 10.1037/h0077525. [DOI] [PubMed] [Google Scholar]
- Siegel S. Opioid expectation modifies opioid effects. Fed Proc. 1982;41(7):2339–2343. [PubMed] [Google Scholar]
- Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol. 2000;8(3):276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- Siegel S, Hinson RE, Krank MD. The role of predrug signals in morphine analgesic tolerance: support for a Pavlovian conditioning model of tolerance. J Exp Psychol Anim Behav Process. 1978;4(2):188–196. doi: 10.1037//0097-7403.4.2.188. [DOI] [PubMed] [Google Scholar]
- Siegel S, Hinson RE, Krank MD, McCully J. Heroin “overdose” death: contribution of drug-associated environmental cues. Science. 1982;216(4544):436–437. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
- Siegel S, Ramos BM. Applying laboratory research: drug anticipation and the treatment of drug addiction. Exp Clin Psychopharmacol. 2002;10(3):162–183. doi: 10.1037//1064-1297.10.3.162. [DOI] [PubMed] [Google Scholar]
- Sizemore GM, Co C, Smith JE. Ventral pallidal extracellular fluid levels of dopamine, serotonin, gamma amino butyric acid, and glutamate during cocaine self-administration in rats. Psychopharmacology (Berl) 2000;150(4):391–398. doi: 10.1007/s002130000456. [DOI] [PubMed] [Google Scholar]
- Smith JE, Koves TR, Co C. Brain neurotransmitter turnover rates during rat intravenous cocaine self-administration. Neuroscience. 2003;117(2):461–475. doi: 10.1016/s0306-4522(02)00819-9. [DOI] [PubMed] [Google Scholar]
- Smith JE, Vaughn TC, Co C. Acetylcholine turnover rates in rat brain regions during cocaine self-administration. J Neurochem. 2004;88(2):502–512. doi: 10.1046/j.1471-4159.2003.02222.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ward SJ, Roberts DC. Lesions of the dorsomedial frontal cortex block sensitization to the positive-reinforcing effects of cocaine. Pharmacol Biochem Behav. 2008;88(3):238–246. doi: 10.1016/j.pbb.2007.08.007. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Swerdlow NR, Koob GF. Paradoxical reinforcing properties of apomorphine: effects of nucleus accumbens and area postrema lesions. Brain Research. 1983;259(1):111–118. doi: 10.1016/0006-8993(83)91071-5. [DOI] [PubMed] [Google Scholar]
- Vila J. Protection from pentobarbital lethality mediated by Pavlovian conditioning. Pharmacol Biochem Behav. 1989;32(1):365–366. doi: 10.1016/0091-3057(89)90257-8. [DOI] [PubMed] [Google Scholar]
- Vogel JR, Nathan BA. Learned taste aversions induced by hypnotic drugs. Pharmacol Biochem Behav. 1975;3(2):189–194. doi: 10.1016/0091-3057(75)90147-1. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Intra-ventral tegmental area heroin-induced place preferences in rats are potentiated by peripherally administered alprazolam. Pharmacol Biochem Behav. 2005;82(3):470–477. doi: 10.1016/j.pbb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Weise-Kelly L, Siegel S. Self-administration cues as signals: drug self-administration and tolerance. J Exp Psychol Anim Behav Process. 2001;27(2):125–136. [PubMed] [Google Scholar]
- Wheeler DS, Miller RR. Contrasting reduced overshadowing and blocking. J Exp Psychol Anim Behav Process. 2007;33(3):349–359. doi: 10.1037/0097-7403.33.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- White N, Sklar L, Amit Z. The reinforcing action of morphine and its paradoxical side effect. Psychopharmacology (Berl) 1977;52(1):63–66. doi: 10.1007/BF00426601. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Effect of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. J Abnorm Psychol. 2008;117(2):428–434. doi: 10.1037/0021-843X.117.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191(4233):1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]