Abstract
While advanced stage melanoma patients have a median survival of less than a year, adoptive T cell therapy can induce durable clinical responses in some patients. Successful adoptive T cell therapy to treat cancer requires engraftment of anti-tumor T lymphocytes that not only retain specificity and function in vivo but also display an intrinsic capacity to survive. To date, adoptively transferred anti-tumor CD8+ T lymphocytes (CTL) have had limited life spans unless the host has been manipulated. To generate CTL that possess an intrinsic capacity to persist in vivo, we developed a human artificial antigen presenting cell system that can educate anti-tumor CTL to acquire both a central memory and effector memory phenotype as well as the capacity to survive in culture for prolonged periods of time. In the present report, we examined whether anti-tumor CTL generated using this system could function and persist in patients. Here, we showed that MART1-specific CTL, educated and expanded using our artificial antigen presenting cell system, could survive for prolonged periods in advanced stage melanoma patients without previous conditioning or cytokine treatment. Moreover, these CTL trafficked to the tumor, mediated biological and clinical responses, and established anti-tumor immunologic memory. Therefore, this approach may broaden the availability of adoptive cell therapy to patients both alone and in combination with other therapeutic modalities.
Introduction
The diagnosis of melanoma with distant metastases carries a median survival of less than one year (1). However, recent clinical trials suggest that adoptive T cell therapy can induce long-lasting clinical responses and may prolong overall survival (2). Successful adoptive T cell immunotherapy necessitates the generation of tumor-specific T lymphocytes that have the capacity to eliminate or control the growth of cancer cells (3-8). Investigators have developed strategies to isolate and expand large numbers of CD8+ T lymphocytes (CTL) that exhibit both anti-tumor specificity and effector function. Although these CTL have been adoptively transferred to cancer patients without significant toxicity, biological and clinical activity was limited in early studies (9-12). Considerable evidence suggests that one of the mechanisms limiting their efficacy is the failure of these CTL to persist in vivo (3, 10, 13-15).
To address the failure of CTL to persist when adoptively transferred, investigators have developed strategies to expand engrafted CTL in vivo. Administration of IL-2 after adoptive T cell transfer significantly increases both T cell survival and biologic activity (10, 12, 16, 17). Pre-infusion lymphodepletion employing myeloablative therapy combined with IL-2 administration further improves persistence of engrafted anti-tumor T cells and, more importantly, has been associated with durable clinical responses (2, 13, 18). Lymphodepletion is thought to increase access to homeostatic cytokines such as IL-7 and IL-15, eliminate suppressive regulatory T cells, and provide T cells space to expand (2, 18-21).
We have developed an alternative strategy to overcome the failure of adoptively transferred CTL to persist that requires the generation of anti-tumor CTL with a central memory and effector memory phenotype and an intrinsic capacity to survive. Previously, we reported the development of a human cell-based artificial antigen presenting cell (aAPC) genetically engineered to express HLA-A*0201 (A2), CD80, and CD83. These aAPCs expanded large numbers of CTL restricted to various tumor-associated antigens in vitro from peripheral CD8+ T cells in the presence of IL-2/IL-15 (22, 23). These antigen-specific CTL demonstrated a central memory and effector memory phenotype and were remarkably long-lived in vitro, persisting more than a year without allogeneic feeder cells or cloning (23).
In the present report, we tested whether these unique anti-tumor CTL generated with gene-engineered aAPC and IL-2/IL15 could persist in humans. MART1-specific CTL were generated in vitro from melanoma patients and then infused back without lymphodepletion or IL-2 administration. We chose the melanoma-associated antigen MART1 as our target since necessary immune assessment technologies to evaluate persistence and localization of infused MART1 T cells are widely available (10, 12). We report that CTL with a memory phenotype generated using the aAPC-based system could be safely infused and functioned as memory T cells, persisting long-term, trafficking to tumors, and inducing anti-tumor biologic and clinical responses in humans.
Results
Adoptive transfer of autologous MART1-specific CD8+ T cells generated in vitro using aAPC and IL-2/IL-15 was well tolerated
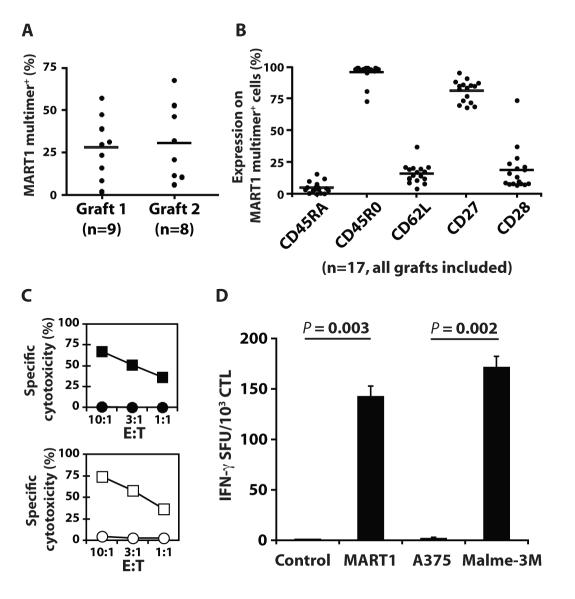
Nine patients with metastatic melanoma received a total of 17 infusions of autologous MART1-specific CTL generated from peripheral CD8+ T cells using aAPC and IL-2/IL-15 over a three-week period. The first infusion (28.0% MART1 multimer positivity, mean) was given on day 0, and the second infusion (30.7% MART1 multimer positivity, mean) was given on day 35 (Fig. 1A). 1.8×109 or greater MART1-specific CTL were successfully generated for all patients, and all but one patient received two infusions of cells (Table 1). The second graft was produced from CD8+ T cells harvested by leukapheresis two weeks after the first infusion (fig. S1). As previously published, autologous MART1-specific CTL generated over a three-week period displayed a central memory and effector memory phenotype (CD45RA− CD45RO+ CD62L+/−) (23) (Fig. 1B). Antigen-specific cytotoxicity and interferon-γ (IFN-γ secretion was demonstrated against both peptide-pulsed targets and tumor cells, demonstrating their specificity and sufficient avidity (Fig. 1C and 1D). Similar data was observed for all patients and grafts.
Fig. 1. Infused MART1-specific CTL grafts possessed a central memory and effector memory phenotype and effector function.
Seventeen MART1-specific CTL grafts were generated and administered to 9 patients. (A) The MART1 multimer positive percentage of infused CTL grafts is shown separately for grafts 1 (n=9) and 2 (n=8) for all patients. Bars represent the mean values. (B) Percent expression for the indicated molecules on MART1 multimer+ CTL is shown for all grafts (n=17). (C and D) A representative example of functional assays for infused MART1-specific CTL grafts is shown (Subject 5). (C) Antigen-specific cytotoxicity was demonstrated for MART1 peptide pulsed T2 (■) versus control peptide pulsed T2 targets (•), and the HLA-A2+ MART1+ melanoma line, Malme-3M (□), versus the HLA-A2+ MART1− melanoma line, A375 (○). (D) IFN-γ ELISPOT showed antigen-specific IFN-γ secretion using peptide pulsed T2 cells and tumor cell line targets. Mean values ±SD of triplicates are shown.
Table 1.
Patient characteristics, CTL infusions, and clinical status.
| No. | Age/Sex | Metastatic disease at study entry |
Prior therapy | Total cells infused graft 1 graft 2 |
Status on day 70 |
Time to next therapy |
Outcome after CTL or next therapy |
Duration of response (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 74/M | Liver, adrenal, spleen, lung, skin |
LND; carboplatin, paclitaxel, sorafenib; gp100 vaccine |
4.0×108 no graft 2 |
Death on day 51 |
- | Died without therapy |
- |
| 2 | 69/M | Lung, skin | WLE; LND; temozolomide; melphalan limb perfusion |
4.0×108 4.0×108 |
PD | Day 103 ipilimumab (10 mg/kg) |
PR | 16 |
| 3 | 49/F | Lung, adrenal | WLE; LND; RT; HD IL-2 |
4.3×108 4.3×108 |
MR | Day 146 ipilimumab (10 mg/kg) |
PR | 31+ |
| 4 | 68/M | Skeletal muscle, lung, mediastinum, cardiac |
Small bowel resection; HD IL-2; ipilimumab vs gp100 vs both |
3.8×108 3.8×108 |
SD | Day 140 RAF265 |
SD | 3 |
|
| ||||||||
| 5 | 66/M | Lymph nodes | WLE; LND | 4.4×109 2.5×109 |
PR | No other therapy |
CR to CTL day 140 |
25+ |
| 6 | 55/M | Lung | WLE; LND; pulmonary nodule resection |
1.8×109 3.4×109 |
SD | Day 287 high dose IL-2 |
Death due to line sepsis |
- |
| 7 | 70/F | Lung, skin | WLE; LND; adjuvant IFN |
4.0×109 4.0×109 |
PD | Day 335 ipilimumab (3 mg/kg) |
SD | 6 |
| 8 | 80/M | Lung, mediastinum |
LND; RT; temozolomide |
3.6×109 3.6×109 |
SD | Day 372 ipilimumab (3 mg/kg) |
SD | 5 |
| 9 | 64/M | Lung, skin | WLE; LND; adjuvant IFN |
4.4×109 4.4×109 |
PD | Day 146 ipilimumab (10 mg/kg) + bevacizumab |
PR | 13+ |
WLE, wide local excision; LND, lymph node dissection; HD IL-2, high dose IL-2; IFN, interferon-alpha; RT, radiation therapy; PD, progressive disease; SD, stable disease; CR, complete response; PR, partial response; MR, mixed response.
We were able to generate targeted numbers of MART1 peptide-specific CTL from all enrolled patients, and all patients received CTL infusions. Subjects 1-4 received 2×108 cells/m2 per infusion (Dose Level 1), whereas Subjects 5-9 were assigned to 2×109 cells/m2 per infusion (Dose Level 2). Subject 1 alone did not receive a second infusion due to disease progression. No dose limiting toxicities were observed in any patient. Possibly related grade 1 toxicities were limited to fatigue, pruritis (general or localized to DTH sites), and pain at sites of tumor. One patient experienced transient, asymptomatic peripheral blood eosinophilia (1,100/μl, normal range 0-400/μl) three weeks after the second infusion.
Adoptive transfer of MART1 CTL with a memory phenotype induced long-term increases in numbers of circulating MART1-specific T cells
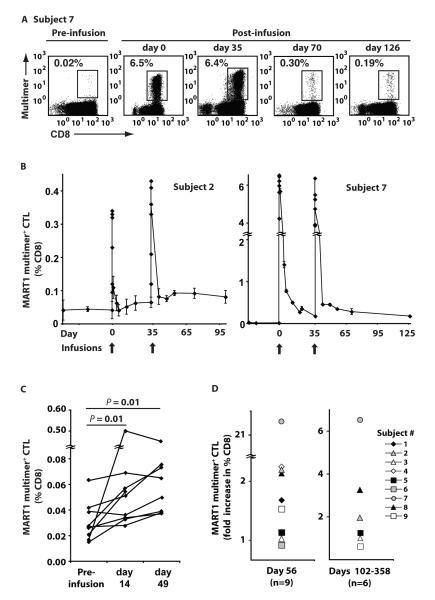
Because CTL expanded in vitro with aAPC and IL-15 could be maintained in vitro for prolonged periods, we examined whether these CTL persisted in patients upon adoptive transfer without further host manipulation. Multimer staining of peripheral CD8+ T cells revealed that, immediately post infusion, the percentage of CD8+ T cells specific for MART1 increased in all patients (Fig. 2A, 2B, fig. S2). For patients receiving 2×108 cells/m2 per dose, the frequency of MART1-specific T cells increased by a median of 6.1 fold (range 3.6-10.2), and for patients receiving 2×109 cells/m2, the MART1 T cell frequency increased by a median of 9.8 fold (range 4.0-383.5). Previous reports have suggested that adoptive transfer of tumor-specific CD8+ T cells without pre-infusion lymphodepletion or IL-2 administration persisted for less than a week (median) and were undetectable at two weeks (9, 10, 12). In contrast, we routinely observed increases in circulating MART1 multimer staining T cells two weeks after infusion of CTL (Fig. 2C, P < 0.05). We also observed sustained increases in the frequency of MART1-specific T cells by more than two-fold in 4 patients for 21 days after infusion 2 (Fig. 2D, left). Samples at later time points were available for 6 patients (Subjects 2, 3, 5, 7, 8, and 9), and in three of these patients, 2, 7, and 8, we observed increased MART1-specific T cell frequencies on days 102, 258, and 358 respectively (Fig. 2D, right). Patients received no other therapy, including CTLA-4 blockade, during this period.
Fig. 2. Adoptive transfer induced sustained increases in the frequency of circulating MART1-specific CD8+ T cells.
The frequency of MART1-specific T cells was determined by MART1 multimer staining of circulating CD8+ T cells pre- and post-infusion without any in vitro expansion. Note that patients received no other therapies such as CTLA-4 blockade during the indicated time periods. (A) Representative MART1 multimer staining for Subject 7 is shown. Day 0 and 35 analyses were performed on blood samples drawn 30 minutes post-infusion of CTL grafts. (B) The frequency of MART1-specific CD8+ T cells over the course of the clinical study is shown for Subjects 2 and 7. Mean values ±SD of quadruplicates are shown. (C) The frequency of MART1-specific CD8+ T cells for all patients is shown for time points pre-infusion and 14 days post-infusion. The average of three pre-infusion time points was used as the baseline. (D) A sustained increase in the frequency of MART1-specific T cells on day 56 was observed and is expressed as the ratio of multimer positive CD8+ T cells on day 56/pre-infusion baseline (left). Data for day 21 was included for Subject 1, who did not receive a second infusion. The ratio of multimer positive CD8+ T cells at later time points/pre-infusion baseline is shown (right). Post-infusion samples were obtained for Subjects 2, 3, 5, 7, 8, and 9 on days 102, 145, 134, 258, 358, and 133 respectively.
Persistent MART1-specific T cells after adoptive transfer were phenotypically and functionally memory T cells
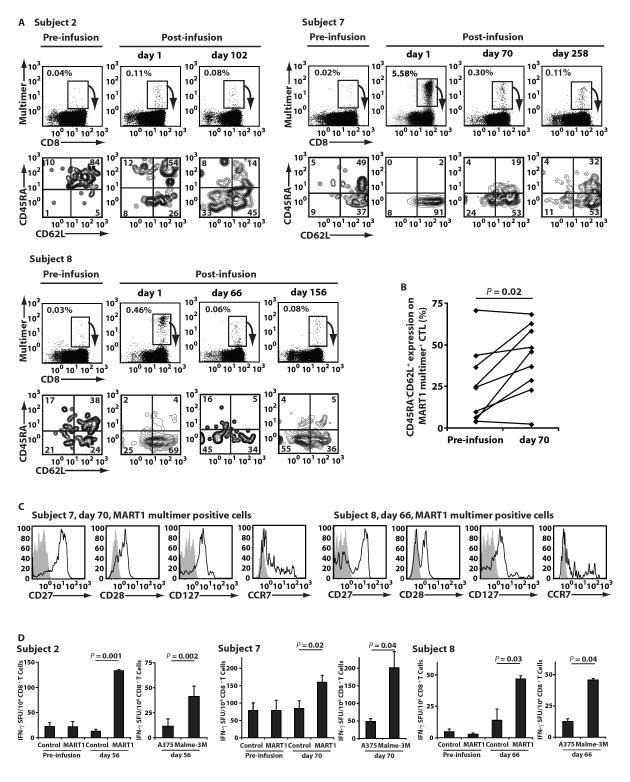
We characterized the naïve/memory immunophenotype of peripheral MART1-specific T cells, prior to and after CTL infusion. Infused MART1 CTL harbored a central memory and effector memory phenotype (CD45RA− CD62L+/−) (Fig 1B). In contrast, as seen for Subjects 2, 7 and 8 (Fig. 3A, pre-infusion), a large proportion of MART1 precursor T cells in healthy individuals’ and cancer patients’ circulation possesses a naïve phenotype (CD45RA+ CD62L+) (24). Therefore, we were able to distinguish infused MART1 CTL from pre-existing MART1 T cells by monitoring the difference in the phenotype of MART1-specific T cells. Immediately after infusion, we detected infused MART1 T cells with a memory phenotype in all patients as shown for 3 representative examples (Fig. 3A). In Subjects 2, 7, and 8, increases in the percentage of MART1-specific T cells with a central memory phenotype (CD45RA− CD62L+) were demonstrated on days 102, 258 and 156, respectively (Fig. 3A). The frequency of MART1-specific T cells displaying a central memory phenotype was determined in all patients pre- and post-infusion (Fig. 3B). Sustained increases were observed 5 weeks after transfer in 7 of 9 patients, suggesting that in vitro generated CTL with a memory phenotype engrafted upon adoptive transfer (P < 0.05). For Subjects 7 and 8, persisting MART1-specific T cells on days 70 and 66, respectively (Figure 3A) were also substantially positive for CD27, CD28, and CD127, and only a subpopulation was positive for CCR7 (Fig. 3C). This result suggests that CCR7+ MART1-specific T cells trafficked to lymph nodes and that primarily CCR7− MART1-specific T cells persisted in the circulation. Sufficient numbers of cells for Subject 2 were not available for this analysis. It should be noted that, during this period, patients received no other therapeutic or immune modulating interventions including anti-CTLA-4 mAb treatment.
Fig. 3. Adoptive transfer increased the number of MART1-specific T cells with a central memory phenotype and memory function.
(A) The phenotype of circulating MART1-specific T cells was assessed for all 9 patients pre- and post-infusion. In the depicted examples (Subjects 2, 7, and 8), the CD45RA/CD62L phenotype of fresh, circulating MART1 multimer+ cells is shown below the multimer stain for each indicated time point. Note that patients received no other anti-melanoma therapies such as anti-CTLA-4 mAb treatment during the indicated periods. (B) The percentage of peripheral CD45RA− CD62L+ MART1 multimer+ CTL is shown for all 9 patients pre- and post-infusion (P<0.05). Data for day 35 was included for Subject 1, who did not receive a second infusion. (C) The phenotype of MART1-specific CD8+ T cells for Subject 7, day 70, and Subject 8, day 66, was demonstrated. The phenotype of gated MART1 multimer staining cells is shown (open) with isotype control mAb staining (shaded). (D) Pre- and post-infusion MART1-specific recall responses are shown in IFN-γ ELISPOT assays for Subjects 2, 7, and 8. Without any prior expansion, freshly purified, peripheral CD8+ T cells were stimulated with T2 targets pulsed with MART1 or control peptide (left). T cells were also incubated with melanoma cell lines, A375 (HLA-A2+ MART1−) and Malme-3M (HLA-A2+ MART1+) (right). Data shown represents mean values ±SD of triplicates.
To demonstrate that persisting peripheral MART1-specific CD8+ T cells with a memory phenotype were functionally memory T cells, we performed an IFN-γ ELISPOT assay using peripheral blood freshly drawn from three patients shown in Figure 3A. Purified CD8+ T cells were stimulated with target cells without any in vitro sensitization in order to strictly assess memory responses. In Subjects 2, 7, and 8, we detected MART1 peptide-specific IFN-γ secretion on days 56, 70, and 66, respectively, but not prior to CTL infusion (Fig. 3D). IFN-γ secretion was also induced by HLA-A2+ MART1+ tumor cells but not by HLA-A2+ MART1− tumor cells. These results suggest that persisting peripheral MART1-specific CD8+ T cells with a memory phenotype were functionally memory T cells that possessed sufficient avidity to recognize tumor cells endogenously presenting MART1 peptide via HLA-A2 molecules.
We also conducted delayed type hypersensitivity (DTH) testing by administering intradermal injections of MART1 peptide 3 weeks pre- and post-infusion 1. We observed enhanced DTH reactions to peptide injections performed post-infusion in Subjects 1, 2, 5, and 7. In Subject 7, histologic examination showed recruitment of mononuclear cells admixed with eosinophils accumulating around blood vessels. Immunohistochemistry analysis demonstrated recruitment of CD3 and CD8 positive lymphocytes consistent with a memory recall response mediated by infused MART1-specific CD8+ T cells (fig. S3). Taken all together, these in vivo data suggest that persisting MART1-specific T cells in the circulation were not only phenotypically but also functionally memory T cells.
Infused MART1 CTL could traffic to tumor sites after adoptive transfer
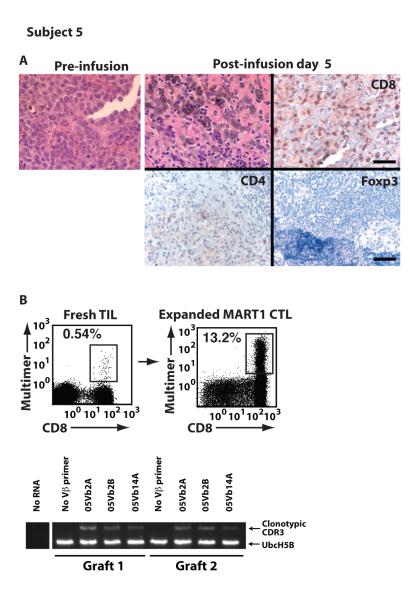
To induce clinical responses, transferred CTL must traffic to tumor sites and mediate effector responses within the tumor microenvironment. We therefore assessed whether infused CTL trafficked to tumor by analyzing post-infusion tumor biopsies. Subject 5, who experienced an objective clinical response (fig. S4), underwent a tumor biopsy on day 5 of cycle 1. A pre-infusion tumor biopsy showed minimal lymphocytic infiltration at 4 lymphocytes/mm2 (Fig. 4A, left). In contrast, post-infusion, lymphocyte infiltration of tumor was brisk in both the central and peripheral regions at 225 lymphocytes/mm2 (Fig. 4A, upper middle). Near complete tumor destruction occurred with tumor/lymphocyte satellitosis, hemorrhagic cell necrosis, and fibrosis, consistent with a strong, overwhelming anti-tumor immune response. Almost all infiltrating T cells were strongly positive for CD8 (135 cells/mm2), with fewer stained by CD4 (56 cells/mm2). Foxp3 staining was negative, suggesting a lack of regulatory T cells (Fig. 4A, upper right and lower panels). Direct multimer staining of tumor infiltrating lymphocytes (TIL) without any in vitro expansion identified the presence of MART1-specific T cells (Fig. 4B, left).
Fig. 4. Transferred CTL trafficked to sites of antigen expression and mediated anti-tumor responses.
Pathologic analysis was performed to determine whether transferred T cells were able to traffic to sites of antigen expression. (A) Infiltration of tumor by lymphocytes was morphologically assessed pre-infusion and post-infusion for Subject 5. Immunohistochemical staining of the post-infusion biopsy for CD8, CD4 and Foxp3 is shown. Scale bar, 50 μm, upper right, applies to upper panels. Scale bar, 100 μm, lower right, applies to lower panels. (B) Without in vitro expansion, MART1-specific T cells were identified in fresh TIL by MART1 multimer staining. Three MART1-specific clonotypes derived from TIL were identified. The presence of all three MART1-specific T cell clonotypes in both CTL grafts was shown by RT-PCR.
In order to evaluate whether CTL mediating the anti-tumor response were present in the CTL grafts, we performed clonotypic analysis of tumor infiltrating MART1-specific T cells using their CDR3 sequences as a molecular marker. Three MART1-specific CTL clonotypes, 05Vb2A, 05Vb2B, and 05Vb14A, were identified from MART1-specific T cells expanded from the Subject 5 tumor biopsy (Fig. 4B). CDR3-specific RT-PCR analysis showed that all three clones existed in both CTL grafts. Similar analysis was performed for Subject 3, who experienced a mixed clinical response (fig. S5A). Five MART1-specific CTL clonotypes were identified from resected tumor samples, and 4 of the 5 clones were detected in both CTL grafts (fig. S5B). Since infused CTL were not gene-marked, we cannot exclude the possibility that MART1-specific T cells at the tumor sites were derived endogenously and not from infused grafts. However, combined with the data that infused MART1 CTL did persist and expand in patients upon transfer (Fig. 2 and 3), these data suggest that anti-tumor CTL expanded in T cell grafts could traffic to sites of tumor and mediate anti-tumor functions after adoptive transfer.
Clinical responses occurred after CTL infusions and subsequent therapies
Patients received CTL infusions without any additional therapy including lymphodepletion, cytokine administration, or vaccination. Responses to CTL infusions were assessed by physical examination and radiographic measurements during the tenth week of the study (Table 1, Status on day 70). On day 70, Subject 5 had achieved a partial response by CT scan with a reduction in the size of tumor-involved lymph nodes by 33%. Follow-up on day 140 showed no evidence of FDG-avid disease by PET/CT with normalization in the size of lymph nodes previously involved by tumor (fig. S4). This response has been confirmed by multiple subsequent high resolution CT and PET/CT scans, and, without any additional anti-tumor therapy, the patient has continued to remain without evidence of disease for over 25 months. For the remaining patients on day 70, 1 had died without receiving a second CTL infusion, 3 had progressive disease, and 4 were stable by RECIST criteria. Subject 3, who had stable disease overall, had a mixed response with reduction in the size of one metastatic pulmonary lesion (fig. S5A). It should be noted that Subjects 7 and 8 experienced clinical stabilization and did not require any other anti-cancer therapy for 11 and 12 months, respectively. Both experienced delayed mixed responses by PET/CT during this time period, a phenomenon that has previously been reported with other immune based therapies (25). These clinical responses suggest that infused CTL with a memory phenotype could traffic to and attack tumor.
Seven patients received additional therapy upon disease progression (Table 1). Five were treated with the anti-CTLA-4 mAb, ipilimumab. Three patients (Subjects 2, 3, and 9) achieved partial responses that were durable, lasting up to 1-2 years, and two (Subjects 7 and 8) had stable disease, lasting 5-6 months. Notably, Subject 3 achieved near complete resolution of pulmonary and adrenal metastases and is considered to have a partial response only due to subcentimenter radiographic abnormalities detected on CT. Yet, despite the fact that anti-CTLA-4 therapy was halted due to autoimmune toxicity, she has remained free of disease progression for over 31 months.
Anti-CTLA-4 mAb therapy may promote the expansion of infused CTL as memory T cells
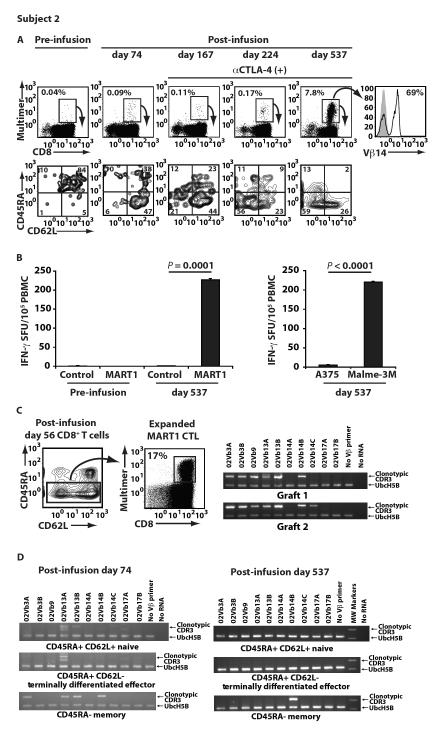
As described above, Subject 2, who had progressive disease after CTL infusion, was treated with multiple cycles of CTLA-4 blockade and achieved a partial response. In this patient, before CTL infusion, all MART1 multimer positive CD8+ T cells had a naïve phenotype (Fig. 5A, left). However, MART1 multimer positive T cells with a memory phenotype persisted and demonstrated memory function after CTL transfer (Fig. 3D and 5A). After CTLA-4 blockade, there was a marked further expansion of peripheral MART1-multimer positive T cells, which continued to display a central memory and effector memory phenotype (Fig. 5A, middle). On day 537, MART1 T cells increased to 7.8% of circulating CD8+ T cells and were oligoclonal, consisting of Vβ 14-positive and negative clones (Fig. 5A, right). Furthermore, these circulating day 537 MART1-specific T cells were able to recognize MART1+ tumor cells and demonstrated potent antigen-specific recall responses without in vitro sensitization (Fig. 5B). A similar effect of CTLA-4 blockade was observed for Subject 8 (fig. S6).
Fig. 5. Adoptively transferred CTL expanded in a patient treated with anti-CTLA-4 (ipilimumab) therapy.
After two infusions of MART1 CTL, Subject 2 began anti-CTLA-4 mAb therapy on post-infusion day 103. (A) The frequency of peripheral MART1-specific T cells is shown pre- and post-infusion of CTL and after initiation of anti-CTLA-4 mAb therapy. The CD45RA/CD62L phenotype of multimer positive cells is shown below the multimer stain for each time point. TCR Vβ 14 staining of markedly expanded MART1 multimer+ T cells on day 537 is shown (right). (B) Without any prior in vitro expansion, MART1-specific recall responses on day 537, but not pre-infusion, were demonstrated with the IFN-γ ELISPOT assay using PBMC incubated with control or MART1 peptide (left). Fresh day 537 PBMC were also incubated with the melanoma cell lines, A375 (HLA-A2+ MART1−) and Malme-3M (HLA-A2+ MART1+) (right). Data shown represents mean values ±SD of triplicates. (C) MART1-specific CTL were expanded from the memory fraction (CD45RA−) of CD8+ T cells on day 56, prior to CTLA-4 blockade, to identify 10 MART1-specific clonotypes. The presence of seven of these 10 clones in CTL grafts 1 or 2, including Vβ 14 clones, 02Vb14B and 02Vb14C, is shown. (D) Identification of the Vβ 14 clone, 02Vb14B, exclusively in the CD45RA− memory subpopulation only is shown on day 74, prior to CTLA-4 blockade (left), and on day 537, after CTLA-4 blockade (right).
To molecularly track persisting MART1-specific T cells in Subject 2, we isolated 10 circulating individual MART1-specific T cell clonotypes from the CD45RA− CD8+ T cell memory fraction on day 56 (Fig. 5C, left). CDR3-specific RT-PCR revealed that 7/10 of these clonotypes were present in the infused CTL grafts, including a Vβ 14 clone, 02Vb14B (Fig. 5C, right). On day 74, after CTL transfer and before CTLA-4 blockade, we detected the Vβ 14 clone, 02Vb14B, in the CD45RA− memory fraction, but not in the CD45RA+ CD62L+ naïve or the CD45RA+ CD62L− terminally differentiated fractions (Fig. 5D, left). Intriguingly, on day 537 after CTLA-4 blockade, the Vβ 14 clone, 02Vb14B, was again detected exclusively in the CD45RA− memory fraction (Fig. 5D, right). These data suggest that 02Vb14B CTL were included in Vβ 14-positive MART1-tetramer positive cells with a memory phenotype shown in Figure 5A, right. These results provide strong molecular evidence that MART1 CTL clonotypes with sufficient avidity to attack tumors could be selected in vivo from adoptively transferred polyclonal MART1 CTL and then persist and expand as memory T cells. Furthermore, CTLA-4 blockade may augment anti-tumor immunological memory and responses established by adoptive transfer of MART1 CTL.
Discussion
In this “proof of concept” clinical trial, we tested the hypothesis that anti-tumor CTL educated in vitro using our gene-engineered aAPC would establish anti-tumor immunological memory in patients when adoptively transferred without lymphodepletion or cytokine therapy. We successfully showed that infused CTL with a memory phenotype were able to persist as memory T cells for long periods of time, thereby establishing anti-tumor immunological memory. Furthermore, persisting CTL had anti-tumor function, and molecular evidence indicated that CTL trafficked to and mediated biological and clinical responses. Adoptive transfer of CTL generated using the aAPC-based system enables the establishment of anti-tumor memory that alone, or in combination with other immune modulators, can promote anti-tumor immunity.
The aAPC-based system was specifically designed to be feasible, transferrable, and to educate CTL so that they could be long-lived in vitro and persistent in vivo. First, we ectopically expressed CD83 on aAPC in conjunction with HLA-A2 and CD80. We and others previously demonstrated that CD83 is critical for the longevity of lymphocytes both in vitro and in vivo (22, 26). Second, we added IL-15 to cultures for T cell expansion. Unlike IL-2, IL-15 has been reported to favor the expansion of antigen-specific T cells with a central memory phenotype (27). Furthermore, it has been shown that in vitro exposure to IL-15 enables T cells to be long-lived in vivo (28). These unique attributes of the aAPC-based system may have enabled the generation of anti-tumor CTL that were capable of prolonged persistence and survival as memory T cells after adoptive transfer.
The percentage of peripheral MART1-specific T cells with a CD45RA− CD62L+ central memory phenotype increased after transfer, even though only a minority of infused CTL expressed CD62L in some grafts. This result supports that CD45RA− CD62L+ CTL generated in vitro can in fact behave as central memory T cells in vivo and thus preferentially persist, whereas MART1 CTL with CD45RACD62L− effector memory and CD45RA+ CD62L− terminally differentiated effector phenotypes may not engraft or expand. Persistent MART1 T cells with a memory phenotype also demonstrated recall responses without any in vitro expansion, suggesting that they were functionally memory T cells. These findings are in accordance with animal studies showing that CTL with a central memory phenotype are more likely to retain memory T cell properties that enable superior persistence and expansion in vivo (27, 29). Our findings further support that CTL with a central memory phenotype can mediate memory responses and can persist after adoptive transfer without lymphodepletion or IL-2 administration.
In this early phase study, the degree of persistence of MART1 T cells in the peripheral blood was not strictly correlated with clinical responses. Indeed, other factors may contribute to the success of adoptive T cell transfer, including the removal of negative regulatory cells. However, we did observe the induction of inflammatory infiltrates within tumors and detected the presence of identical clonotypic CTL in both infused grafts and tumor T cell infiltrates. Combined with data that persistent CTL have anti-tumor function, this strongly indicates that transferred CTL trafficked to tumor and modulated the host anti-tumor immune response. No patient experienced any untoward side effects, and we did observe beneficial biological and clinical responses. Subject 5 had a partial clinical response on day 70 and continues to have complete resolution of disease for more than 25 months. Three other patients experienced mixed responses or long-lasting disease stabilization. One patient showed a mixed response with marked reduction of a pulmonary lesion. Two other patients, who both showed increases in circulating MART1 T cells (Fig. 2D), had mixed clinical responses in the months after CTL transfer and did not require further treatment for more than 300 days. With the exception of Subject 5, who has not needed any further therapy, CTL infusion alone, however, was not sufficient to prevent eventual tumor progression.
As shown in Table 1, upon disease progression five patients were treated with ipilimumab, which blocks CTLA-4 and potentiates antitumor T cell responses (1, 30, 31). Among 5 patients, three achieved partial responses (Subjects 2, 3, and 9) and two other patients had stable disease (Subjects 7 and 8). Although we cannot draw definite conclusions due to the small number of patients and the potential for selection bias, we found these responses noteworthy because the reported overall response rate for ipilimumab is less than 16% (1, 32). Moreover, cellular and molecular evidence indicates that CTLA-4 blockade can induce a marked expansion in vivo of adoptively transferred anti-tumor CTL with memory phenotype and function. Either adoptive cell therapy or CTLA-4 blockade alone may be ineffective for most advanced melanoma patients. However, our data suggests that, by first establishing anti-tumor memory responses with adoptive transfer, anti-CTLA-4-induced immune activation can result in enhanced biological responses.
By intensive analysis of infused MART1 CTL at both the cellular and molecular levels, we demonstrated that anti-tumor CTL with a memory phenotype educated in vitro using gene-engineered aAPC and IL2/IL15 can persist in vivo as memory T cells. Although anti-tumor biological effects were ultimately insufficient to prevent progression for most patients, establishment of anti-tumor memory by engraftment of infused CTL may shift the balance between tolerance and anti-tumor immunity. Thus primed, anti-tumor immune responses could be enhanced by CTLA-4 blockade, resulting in the observed tumor regressions seen in patients with previously progressive disease. Further improvement in clinical activity may be achieved by combining CTL transfer with approaches such as lymphodepletion, which can eliminate immune suppressive elements such as regulatory T cells. Adoptive transfer of CTL generated with aAPC therefore provides a platform for establishing anti-tumor memory, which can then be combined with immune modulators such as checkpoint inhibitors, lymphodepletion, cytokines, or vaccination to improve tumor regression and patient prognosis.
Materials and Methods
Patients
This Dana-Farber/Harvard Cancer Center phase I clinical protocol (NCT00512889) received approval from the local institutional review board and biosafety committees, the National Institutes of Health Recombinant DNA Advisory Committee, and the United States Food and Drug Administration. All patients had Stage IV metastatic melanoma with baseline biopsies positive for MART1 staining by immunohistochemistry (Covance). MART1 expression was >90% for Subjects 3, 7, 8, and 9; 50-90% for Subjects 1, 2, and 5; and 10-50% for Subjects 4 and 6. All patients were HLA-A*0201 positive by high resolution HLA DNA typing (American Red Cross). Patients received no other anti-cancer therapy, including lymphodepletion, IL-2 administration, or antibody treatment, within four weeks of the leukapheresis and during study participation. Primary study endpoints were to define the feasibility and toxicity of administering MART1-specific CTL at two dose levels (2×108 cells/m2 and 2×109 cells/m2). Secondary endpoints included analyzing the phenotype and function of in vitro expanded CTL, evaluating the in vivo frequency and function of MART1-specific CTL after adoptive transfer, and evaluating whether transferred CTL can traffic to sites of tumor.
Adoptive transfer of MART1-specific CTL generated in vitro using aAPC and IL-2/IL-15 to patients with advanced melanoma
Patient PBMC were leukapheresed and CD8+ T cells were selected using CliniMACs (Miltenyi). The generation of clinical grade aAPC has previously been reported (23). Purified CD8+ T cells were stimulated with clinical grade aAPC pulsed with MART1 peptide (ELAGIGILTV) obtained from Clinalfa (23). Between stimulations, cultures were supplemented with IL-2 at 10-50 IU/ml (Novartis) and IL-15 at 10-50 ng/ml (Peptrotech). Stimulation and expansion of CTL were performed in gas permeable FEP culture bags to allow consistent and reproducible production of CTL grafts (American Fluoroseal Corporation). After three weekly stimulations, CTL were harvested and infused to patients on day 0 at either dose level 1 (2×108 cells/m2) or dose level 2 (2×109 cells/m2) without any other therapies such as lymphodepletion, IL-2 administration or vaccination. Two weeks after the first CTL infusion, patients underwent a second leukapheresis to generate a second CTL graft. MART1-specific CTL for the second CTL graft were similarly generated and were infused on day 35. The date of the first infusion is defined as day 0 throughout the manuscript.
Phenotype analysis of MART1-specific T cells and CTL grafts
To determine the phenotype of in vitro expanded MART1-specific T cells, CTL from grafts were stained with HLA-A2/peptide multimers (ProImmune) as previously described (23). Multimer positive T cells were costained with the following anti-human mAbs: CD8, CD28, CD45RO, CD62L (Beckman-Coulter); CD27, CD45RA (Invitrogen); CD127 (BD Biosciences); CCR7 (R&D Systems).
Functional assays
IFN-γ ELISPOT and standard chromium release assays were performed as described elsewhere (22, 23). In these in vitro assays, the native MART1 peptide (27AAGIGILTV35) and the control HIV pol peptide (476ILKEPVHGV484) were used (New England Peptide). T2 cells, A375 (HLA-A2+ MART1−), and Malme-3M (HLA-A2+ MART1+) were obtained from American Type Cell Collection. Assays were performed with a portion of infused CTL grafts and with CD8+ T cells or PBMC freshly drawn from patients without any in vitro stimulation.
Phenotypic analysis of peripheral and tumor infiltrating MART1-specific CD8+ T cells prior to and post CTL infusion
Without any in vitro expansion, peripheral CD8+ T cells were isolated from freshly drawn PBMC and stained with HLA-A2/MART1 peptide multimer (ProImmune) as previously described (23). Positive staining was confirmed by concurrently staining samples with control HLA-A2/HIV pol peptide multimer. Where indicated, multimer positive cells were costained with anti-CD45RA, CD62L, CD27, CD28, CD127, CCR7, and isotype control mAbs. TCR Vβ subtype analysis was performed on MART1 multimer+ T cells using the Beta Mark kit and anti-Vβ 14 mAb (Beckman Coulter) without any in vitro expansion.
Molecular analysis of clonotypic MART1-specific T cells
MART1 multimer+ T cells were highly purified using flow cytometry-guided sorting from short-term expanded TIL or CD45RA− CD8+ T cells. Based on the TCR Vβ subtypes of MART1 multimer+ cells determined by the Beta Mark kit, CDR3 regions of positive Vβ subtypes were amplified by RT-PCR using SuperScript reverse transcriptase (Invitrogen) and Phusion high fidelity DNA polymerase according to the manufacturers’ instruction. Sequences of Vb primers are published elsewhere (33). Amplified fragments were cloned to plasmid vectors and their sequence was determined.
For the detection of circulating MART1-specific clonotypic T cells, without any in vitro culture, fresh CD45RA+CD62L+ naïve, CD45RA− memory, and CD45RA+CD62L− terminally differentiated effector CD8+ T cells were isolated by flow cytometry-guided sorting at the time points indicated. CDR3-specific RT-PCR was performed using SuperScript One-Step RT-PCR (Invitrogen) according to the manufacturer’s protocol to determine the existence of clonotypic MART1 T cells in each subpopulation. To detect MART1-specific clonotypic T cells in each CTL graft, MART1 multimer+ CTL were purified and CDR3-specific RT-PCR was performed similarly. A housekeeping gene, UbcH5B, was used as an internal control and its primer sequences are; 5′-TCTTGACAATTCATTTCCCAACAG-3′ (sense) and 5′-TCAGGCACTAAAGGATCATCTGG-3′ (antisense).
Pathologic evaluation of tumor biopsies
When tumor was accessible, biopsies were performed during the first week of cycle 1. Tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. 5-μm sections were stained with hematoxylin and eosin, and immunohistochemical staining was performed on paraffin sections using antibodies against CD3, CD4, and CD8 (Ventana Medical Systems) and Foxp3 (Abcam). Tumor lymphocyte reactivity was quantitatively assessed according to criteria previously described (34).
Pathologic evaluation of DTH reactions
Delayed-type hypersensitivity (DTH) tests were performed by intradermal injections of 50 μg MART1 peptide (ELAGIGILTV) on day -21 immediately after leukapheresis and on cycle 1 day 21. Injection sites were inspected two days later, and biopsies were performed. Tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. 5-μm sections were stained with hematoxylin and eosin for pathologic analysis. Immunohistochemical staining was performed on paraffin sections using antibodies against CD3 and CD8 (Ventana Medical Systems). DTH reactions were assessed according to criteria previously described (35).
Statistical analysis
Welch’s t-test was used for two-sample comparisons and the Wilcoxon signed-rank test was used for paired comparisons. All p-values are two-sided and considered significant at the 0.05 level.
Supplementary Material
Fig. S1. Clinical protocol time line.
Fig. S2. Verification of HLA-A2/MART1 peptide multimer staining.
Fig. S3. Adoptive transfer increased delayed-type hypersensitivity reaction to injected MART1 peptide.
Fig. S4. Clinical response to adoptive transfer of MART1-specific T cells in Subject 5.
Fig. S5. Trafficking of transferred CTL to pulmonary lesions, Subject 3.
Fig. S6. Expansion of adoptively transferred MART1 CTL in Subject 8 treated with anti-CTLA-4 (ipilimumab) therapy.
Acknowledgements
We thank the DFCI Connell-O’Reilly Cell Manipulation Core Facility for the generation of CTL grafts. We thank John Daley and Suzan Lazo-Kallanian at the flow cytometry core for expert technical assistance, and Stephen Conley for photography. Funding: Funding was provided by the Immunotherapy Fund 1 (LMN), the S. Craig Lindner Fund for Cancer Research (LMN), the Rudolf E. Rupert Foundation for Cancer Research (LMN), the Cancer Research Institute/Ludwig Institute for Cancer Research Cancer Vaccine Collaborative (MOB, LMN, and NH), Friends of the Dana-Farber Cancer Institute (MOB and NH), and Dunkin’ Donuts Rising Stars Program (MOB and NH). NH was funded by NIH grants K22CA129240 and R01CA148673 and the American Society of Hematology Scholar Award. Pre-clinical scale-up was supported by the Center for Human Cell Therapy Boston (NIH 5U24HL074355). Subjects received ipilimumab on clinical trials supported by Bristol-Myers Squibb (FSH).
Footnotes
Author contributions: MOB, NH, LMN conceived the study and wrote the paper. MOB performed the clinical trial as the principal investigator. PF, LD, LB, MF, DL, FSH, MJ, and SR assisted with conducting the clinical trial. DN and KS performed statistical analysis. MOB and NH designed and conducted laboratory experiments. MIM, MMM, GM, MT, APM, AB, and OI helped to perform experiments. EV and MM analyzed histopathological specimens. All authors approved the manuscript.
Competing interests: The Dana-Farber has filed a patent related to aAPC, application number 10/850,294 entitled “Modified Antigen-Presenting Cells,” on which MOB, LMN, and NH are named as inventors. FSH is a non-paid consultant to Bristol-Myers Squibb. The other authors declare that they have no competing interests.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific t cell immunity. Curr Opin Immunol. 2009;21:224–232. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner MK, Heslop HE. Adoptive t cell therapy of cancer. Curr Opin Immunol. 2010;22:251–257. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulos CM, Suhoski MM, Plesa G, Jiang T, Basu S, Golovina TN, Jiang S, Aqui NA, Powell DJ, Jr., Levine BL, Carroll RG, Riley JL, June CH. Adoptive immunotherapy: Good habits instilled at youth have long-term benefits. Immunol Res. 2008;42:182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt TM, Ragnarsson GB, Greenberg PD. T cell receptor gene therapy for cancer. Hum Gene Ther. 2009;20:1240–1248. doi: 10.1089/hum.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yee C. Adoptive therapy using antigen-specific t-cell clones. Cancer J. 2010;16:367–373. doi: 10.1097/PPO.0b013e3181eacba8. [DOI] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, Leitman SF, Rosenberg SA. Adoptive transfer of cloned melanoma-reactive t lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive t cell therapy using antigen-specific cd8+ t cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred t cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell MS, Darrah D, Yeung D, Halpern S, Wallace A, Voland J, Jones V, Kan-Mitchell J. Phase i trial of adoptive immunotherapy with cytolytic t lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20:1075–1086. doi: 10.1200/JCO.2002.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 12.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase i study of adoptive t-cell therapy using antigen-specific cd8+ t cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 13.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr., Rosenberg SA. Cutting edge: Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific t-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 17.Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: Direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred cd8 t cells in patients with metastatic melanoma. PLoS One. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (il)-15 and il-7 jointly regulate homeostatic proliferation of memory phenotype cd8+ cells but are not required for memory phenotype cd4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific cd8+ t cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. Cd8+ t cell immunity against a tumor/self-antigen is augmented by cd4+ t helper cells and hindered by naturally occurring t regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano N, Butler MO, Xia Z, Ansen S, von Bergwelt-Baildon MS, Neuberg D, Freeman GJ, Nadler LM. Engagement of cd83 ligand induces prolonged expansion of cd8+ t cells and preferential enrichment for antigen specificity. Blood. 2006;107:1528–1536. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler MO, Lee JS, Ansen S, Neuberg D, Hodi FS, Murray AP, Drury L, Berezovskaya A, Mulligan RC, Nadler LM, Hirano N. Long-lived antitumor cd8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin Cancer Res. 2007;13:1857–1867. doi: 10.1158/1078-0432.CCR-06-1905. [DOI] [PubMed] [Google Scholar]
- 24.Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Lienard D, Lejeune F, Fleischhauer K, Cerundolo V, Cerottini JC, Romero P. High frequencies of naive melan-a/mart-1-specific cd8(+) t cells in a large proportion of human histocompatibility leukocyte antigen (hla)-a2 individuals. J Exp Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 26.Prazma CM, Yazawa N, Fujimoto Y, Fujimoto M, Tedder TF. Cd83 expression is a sensitive marker of activation required for b cell and cd4+ t cell longevity in vivo. J Immunol. 2007;179:4550–4562. doi: 10.4049/jimmunol.179.7.4550. [DOI] [PubMed] [Google Scholar]
- 27.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive cd8+ t cells confer superior antitumor immunity compared with effector memory t cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. Il-15 as a mediator of cd4+ help for cd8+ t cell longevity and avoidance of trail-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector cd8+ t cells derived from central memory cells establishes persistent t cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber J. Ipilimumab: Controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong L, Small EJ. Anti-cytotoxic t-lymphocyte antigen-4 antibody: The first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 32.Agarwala SS, Ribas A. Current experience with ctla4-blocking monoclonal antibodies for the treatment of solid tumors. J Immunother. 2010;33:557–569. doi: 10.1097/CJI.0b013e3181dcd260. [DOI] [PubMed] [Google Scholar]
- 33.Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current protocols in immunology. Wiley; New York: 2010. p. v. (loose-leaf) [Google Scholar]
- 34.Mihm MC, Jr., Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: A histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 35.Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, Hodi FS, Jaklitsch M, Mentzer S, Swanson S, Lukanich J, Bueno R, Wain J, Mathisen D, Wright C, Fidias P, Donahue D, Clift S, Hardy S, Neuberg D, Mulligan R, Webb I, Sugarbaker D, Mihm M, Dranoff G. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;21:624–630. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Clinical protocol time line.
Fig. S2. Verification of HLA-A2/MART1 peptide multimer staining.
Fig. S3. Adoptive transfer increased delayed-type hypersensitivity reaction to injected MART1 peptide.
Fig. S4. Clinical response to adoptive transfer of MART1-specific T cells in Subject 5.
Fig. S5. Trafficking of transferred CTL to pulmonary lesions, Subject 3.
Fig. S6. Expansion of adoptively transferred MART1 CTL in Subject 8 treated with anti-CTLA-4 (ipilimumab) therapy.