Abstract
Androgens regulate the development and functioning of male reproductive organs and play a crucial role in the onset and progression of prostate cancer. Androgen action is primarily mediated through the nuclear androgen receptor (AR) that acts as a ligand-dependent transcription factor. This mode of androgen action takes hours to manifest and is referred to as the genomic pathway. The androgen-mediated genomic responses require activity of cAMP-dependent protein kinase (PKA). Androgens also act through non-genomic pathways in certain cell types to evoke rapid (manifested in minutes) responses that are mediated through changes in ion currents and second messengers. Here, we show that androgen causes the rapid and cAMP-dependent activation of PKA in prostate cells. The androgen-induced PKA activation is not inhibited by nuclear AR antagonist bicalutamide, and can be observed in cells that do not express nuclear AR gene. Reduction of Gαs expression with siRNA attenuates the androgen-mediated activation of PKA, which is required for the androgen-induced prostate cell proliferation. We conclude that androgen actively evokes a non-genomic signaling pathway to activate PKA that is needed for the genomic functioning of nuclear AR. The inhibition of PKA activation, together with standard AR-targeted therapies, may be more efficacious for treatment of patients with prostate cancer.
Keywords: G protein, protein kinase A, androgen receptor, testosterone, prostate cancer
Introduction
Androgens manifest their effects on target cells by binding the androgen receptor (AR), which plays a critical role in normal development of the prostate gland as well as the initiation and progression of prostate cancer. Indeed, mainstay treatment of locally advanced and metastatic prostate cancer is achieved with endocrine therapies aimed to: [i] decrease circulating androgen levels via chemical or physical castration (1), and [ii] block AR activation with anti-androgens (2). The AR is a ligand-activated transcription factor that mediates the biological effects of male sex steroids in the target cells by activating transcription of androgen-regulated genes. Upon ligand binding, the AR dimerizes, translocates into the nucleus, binds to androgen response elements (specific DNA sequences situated in the promoter/enhancer regions of target genes), and associates with various regulatory proteins and members of the transcription initiation complex to initiate transcription (3). This is the classical view of AR signaling, or the “genomic pathway.” This mode of androgen action requires hours to manifest fully and can be inhibited by agents that block gene transcription or protein translation (4).
Emerging evidence indicates that stimulation with androgens can also initiate rapid (manifested in minutes), non-genomic signaling events such as increased intracellular Ca+2 in skeletal muscle cells (5), activation of extracellular signal-regulated kinase (ERK) in skeletal muscle or sertoli cells (5, 6), and activation of phosphatidylinositol 3-kinase in osteoblasts (7). The signaling steps involved in the non-genomic actions of androgen are not understood completely; however, they are likely mediated through multiple, cell type-specific mechanisms. Significantly, the non-genomic action of androgen may or may not involve nuclear AR. For instance, the androgen-mediated activation of ERK in androgen-dependent (AD) LNCaP prostate cancer cells appears to require nuclear AR (8), whereas the release of intracellular Ca+2 in skeletal muscle cells (5) or IC21 macrophages (9) occurs through nuclear AR-independent mechanisms. It is possible that these non-genomic actions of androgen contribute to and/or complement the genomic responses mediated through the classical pathway. In support of this view, a recent report suggested that genomic nuclear AR function (to mediate cell proliferation) requires non-genomic activity of AR to activate c-Src (10).
We previously showed that androgen-mediated activation of nuclear AR (through the genomic pathway) is dependent upon the activation of cAMP-dependent protein kinase (PKA) (11). However, it is not known whether the existing pool of activated PKA inside the cell is used for this purpose, or if androgen stimulation per se provokes PKA activation. In this study, we tested the hypothesis that androgen activates PKA in prostate cells. We show that androgen promotes the PKA activation via a signaling cascade that involves heterotrimeric Gs protein, adenylyl cyclase and cAMP, but is independent of nuclear AR. We also find that PKA activity is required for the androgen-induced activation of nuclear AR and proliferation of prostate cells.
Materials and Methods
Cell culture and transfection
Androgen-dependent LNCaP, prostate epithelial RWPE1 and mouse macrophage IC21 cell lines were obtained from the American Type Culture Collection (Manassas, VA). The androgen-dependent LAPC4 and androgen-independent PC3M prostate cancer cells were generous gifts from C. L. Sawyers (MSKCC), and P. Kelly (Duke University), respectively. LNCaP, LAPC4 and IC21 cells were maintained in RPMI 1640 containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 10 mM HEPES, pH 7.5, 1 mM sodium pyruvate, and 1.26 g/l glucose. The PC3M cells were cultured in RPMI 1640 containing 10% FBS, 1% penicillin-streptomycin, 10 mM HEPES. The RWPE1 cells were cultured in keratinocyte-serum free medium (Gibco-BRL) supplemented with 5 ng/ml human recombinant EGF and 0.05 mg/ml bovine pituitary extract. Cells at about 60-80% confluence were used for transfection in 100 mm dishes using the appropriate cDNAs and transfection reagents (DMRIE-C for LNCaP and LAPC4 cells; lipofectamine for PC3M and RWPE1 cells). After transfection, cells were equally divided to ensure the identical cell populations. The agonist-regulated cell proliferation was determined by counting cells using trypan blue and hemocytometer, as previously described (12).
DNA constructs
Mammalian expression plasmids encoding phosphodiesterase (PDE) 4D, Flag epitope-tagged vasodilator-stimulated phosphoprotein (VASP), and the regulator of G protein signaling (RGS) domain of Axin were kindly provided by R. J. Lefkowitz (Duke University) and J. S. Gutkind (NIH). Plasmids encoding AR reporters (ARR2-luciferase or probasin-luciferase) were described before (11). Two different siRNA duplexes were designed using the OligoEngine siRNA design tool (Seattle, WA) based on sequence of the long and short isoforms of Gαs mRNAs. The sequence of shRNA 1625 was 5′- GATCCCCCAAACAACTCCAGGACGAATTCAAGAGATTCGTCCTGGAGTTGTT TGTTTTTA-3′, and the sequence of shRNA 2051 was 5′- GATCCCCCCAGCTGATTGACTGTGCCTTCAAGAGAGGCACAGTCAATCAGCT GGTTTTTA-3′. Complementary oligomers were annealed and cloned into pSUPER vectors obtained from OligoEngine, according to their instructions.
PKA assay
LNCaP cells were grown in 60 mm plates to about 60-80% confluence and starved overnight in phenol-red free RPMI medium containing 0.1% (w/v) bovine serum albumin (BSA). The cells were stimulated for 10 min with androgen (including testosterone and dihydrotestosterone that we used interchangeably), or isoproterenol (ISO) that we used as a positive control. Ethanol at 0.1 % (v/v) was used as a control. After stimulation, cells were washed with PBS and lysed in extraction buffer containing 25 mM Tris-HCl, pH 7.4, 0.5 mM EGTA, 10 μM β-mercaptoethanol, aprotenin and leupeptin. PKA activity was determined by measuring the transfer of 32P from [γ32P]ATP (Amersham Pharmacia Biotech) to a specific PKA peptide substrate (Upstate). The reaction mixture contained 0.1 mM ATP (Sigma), 10 μM [γ32P]ATP, 50 μM PKA substrate, 25 mM Tris-HCl, pH 7.4, 0.1 mg/ml BSA, 20 mM MgCl2 and 10 μl of crude cell lysate. The reaction was allowed to proceed for 5 min at 30 °C and was terminated by adding 20 μl of 20% (v/v) trichloroacetic acid. To assay PKA activity, 35 μl of reaction mixture was spotted on cellulose phosphate membranes (Whatman P81) that were pre-washed three times (for 10 min each) using 1% H3PO4 and once with distilled water. The filters were air dried and counted in a scintillation counter (Tri-Carb 2700 TR, Packard).
In each set of experiments, a PKA-positive control (in which about 25 ng of pure PKA (Sigma) was added instead of extract) and two negative control (enzyme control and substrate control) reactions were set up to ascertain specificity of results. The assays were performed in triplicate and repeated at least three times to calculate standard error of the means (SEM).
Cyclic AMP assay
LNCaP cells were seeded equally in 24-well plates and allowed to grow overnight. The cells were starved for 2 - 4 hr followed by stimulation for 10 min at 37 °C with the indicated concentration of ligand. Cells were washed using PBS and lysed using 0.1 N HCl, and the cAMP assay was performed using the correlate-EIA direct cAMP enzyme immunoassay kit (Assay Designs, Inc.) according to the manufacturer’s instructions. The assay was performed in duplicate and repeated three times.
AR reporter assay
Cells were transfected with cDNAs encoding luciferase gene under control of AR (ARR2 or probasin; 1 μg) and SV40-Renilla luciferase (10 ng). At the end of transfection, cells were divided equally into 24-well plates, allowed to attach for 24 hr and the culture medium was replaced with starvation medium (phenol red-free RPMI 1640 containing 0.1% BSA, 1% penicillin-streptomycin, and 10 mM HEPES buffer, pH 7.5). Identical cell populations, in triplicates, were cotreated with rolipram (100 nM) or H89 (10 μM) together with androgen for 24 hr. Luciferase activities in cell lysates were measured using the dual luciferase assay kit (Promega) and were normalized by the Renilla luciferase activities and protein concentrations of the samples (11).
Immunoblotting
Appropriately treated cells were lysed in radioimmunoprecipitation buffer (150 mM NaCl, 50 mM Tris-HCl, pH 8, 1 mM EDTA, 0.25 % (w/v) sodium deoxycholate, 0.1% (v/v) Nonidet P40, 1 mM NaF, 1 mM sodium pyrophosphate, 100 μM Na3VO4, 1 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 0.7 μg/ml pepstatin) and analyzed by SDS-PAGE and Western blotting (10, 12). M2 anti-Flag monoclonal antibody (Sigma) and anti-Gαs polyclonal antibody (Calbiochem) were used at a dilution of 1:5000 and 1:1000, respectively.
Immunostaining
LNCaP cells grown on glass coverslips were treated with H89 (10 μM) and androgen for 24 hr, fixed and processed for immunostaining with 1:200 dilution of anti-prostate specific antigen (PSA) antibody (11, 12). Specific signal was visualized with 3,3′-diaminobenzidine and cells were counter stained with DAPI to visualize nuclei.
Results
Androgen activates PKA
In monkey kidney CV1 (13) and prostate cancer LNCaP (14) cells, the AR can be aberrantly transactivated (i.e. in the absence of exogenous androgens) by the PKA activator forskolin. Further, forced expression of the catalytic subunit of PKA in the CV1 cells leads to activation of AR in the absence of added androgen (13). Importantly, PKA activation appears to be required for classical androgen-mediated activation of nuclear AR in prostate cancer cells (11). Just how PKA becomes activated to control the androgen-mediated activation of AR remains undetermined. We tested whether direct stimulation with androgen (including testosterone (T) and dihydrotestosterone (DHT)) could activate PKA. LNCaP cells were treated with escalating concentrations of androgen for 10 min at 37 °C, and cell extracts were obtained and used for in vitro PKA kinase assay that measures transfer of 32P from [γ32P]ATP to a specific PKA peptide substrate. T induced the concentration-dependent increase in PKA activity with maximal (35% above basal) effects achieved at 1 μM (Fig. 1A), consistent with a receptor-mediated signaling event.
Fig. 1.
Testosterone stimulation activates PKA in LNCaP cells. (A) T induces the dose-dependent increase in PKA activity. LNCaP cells were stimulated with T for 10 min, lysed and cell lysates used for in vitro PKA kinase assay. (B) H89 inhibits T-mediated PKA activation. LNCaP cells were pretreated with H89 for 30 min, stimulated with T (1 μM) for 10 min, and in vitro PKA kinase assay performed, as described. (C) T stimulates the dose-dependent increase in VASP phosphorylation. LNCaP cells were transfected with a cDNA encoding Flag-VASP (1 μg/100 mm dish) and stimulated with T or ISO (10 μM) for 10 min. Total cell lysates were immunoblotted with M2 anti-Flag antibody. P-VASP and VASP indicate, respectively, the phosphorylated and unphosphorylated forms of VASP proteins. (D) H89 inhibits T-mediated phosphorylation of VASP. LNCaP cells expressing Flag-VASP were pretreated with H89 for 30 min prior to stimulation with agonist and P-VASP band intensities were measured and used to calculate the agonist-induced P-VASP fold increase. For data shown in A, B and D, each point represents mean ± SEM of values obtained from three different experiments, and *, p < 0.05 versus cells treated with 0.1 % (v/v) ethanol alone.
We used isoquinoline H89 (15) as a diagnostic tool to assess specificity of the T-mediated activation of PKA. Exposure of the LNCaP cells to H89 inhibited, in a dose-dependent manner, the T-mediated activation of PKA (Fig. 1B), with a total inhibition of PKA activity achieved at a H89 concentration of 10 μM. The VASP protein serves as a substrate for PKA (16), and we used VASP phosphorylation in intact cells as another measure to confirm the androgen-mediated activation of PKA. Consistent with the in vitro PKA kinase results (Fig. 1A, 1B), treatment of LNCaP cells with T promoted VASP phosphorylation (Fig. 1C), and that was dose-dependently inhibited by H89 (Fig. 1D).
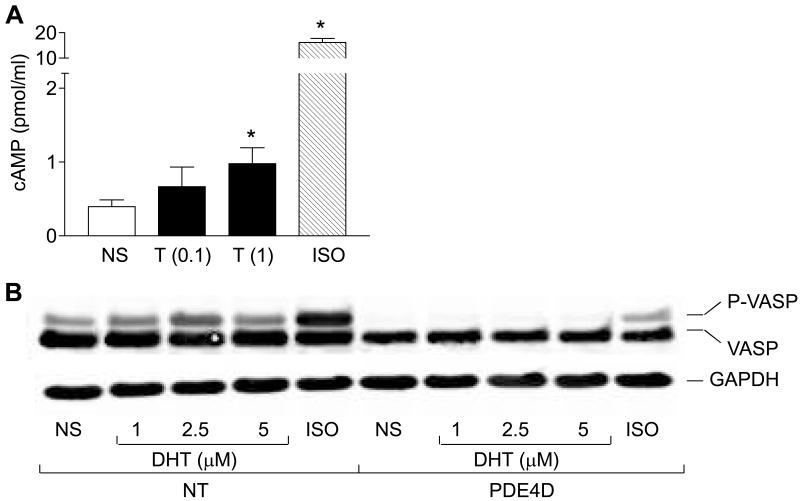
Androgen causes the cAMP accumulation
Although activation of PKA often occurs due to accumulation of intracellular cAMP (17), some studies have reported cAMP-independent mechanisms to activate PKA (18-20). Stimulation with T consistently induced the modest, but significant increases in cAMP formation (Fig. 2A). Stimulation of cells with ISO served as a positive control. To gain more confidence in these results, and to directly implicate cAMP in the androgen-mediated activation of PKA, we assessed effect of expressing the selective cAMP-degrading enzyme phosphodiesterase 4D (PDE4D) (21). Transient expression of PDE4D in LNCaP cells abrogated the ISO- and androgen-mediated VASP phosphorylation (Fig. 2B). These results show that androgen promotes the intracellular accumulation of cAMP that is needed for PKA activation (11).
Fig. 2.
Androgen activation of PKA requires cAMP. (A) T induces accumulation of cAMP. LNCaP cells were stimulated with T (0.1 μM and 1 μM) or ISO (10 μM) for 10 min. Cyclic AMP levels were determined using EIA, as described. Each point represents mean ± SEM of values obtained from three different experiments. *, p < 0.05 versus not stimulated (NS) cells. (B) PDE4D inhibits the DHT-mediated VASP phosphorylation. LNCaP cells were co-transfected with cDNAs encoding VASP (1 μg/100 mm dish) alone, or together with PDE4D (5 μg/100 mm dish), and stimulated with ISO (10 μM) or DHT for 10 min. Cell lysates were obtained and analyzed for VASP phosphorylation by immunoblotting. P-VASP and VASP indicate, respectively, the phosphorylated and unphosphorylated forms of VASP proteins. NT, not transfected with PDE4D; NS, not stimulated.
Gαs mediates PKA activation by androgen
The α subunit of heterotrimeric Gs proteins (Gαs) is the best studied receptor-induced regulator of cAMP-generating enzymes, adenylyl cyclases. However, the adenylyl cyclases may also be activated by receptor-independent events, such as the direct binding to forskolin (22). To determine whether androgen-induced cAMP accumulation occurred in a Gαs-dependent manner, Gαs-specific siRNAs were designed and used to knock-down expression of the Gαs proteins. LNCaP cells were transfected with Flag-VASP and plasmids expressing Gαs siRNAs (1625 and 2051) that were synthesized based on the sequence of the long and short isoforms of Gαs mRNAs. Forced expression of the Gαs siRNAs reduced expression of Gαs protein in LNCaP cells by more than 50%, but showed no measurable effect on the expression of clathrin heavy chain protein (Fig. 3A). Importantly, expression of the Gαs siRNAs inhibited the VASP phosphorylation induced by both ISO (Fig. 3B) and androgen (Fig. 3C), as compared to cells that were transfected with an unrelated siRNA. Contribution of Gαs to androgen-induced PKA activation was confirmed through the forced expression of Gαs signaling inhibitor, RGS domain of Axin (Fig. 3D). Together, these results clearly indicate involvement of Gαs protein in androgen-mediated PKA activation.
Fig. 3.
Gαs mediates androgen-induced PKA activation. (A) Reduction of Gαs expression by siRNA. LNCaP cells were transfected with a cDNA encoding Gαs 1625, 2051 or control siRNAs (EV), and the expression of Gαs protein was determined by immunoblotting. The filter was stripped of immunoglobulin and re-blotted with anti-clathrin antibodies to demonstrate specificity of the Gαs siRNA and the equal loading of proteins in each lane. Expression of Gαs siRNA attenuates the ISO (B) and androgen (C) mediated VASP phosphorylation. LNCaP cells were co-transfected with cDNAs encoding Flag-VASP (1 μg/100 mm dish) and empty vector, or together with Gαs siRNA 1625 (10 μg/100 mm dish) or Gαs siRNA 2051 (10μg/100 mm dish). Cells were stimulated with 10 μM ISO (B) or 1 μM T (C) and VASP phosphorylation determined by immunoblotting, as described. To quantitate agonist-induced phosphorylation, densities of the phosphorylated and unphosphorylated VASP bands were determined using image analysis tools from AlphaImager™. Data are presented as percent increase in VASP phosphorylation above control, and 100 represents basal VASP phosphorylation. Each point represents mean ± SEM of values obtained from three different experiments. *, p < 0.05 versus values obtained from empty vector transfected cells. (D) Forced expression of RGS domain of Axin attenuates androgen-induced PKA activation. LNCaP cells were co-transfected with cDNAs encoding Flag-VASP (1 μg/100 mm dish) and empty vector, or together with RGS domain of Axin (5 μg/100 mm dish). Cells were stimulated with 1 μM T and VASP phosphorylation determined by immunoblotting, as described. NT, not transfected with Flag-VASP; NS, not stimulated.
Nuclear AR is not required for androgen-mediated PKA activation
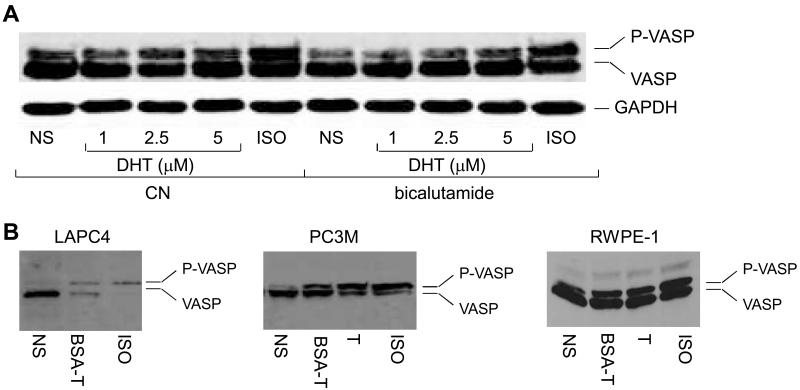
Nuclear AR has been shown to transduce some, but not all, of the non-genomic actions engendered by androgens (8, 9). We determined the androgen-induced VASP phosphorylation using LNCaP cells pre-treated with nuclear AR antagonist bicalutamide. No consistent change in VASP phosphorylation status was observed in the presence of 10 μM bicalutamide (Fig. 4A), suggesting nuclear AR-independent activation of PKA. However, the similar treatment of LNCaP cells with bicalutamide (10 μM) abrogated the androgen-regulated transcriptional activity of nuclear AR as determined by expression of PSA protein (data not shown). To strengthen the argument that androgen activates PKA in a nuclear AR-independent manner, we made use of IC21 macrophages that do not express nuclear AR gene (9). The IC21 cells were starved, stimulated with androgen for 10 min at 37 °C, and PKA activity determined. Treatment of the IC21 cells with androgen, like ISO, promoted the PKA activation (data not shown). Together, these results show that androgen-induced PKA activation does not require nuclear AR.
Fig. 4.
Androgen activates PKA by a nuclear AR-independent mechanism. (A) Bicalutamide does not affect PKA activation by androgen. LNCaP cells expressing Flag-VASP were pretreated overnight with bicalutamide (10 μM), followed by stimulation with DHT or ISO (10 μM) for 10 min. VASP phosphorylation was determined by immunoblotting, as described. (B) Androgen stimulation promotes VASP phosphorylation in multiple prostate cells. Androgen-dependent LAPC4, androgen-independent PC3M and normal epithelial RWPE1 prostate cells were transfected with a cDNA encoding Flag-VASP and stimulated with BSA-T (100 nM), T (1 μM), or ISO (10 μM) for 10 min. VASP phosphorylation was determined by immunoblotting, as described.
Androgen activates PKA in prostate epithelial cells
To ascertain generality of the androgen-mediated activation of PKA, we determined effect of androgen stimulation on VASP phosphorylation using two additional prostate cancer cell lines (androgen-dependent LAPC4 and androgen-independent PC3M) and one normal prostate epithelial (RWPE1) cell line. Similar to the situation in LNCaP cells, stimulation with androgen, including BSA-conjugated testosterone (BSA-T) and free testosterone, like ISO, induced the PKA activation in these cells as well (Fig. 4B). Stimulation of the prostate cells with estradiol or BSA-conjugated estradiol did not impact the PKA activity (data not shown). Significantly, results using the PC3M cells that do not express endogenous nuclear AR gene reinforce the idea that androgen stimulation activates PKA independently of nuclear AR.
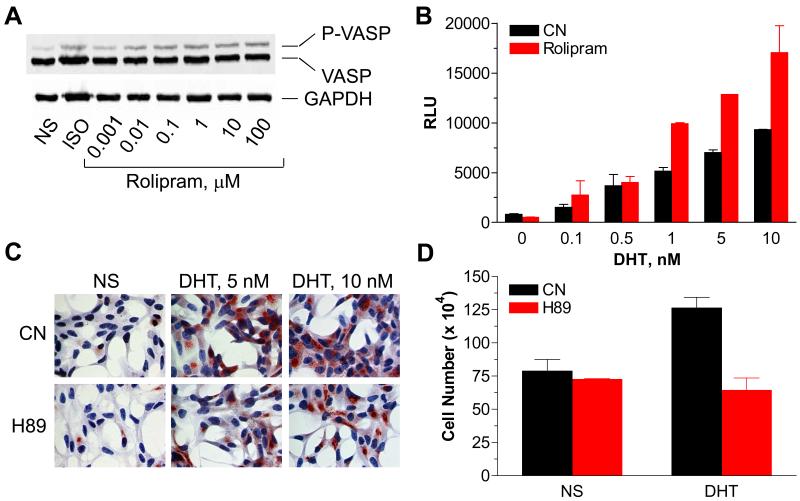
Androgen stimulation of LNCaP cells promotes the cAMP accumulation (Fig. 2A) and PKA activation (Fig. 1). Androgen stimulation also promotes the LNCaP cell proliferation via a signaling cascade that utilizes nuclear AR (23). We therefore asked whether cAMP and PKA contribute to the nuclear AR activation and subsequent cell proliferation. Inhibition of PDE activity with rolipram increased levels of intracellular cAMP (data not shown) and PKA activity (Fig. 5A), and potentiated the androgen induced nuclear AR activation (Fig. 5B). The mechanism of rolipram-mediated AR activation remains unclear, but may involve PKA. Accordingly, treatment of cells with H89 abolished the androgen-induced nuclear AR activation as determined with expression of PSA (Fig. 5C), confirming previous observations (11, 13, 14). To test whether PKA is involved in the androgen-induced LNCaP cell proliferation, cells were treated, or not, with H89 followed by stimulation with vehicle alone, or together with androgen. Figure 5D illustrates that treatment with H89 abolished the androgen-induced LNCaP cell proliferation. Together, these results are consistent with the idea that androgen stimulation promotes the PKA activation that, in turn, is required for nuclear AR function to regulate prostate cell proliferation.
Fig. 5.
Androgen stimulation promotes the PKA-mediated LNCaP cell proliferation. (A) Effect of rolipram on VASP phosphorylation. LNCaP cells were transfected with a cDNA encoding Flag-VASP and treated with ISO (1 μM) or rolipram for 10 min. VASP phosphorylation was determined by immunoblotting, as described. (B) Rolipram potentiates androgen-induced AR-responsive promoter activity. LNCaP cells were co-transfected with firefly and Renilla luciferase genes driven by an artificial promoter that contains two copies of the rat probasin ARE elements and SV40 promoter, respectively. Cells were incubated in starvation medium containing, or not, rolipram (100 nM) in the presence, or absence, of androgen for 24 hr. Each point represents the mean ± SEM of the normalized luciferase activities obtained from three independent experiments performed in duplicate. (C) H89 inhibits the androgen-induced PSA expression. LNCaP cells were treated with H89 (10μM) and androgen for 24 hr. PSA expression was determined by staining cells with anti-PSA antibody (1:200), and nuclei were visualized with DAPI. (D) H89 attenuates the androgen-mediated LNCaP cell growth. Cells were cultured in serum-free medium for two days, and treated, or not, with androgen in the presence or absence of H89 for additional two days. Cells were harvested, and mixed with 0.1% trypan blue stain. Cells excluding the dye were counted under light microscopy. Each point represents the mean ± SEM of values obtained from three independent experiments performed in duplicate. CN, control sample not treated with drug; NS, not stimulated.
Discussion
Androgens control development of the normal prostate, and play a major role in the initiation and progression of prostate cancer via activation of cognate androgen receptors. The main venue for androgen effects on target cells comprises nuclear AR, which acts as a ligand-controlled transcription factor to regulate expression of specific genes involved in cell growth and differentiation. The major finding of this study is that in addition to eliciting genomic responses, androgen stimulation initiates rapid, non-genomic signals to activate PKA that is needed for the proper functioning of nuclear AR as a transcriptional regulator (11-13). As depicted schematically in Figure 6, the signaling intermediates that connect androgen and PKA include heterotrimeric Gαs protein, adenylyl cyclase, cAMP and PKA.
Fig. 6.
A model for androgen-mediated activation of PKA. In the classical paradigm, T transverses the plasma membrane into the cytosol and is converted to DHT that, in turn, binds to and activates nuclear AR (pathway 1). T may transverse the plasma membrane and directly bind to adenylyl cyclase (pathway 2), in a manner similar to forskolin. Alternatively, T may activate PKA by binding to plasma membrane- or cytosolic vesicle-expressed AR that couples to Gαs (pathway 3), in a manner similar to the β2 adrenergic receptor. AC, adenylyl cyclase; AR, androgen receptor; ARBP, AR binding protein; FSK, forskolin; ISO, isoproterenol; PKA, protein kinase A; T, testosterone.
The results offer three possibilities to account for the androgen-mediated activation of PKA (Fig. 6). First, the non-genomic responses of androgens may be mediated through nuclear AR (6, 8), and previous studies have shown that the nuclear AR can be found in lipid rafts that are specialized domains of the plasma membrane (24). We can exclude this possibility based on the observations that androgen activates PKA in cells pretreated with the nuclear AR antagonist bicalutamide and, more to the point, androgen activates PKA in IC21 macrophages and PC3M cells that do not express nuclear AR gene. Second, androgen directly activates adenylyl cyclases, in a manner similar to forskolin (Fig. 6, pathway 2). We tested this possibility using purified membranes expressing various reconstituted adenylyl cyclases, including isoforms 1, 2, 4, and 5 (a generous gift from C. W. Dessauer, UT) and observed that the stimulation with androgen, unlike forskolin, does not increase levels of cAMP (data not shown). These observations argue against the direct activation of adenylyl cyclase by androgen. Third, androgen may cause PKA activation via the binding to a Gs protein-coupled receptor (GPCR), in a manner similar to the β2 adrenergic receptor (Fig. 6, pathway 3). Indeed, the best studied physiologically-relevant cellular mechanism to activate PKA involves a signal transduction cascade comprised of a GPCR → Gαs → adenylyl cyclase → cAMP → PKA, and our data show that the reduction of Gαs expression with siRNA, or inhibition of Gαs signaling with the RGS domain of Axin attenuates androgen-induced PKA activation. Further, inhibition of adenylyl cyclase activation with 2′-5′-dideoxyadenosine (a generous gift from R. Johnson, SUNY) as well as expression of the cAMP-degrading enzyme PDE4D abrogated the androgen-induced PKA activation. Together, these results establish that androgen generates the Gαs/adenylyl cyclase/cAMP-dependent signalsome to activate PKA.
The physiological concentrations of testosterone vary widely in body tissues. Normal prostate tissue contains 1.2 ng/g DHT and cancer tissue contains 4.8 ng/g (25, 26). Prostate fluid obtained from patients with prostate hypertrophy contained 54 ng/ml DHT and 106 ng/g testosterone (27). Recent studies show that testicular androgen concentration in humans is 1236 ± 86 nM as compared to 11.7 ± 0.7 nM in serum (28). More than 70% of this androgen is biologically active and essential for spermatogenesis (6). One of the pathways that is triggered by this “high” testosterone concentration in sertoli cells induces the non-genomic activation of ERK and CREB (6). Hence, in our study the androgen concentrations used fall within the physiologic concentration range present in testes.
Recent studies have shown that non-genomic action of estrogen is mediated through the GPCR GPR30 that is chiefly expressed intracellularly on the endoplasmic reticulum membrane (29, 30). An intracellular GPCR located on an organelle membrane can be functional only if the cognate ligand is capable of transversing the plasma membrane. Stimulation with membrane-impermeable BSA-T induced the Gαs-dependent PKA activation (Fig. 4B), suggesting existence of a plasma membrane-anchored AR (Fig. 6). However, careful analysis of the BSA-T revealed that it contained free T. Hence, two possibilities exist to account for subcellular localization of the Gαs-coupled AR (Fig. 6): the receptor may be expressed on the plasma membrane like the progestin receptor (31), or in vesicles in the cytosol like the estrogen receptor (29, 30).
The androgen-mediated activation of PKA is not cell type specific. We can detect the PKA activation in androgen-dependent (LNCaP and LAPC4) and -independent (PC3M) prostate cancer cells, as well as in non-cancerous prostate epithelial (RWPE1) cells, establishing generality of the pathway. Notably, androgen-independent prostate cancer DU145 cells did not show PKA activation following androgen treatment. Previous studies have shown that DHT induced cAMP accumulation and PKA activation by binding to sex hormone binding globulin (SHBG) and acting through the SHBG receptor (32). However, the androgen-induced PKA activation that we observed appears to be independent of SHBG. First, considerable amounts of exogenous SHBG (50 nM) were needed to initiate the DHT/SHBG-induced activation of PKA (32). In our case, experiments were performed in serum free medium in the absence of exogenously added SHBG, and similar results were obtained when experiments were carried out in medium containing charcoal-stripped serum. Moreover, the deprivation of cells from serum for up to three days had no effect on the androgen-induced activation of PKA. Second, The DHT/SHBG-mediated activation of SHBG receptor to activate PKA was shown to occur only in cancer, but not benign prostate cells (33). We showed that stimulation of benign prostate RWPE1 epithelial cells (and IC21 macrophages) with androgen induces the PKA activation.
Based on our results, and taking into consideration previous findings demonstrating requirement of PKA to propagate nuclear AR function (11-13), we propose presence of a Gαs-coupled androgen binding sites that transduce rapid, non-genomic signals to activate PKA. We further suggest existence of parallel androgen effector pathways; a non-genomic pathway that activates PKA (Fig. 6, pathway 3) and a genomic pathway that is mediated through the direct binding of androgen to nuclear AR (Fig. 6, pathway 1). Activation of PKA is required for the androgen-mediated activation of nuclear AR genomic function. Identification and further characterization of the G protein-coupled AR will enhance our knowledge of non-genomic signals by androgens, and may serve as a drug target for the blockade of (both genomic and non-genomic) pathophysiologic signaling pathways triggered by androgens.
Acknowledgements
We thank Drs R.J. Lefkowitz, J.S. Gutkind, C.L. Sawyers, R. Johnson and C.W. Dessauer for sharing reagents. This work was supported in part by grants from the NIH (AG17952, DK60917). Y.D. is a Georgia Cancer Coalition Distinguished Cancer Scholar.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate (Reprinted from Cancer Res, vol 1, pg 293-297, 1941) J Urol. 2002;167:948–51. [PubMed] [Google Scholar]
- 2.Pirtskhalaishvili G, Hrebinko RL, Nelson JB. The treatment of prostate cancer -An overview of current options. Cancer Pract. 2001;9:295–306. doi: 10.1046/j.1523-5394.2001.96009.x. [DOI] [PubMed] [Google Scholar]
- 3.Gobinet J, Poujol N, Sultan C. Molecular action of androgens. Mol Cell Endocrinol. 2002;198:15–24. doi: 10.1016/s0303-7207(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 4.Norman AW, Mizwicki MT, Norman DPG. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 5.Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–97. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 6.Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:10919–24. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang HY, Cho CL, Huang KL, et al. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J Bone Miner Res. 2004;19:1181–90. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- 8.Unni E, Sun SH, Nan BC, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–68. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 9.Benten WPM, Lieberherr M, Stamm O, Wrehlke C, Guo ZY, Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell. 1999;10:3113–23. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migliaccio A, Varricchio L, De Falco A, et al. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007 doi: 10.1038/sj.onc.1210487. E-Pub. [DOI] [PubMed] [Google Scholar]
- 11.Kasbohm EA, Guo R, Yowell CW, et al. Androgen receptor activation by G(s) signaling in prostate cancer cells. J Bioll Chem. 2005;280:11583–9. doi: 10.1074/jbc.M414423200. [DOI] [PubMed] [Google Scholar]
- 12.Guo R, Kasbohm EA, Arora P, et al. Expression and function of lysophosphatidic acid LPA(1) receptor in prostate cancer cells. Endocrinol. 2006;147:4883–92. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- 13.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–7. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 14.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–83. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 15.Chijiwa T, Mishima A, Hagiwara M, et al. Inhibition of Forskolin-Induced Neurite Outgrowth and Protein-Phosphorylation by a Newly Synthesized Selective Inhibitor of Cyclic Amp-Dependent Protein-Kinase, N-[2-(P-Bromocinnamylamino)Ethyl]-5-Isoquinolinesulfonamide (H-89), of Pc12d Pheochromocytoma Cells. J Biol Chem. 1990;265:5267–72. [PubMed] [Google Scholar]
- 16.Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–39. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 17.Krebs EG. Role of the Cyclic-Amp Dependent Protein-Kinase in Signal Transduction. JAMA. 1989;262:1815–8. doi: 10.1001/jama.262.13.1815. [DOI] [PubMed] [Google Scholar]
- 18.Zhang LZ, Duan CJ, Binkley C, et al. A transforming growth factor beta-induced Smad3 Smad4 complex directly activates protein kinase A. Mol Cell Biol. 2004;24:2169–80. doi: 10.1128/MCB.24.5.2169-2180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu SH, Chiang WC, Shih HM, Wu KJ. Stimulation of c-Rel transcriptional activity by PKA catalytic subunit beta. J Mol Med. 2004;82:621–8. doi: 10.1007/s00109-004-0559-7. [DOI] [PubMed] [Google Scholar]
- 20.Vinciguerra M, Deschenes G, Hasler U, et al. Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell. 2003;14:2677–88. doi: 10.1091/mbc.E02-11-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin SLC, Richard FJ, Kuo WP, D’Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci USA. 1999;96:11998–2003. doi: 10.1073/pnas.96.21.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awad JA, Johnson RA, Jakobs KH, Schultz G. Interactions of Forskolin and Adenylate-Cyclase - Effects on Substrate Kinetics and Protection against Inactivation by Heat and N-Ethylmaleimide. J Biol Chem. 1983;258:2960–5. [PubMed] [Google Scholar]
- 23.Kokontis JM, Hay N, Liao SS. Progression of LNCaP prostate tumor cells during androgen deprivation: Hormone-independent growth, repression of proliferation by androgen, and role for p27(Kip1) in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12:941–53. doi: 10.1210/mend.12.7.0136. [DOI] [PubMed] [Google Scholar]
- 24.Lu ML, Schneider MC, Zheng YX, Zhang XB, Richie JP. Caveolin-1 interacts with androgen receptor - A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–51. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 25.Hammond GL. Endogenous steroid levels in the human prostate from birth to old age: a comparison of normal and diseased tissues. J Endocrinol. 1978;78:7–19. doi: 10.1677/joe.0.0780007. [DOI] [PubMed] [Google Scholar]
- 26.Geller J, Candari CD. Comparison of dihydrotestosterone levels in prostatic cancer metastases and primary prostate cancer. Prostate. 1989;15:171–5. doi: 10.1002/pros.2990150210. [DOI] [PubMed] [Google Scholar]
- 27.Habib FK, Goodman CM, Buck C. Androgen concentrations in expressed prostatic secretions: no correlation with tissue levels. Urol Res. 1992;20:281–4. doi: 10.1007/BF00300259. [DOI] [PubMed] [Google Scholar]
- 28.Jarow JP, Wright WW, Brown TR, Yan XH, Zirkin BR. Bioactivity of androgens within the testes and serum of normal men. J Androl. 2005;26:343–8. doi: 10.2164/jandrol.04100. [DOI] [PubMed] [Google Scholar]
- 29.Filardo EJ, Quinn JA, Frackelton AR, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Molecular Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 30.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Rice CD, Pang YF, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–6. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakhla AM, Khan MS, Rosner W. Biologically-Active Steroids Activate Receptor-Bound Human Sex Hormone-Binding Globulin to Cause Lncap Cells to Accumulate Adenosine-3′,5′-Monophosphate. J Clin Endocrinol Metab. 1990;71:398–404. doi: 10.1210/jcem-71-2-398. [DOI] [PubMed] [Google Scholar]
- 33.Nakhla AM, Khan MS, Romas NP, Rosner W. Estradiol Causes the Rapid Accumulation of Camp in Human Prostate. Proc Natl Acad Sci USA. 1994;91:5402–5. doi: 10.1073/pnas.91.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]