Abstract
Administration of the neuropeptides NPW and NPB in rodents has been shown to influence the activity of a variety of autonomic and neuroendocrine systems. The paraventricular nucleus is a major autonomic and neuroendocrine integration site in the hypothalamus and neurons within this nucleus express the receptor for these ligands, neuropeptide B/W receptor 1 (NPBWR1). Therefore we used whole cell patch clamp recordings coupled with single cell RT-PCR to examine the effects of neuropeptide W-23 (NPW-23) on the excitability of identified paraventricular nucleus neurons. Oxytocin, vasopressin and thyrotropin releasing hormone neurons were all found to be responsive to 10 nM NPW-23, although both depolarizing and hyperpolarizing effects were observed in each of these cell groups. In contrast corticotropin releasing hormone cells were unaffected. Further subdivision of chemically phenotyped cell groups into magnocellular, neuroendocrine or pre-autonomic neurons, using their electrophysiological fingerprints, revealed that neurons projecting to medullary and spinal targets were predominantly inhibited by NPW-23 while those that projected to median eminence or neural lobe showed nearly equivalent numbers of depolarizing and hyperpolarizing cells. The demonstration of particular phenotypic populations of paraventricular nucleus neurons showing NPW-induced effects on excitability reinforces the importance of the NPB/NPW neuropeptide system as a regulator of autonomic function.
Keywords: Single cell RT-PCR, hypothalamus, oxytocin, vasopressin, thyrotropin releasing hormone, corticotropin releasing hormone
Introduction
Neuropeptide B (NPB) and Neuropeptide W (NPW) are endogenous ligands for the Neuropeptide B/W receptor 1 (NPBWR1, formerly called GPR7) in rodents (1,2). This neuropeptide system influences a variety of autonomic and neuroendocrine functions, including activation of the hypothalamic-pituitary-adrenal stress axis, increased feeding activity, increased heart rate and blood pressure and increases in plasma prolactin levels (3–6). Localization of NPBWR1 to the paraventricular nucleus (PVN) and the findings of cell bodies expressing mRNA for prepro-NPB and NPB immunopositive fibers within the PVN make this nucleus a potentially important site where many of these activities arise (7–9). Indeed, direct injection of NPW-23 into the PVN initiated long term increases in feeding, and changes in the membrane potential of PVN neurons in brain slices were measured, during whole cell current clamp recording, following exposure to NPW-23 (10,11).
The PVN is a key integration site within the hypothalamus receiving afferent inputs from a number of brain regions providing information on the autonomic status and arousal state of the animal. These inputs are integrated by the individual PVN neurons whose output is directed to one of two routes. Magnocellular (MNC) and parvocellular neuroendocrine cells (NE) send their axons to either the posterior pituitary or median eminence where neuropeptides are released into the blood circulation (12). Alternatively, parvocellular preautonomic neurons (PA) send axons to the medulla and spinal cord where they directly communicate with their targets (13,14). The neurons that make up these three groups of cells each release one or more neuropeptides which ultimately defines what autonomic response will occur due to the combined, integrated signals received in the PVN.
Because NPB and NPW influence a variety of autonomic and neuroendocrine systems it would be expected that several neuronal cell types within the PVN would be sensitive to NPB and NPW. Therefore, using a combination of electrophysiological and molecular techniques we have obtained current clamp recordings from PVN neurons in rat brain slices, and have correlated the responsiveness of individual neurons to NPW-23 with electrophysiological [magnocellular (MNC), parvocellular neuroendocrine (NE) or preautonomic (PA)], and/or neuropeptide [vasopressin (VP), oxytocin (OT), corticotropin releasing hormone (CRH) and thyrotropin releasing hormone (TRH)] phenotype.
Materials and Methods
Slice preparation
All animal procedures conformed to the standards of the Canadian Council on Animal Care and were approved by the Queen’s University Animal Care Committee. Male Sprague Dawley rats (Charles River, Quebec, Canada) aged postnatal day 21 to 27 (approximately 50–100g) were used in the preparation of hypothalamic slices. Rats were decapitated and the brain dissected and placed into ice cold slicing solution, consisting of (in mM): 87 NaCl, 2.5 KCl, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 25 glucose and 75 sucrose, bubbled with 95% O2/5% CO2. A tissue block containing the hypothalamus was obtained and 300 µm coronal slices cut using vibratome. Slices containing the PVN were then incubated for at least 1 hour, before recordings commenced, in artificial cerebrospinal fluid (ACSF) composed of (in mM): 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 2 MgCl2 1.25 NaH2PO4 and 10 glucose, bubbled with 95% O2/5% CO2.
Electrophysiology
Slices were placed in a chamber that was continuously perfused with carbogenated ACSF at room temperature where PVN neurons were visualized with an infrared differential interference contrast system (Nikon, Japan). Whole cell current clamp recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale CA) and sampled using a Micro1401 interface and Spike2 software (Cambridge Electronic Design, Cambridge UK) for offline analysis. Electrodes were filled with an intracellular solution that was prepared under RNase-free conditions and contained (in mM): 125 potassium gluconate, 2 MgCl2, 5.5 EGTA, 10 KCl, 0.1 CaCl2, 2 NaATP and 10 Hepes (pH 7.2–7.3 with KOH). When filled with this solution electrodes had resistances of 3 to 7 M´Ω. A liquid junction potential calculated to be approximately 14 mV has been subtracted from all membrane potentials and command potentials reported. Electrophysiological fingerprints (MNC, NE, PA) were obtained using a current stepping protocol where the neuronal membrane potential was initially stepped to a potential below −90 mV followed by a step to a series of more positive membrane potentials. The resulting voltage responses were then examined to determine if individual neurons expressed a large A-current (presumptive MNC), a low threshold spike (presumptive PA) or neither of these features (presumptive NE). Cells were classified as responding to NPW-23 if there was a change in membrane potential that exceeded 2X the standard deviation of the average baseline membrane potential measured over a 50s period immediately prior to application of the peptide. For neurons with a high rate of spontaneous action potential activity recordings were smoothed using the Spike2 analysis software to eliminate action potentials and therefore avoid the prospect of false negatives due to the activity.
Single cell RT-PCR
Following the completion of single cell recordings, gentle suction was applied to the electrode interior and, under visual control, the cytoplasmic contents were collected. The electrode was then carefully withdrawn from the cell until the electrode detached, forming an outside-out patch. The electrode tip was subsequently broken and its contents aspirated into a 0.5 ml microcentrifuge tube. Prior to the reverse transcriptase reaction cytoplasm was treated for 30 min at 37°C with DNase (Fermentas, Burlington, Ont., Canada). Immediately following inactivation of the DNase with high temperature (65°C) and Mg2+ chelation, the reverse transcriptase reaction was initiated with addition of the following reagenets (approximate final concentration): dithiothreitol (26 mM), dNTPs (3 mM), random heximer primers (3 µM), MgCl2 (4 mM), RNase inhibitor (20 U) and superscript II reverse transcriptase (100 U) (all from Invitrogen, Burlington, Ontario, Canada). The reverse transcriptase reaction ran overnight at 37°C, after which the cDNA was stored at −80°C until the start of the multiplex reaction.
A multiplex PCR approach was employed to amplify the cDNA obtained from the reverse transcriptase reaction using primers specific for key neuropeptides associated with PVN output (Table 1). In addition to a primer for the housekeeping gene GADPH was included as a positive control. The first step was a multiplex reaction containing primers (outside) for all the genes of interest along with cDNA from the single cell. The second reaction was a nested PCR reaction using a single set of primers (nested) for each gene of interest. The initial multiplex reaction was performed in a 100 µl volume using the reagents provided with the Qiagen Multiplex kit (Qiagen, Mississauga, Ontario, Canada) and 0.2 µM of each primer. Each reaction was denatured at 95°C for 15 min then cycled 20 times through a temperature protocol consisting of 30 s at 94°C, 90 s at 60°C and 90 s at 72°C. The final product in early experiments was diluted 1:1000 and used as the template for the next round of PCR. In later experiments the first round product was used undiluted as template for the second round of PCR. The second round of PCR consisted of individual 50 µl reactions for each of the genes of interest. Reactions were done again using the reagents contained in the Qiagen Multiplex kit and 0.2 µM of each primer. The reaction mixture was cycled 35 times through the same temperature protocol as indicated above, afterwards the PCR products were run out on a 2% agarose gel containing ethidium bromide and sequenced to confirm their identity (Robarts Institute, London, Ontario, Canada).
Table 1.
Inside and outside primer pairs used in multiplex PCR
| Primer | Outside | Inside |
|---|---|---|
| GAPDH | Sense: 51gatggtgaaggtcggtgtg31 | 51taccagggctgccttctct31 |
| Antisense: 51gggctaagcagttggtggt31 | 51ctcgtggttcacacccatc31 | |
| Vasopressin | Sense: 51cctcacctctgcctgctactt31 | 51ccagaactgcccaagagg31 |
| Antisense: 51gcttccgcaaggcttctg31 | 51gcttccgcaaggcttctg31 | |
| Oxytocin | Sense: 51ctgccccagtctcgcttg31 | 51ctgccccagtctcgcttg31 |
| Antisense: 51cctccgcttccgcaaggcttc31 | 51gcgagggcaggtagttctcc31 | |
| TRH | Sense: 51agaggggagacttgggagaa31 | 51attcatgggcagatgaggag31 |
| Antisense: 51ctttgcttcaccagggtctc31 | 51ggcgtttctcaggcattaag31 | |
| CRH | Sense: 51ggggaaaggcaaagaaaagg31 | 51ggagaagagaaaggagaagag31 |
| Antisense: 51gacagagccaccagcagcat31 | 51ggacaccagcagccgcag31 | |
Chemicals and drugs
Peptide and drug application was via the bath perfusion. Owing to the similarity of binding characteristics between NPB and the various forms of NPW at the NPBWR1 receptor, we solely utilized NPW-23 in our experiments. NPW-23 was applied only after a stable baseline had been established lasting at least 100 s. Perfusion rate was approximately 2 ml/min. Salts used in the preparation of the intracellular solution and normal and high sucrose ACSF were obtained from Sigma (Oakville, Ontario, Canada). NPW-23, obtained from Phoenix Pharmaceuticals (Burlingame, CA), was reconstituted in distilled H2O at a concentration of 10 µM, aliquoted and stored at −80°C.
Results
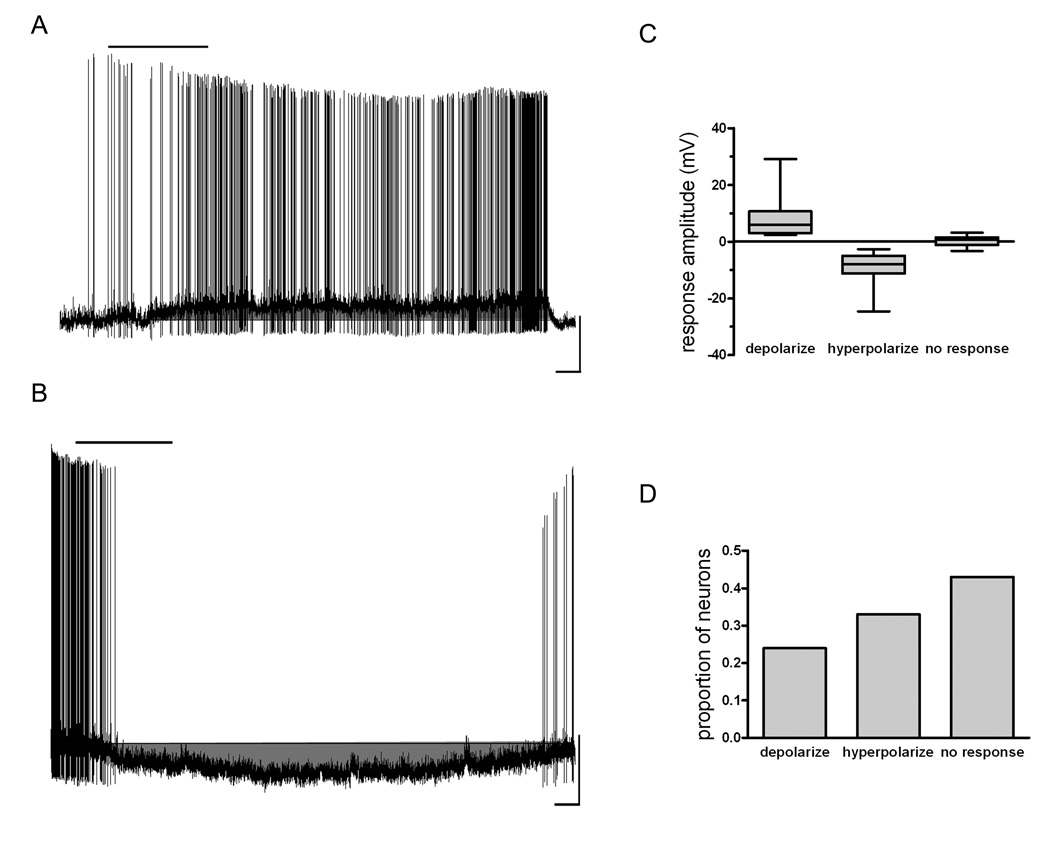
To assess the effects of NPBWR1 activation on the excitability of PVN neurons whole cell current clamp recordings were performed on 115 neurons visualized in brain slices. Bath application of 10 nM NPW-23, a concentration found previously to be at the top of the dose-response curve for this peptide for the depolarization of PVN neurons by NPW-23 (11) and the effects on the excitability of hypothalamic arcuate nucleus neurons (unpublished observations) and well above the published affinity constant (0.44 nM) for [125I]NPW (15), resulted in changes in membrane potential that met or exceeded our criteria for being regarded a response in 57% of neurons tested, with the predominant effects observed in these responsive neurons being hyperpolarization (38/115 neurons, mean 8.6 ± 0.8mV) (Figure 1). We also recorded from a smaller population of PVN neurons which displayed depolarizations (28/115 neurons, mean 7.8 ± 1.3mV), while the remaining cells tested were unaffected by NPW-23. Responses to NPW-23 usually occurred within 30 s of the peptide reaching the slice, lasted for periods of 5 to 25 minutes following return to ACSF perfusion, and were normally accompanied by increases (depolarized cells), or decreases (hyperpolarized cells) in spike frequency (Figure 1,Figure 2,Figure 3,Figure 4,Figure 5).
Figure 1.
NPW has both depolarizing and hyperpolarizing effects on individual PVN neurons. A) Trace from a current clamp recording demonstrating NPW-induced depolarization of a PVN neuron with accompanying increase in action potential firing frequency. 10 nM NPW was applied for 200s, as indicated by the bar over the trace. Scale: 20mV / 50s. B) Trace from a current clamp recording from another PVN neuron demonstrating NPW-induced hyperpolarization and accompanying decrease in action potential firing frequency. 10 nM NPW was applied for 200s, as indicated by the bar over the trace. Scale: 20mV / 50s. C) Box and whiskers plot showing the distribution of response amplitudes for depolarizations and hyperpolarizations induced by 10nM NPW as well as the displacement in membrane potential seen in neurons that did not show a criteria-meeting response. Box boundaries represent the 25th and 75th percentile, while the line represents the median value. D) Histogram showing the proportion of neurons depolarizing, hyperpolarizing and showing no response to 10nM NPW.
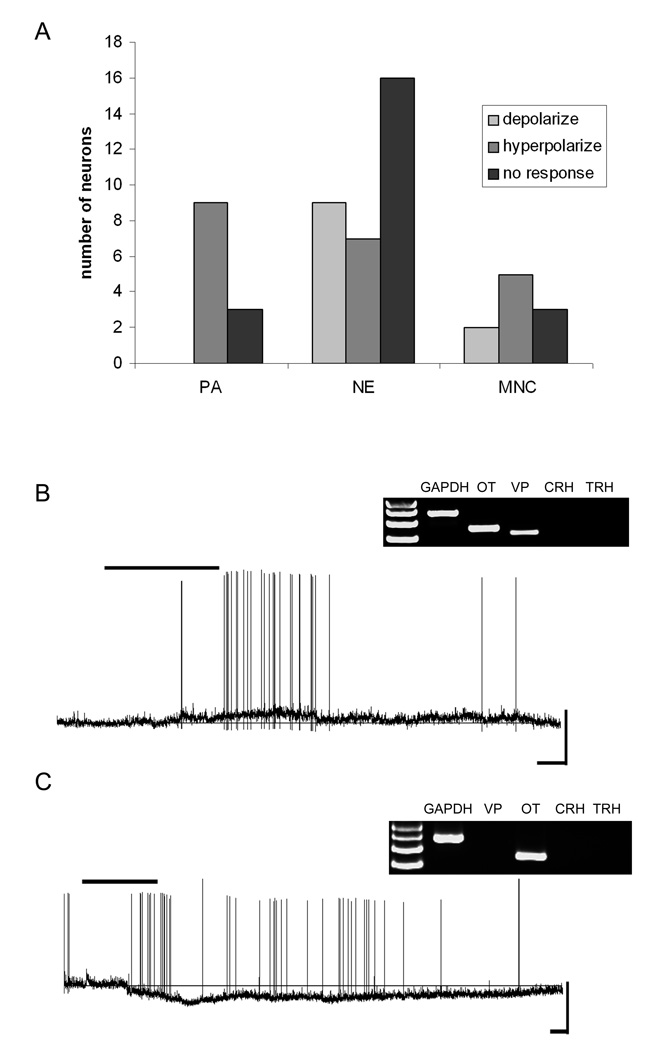
Figure 2.
Oxytocin mRNA-expressing neurons and their responses to NPW. A) Frequency distribution histogram showing the number of PA, NE and MNC OT neurons and their responses to 10nM NPW. B) Current-clamp trace from an oxytocin mRNAexpressing NE cell showing 10nM NPW-induced depolarization (Scale 40mV/50s). Inset, the agarose gel image showing the neuropeptide phenotype of this cell. C) Current-clamp trace from an oxytocin mRNA-expressing PA cell showing 10nM-induced hyperpolarization (scale 50mV/50s). Bar over traces indicates duration of NPW application. Inset, the agarose gel image showing the neuropeptide phenotype of this cell.
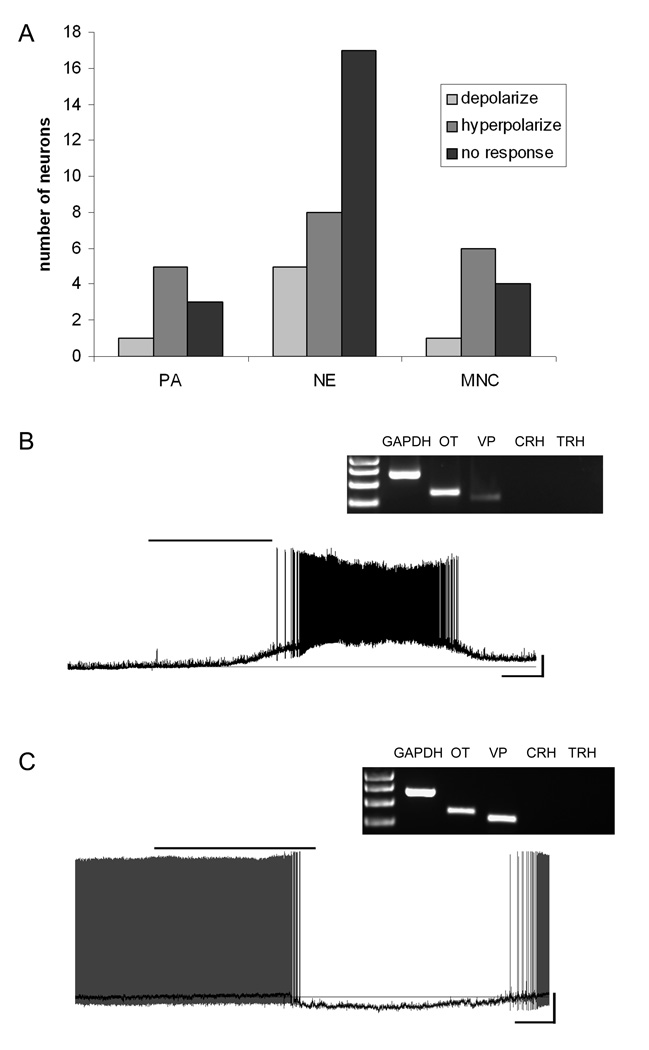
Figure 3.
Vasopressin mRNA-expressing neurons and their responses to NPW. A) Frequency distribution histogram showing the number of PA, NE and MNC VP neurons and how they responded to 10nM NPW. B) Current-clamp trace from a NE cell expressing mRNA for VP showing depolarization induced by 10nM NPW (scale 20mV/50s). Inset, the agarose gel image showing the neuropeptide phenotype of this cell. C) Current-clamp trace from a MNC neuron showing hyperpolarization induced by 10nM NPW. The dark trace is the smoothed image superimposed upon the action potential containing trace to emphasize the change in membrane potential (scale 20mV/50s). Inset, the agarose gel image showing the neuropeptide phenotype of this cell.
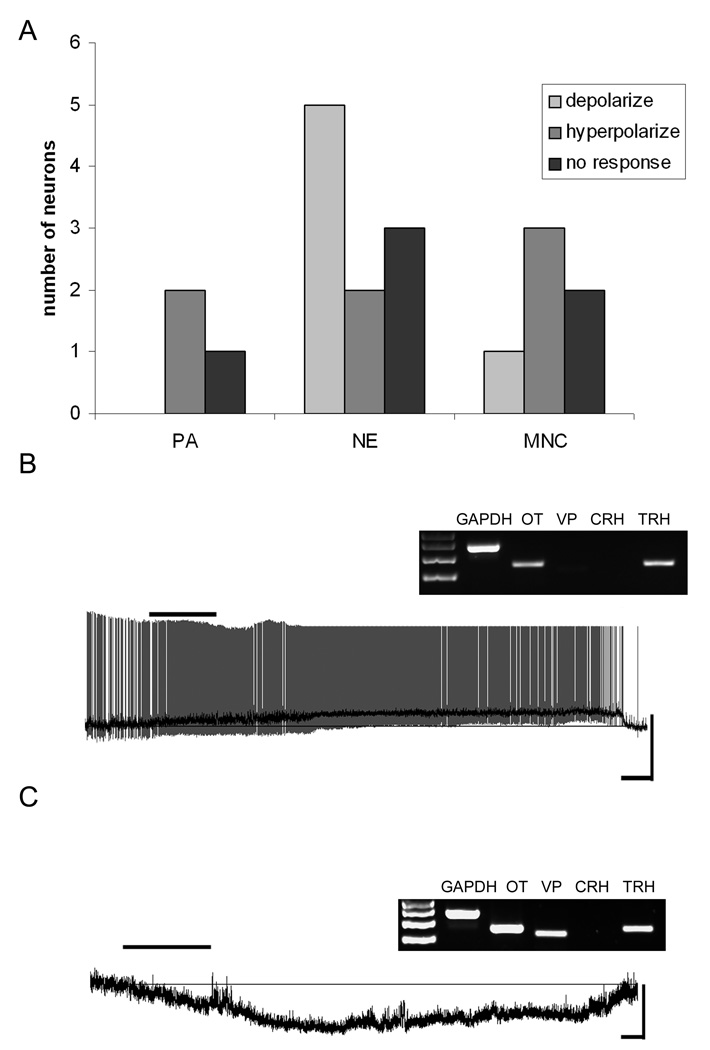
Figure 4.
TRH mRNA-expressing neurons and their responses to NPW. A) Frequency distribution histogram showing the number of PA, NE and MNC TRH neurons and how they responded to 10nM NPW. B) Current-clamp trace from a NE TRH-expressing neuron that depolarized following exposure to 10nM NPW. The dark trace is the smoothed trace superimposed upon the action potential containing trace to emphasize the change in membrane potential (scale 40mV/50s). Inset, the agarose gel image showing the neuropeptide phenotype of this cell. C) Current-clamp trace from a MNC neuron expressing TRH that hyperpolarized following exposure to 10nM NPW (scale 20mV/50s). Inset, the agarose gel image showing the neuropeptide phenotype of this cell.
Figure 5.
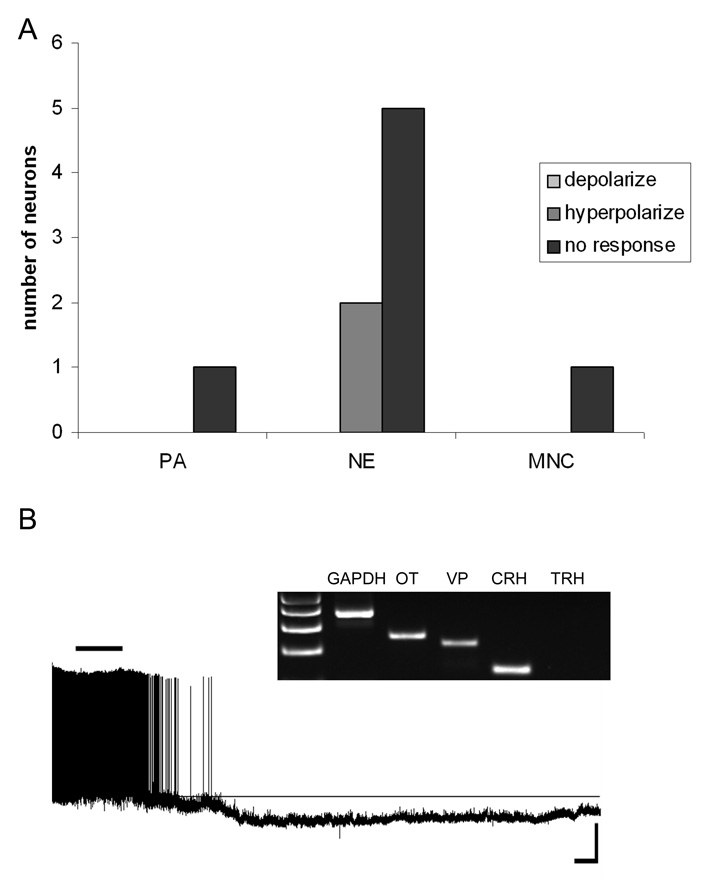
CRH mRNA-expressing neurons were largely unaffected by NPW. A) Frequency distribution histogram showing the number of PA, NE and MNC CRH neurons and how they responded to NPW exposure. B) Current-clamp trace from a NE CRH-expressing neuron that hyperpolarized following exposure to NPW (scale 20mV/100s). Inset, the agarose gel image showing the neuropeptide phenotype of this cell.
The heterogeneity of these responses was not surprising in view of the diversity of functionally and anatomically distinct cell groups which have been described in PVN. We therefore attempted to further understand the specific actions of 10nM NPW-23 on single PVN neurons, by classifying recorded neurons in association with both their chemical phenotype (single cell RTPCR), and putative projection sites (electrophysiological fingerprints). The results of our PCR analysis showed both simple (single mRNA expressing) and complex (multiple mRNA expression) patterns for the four neuropeptide mRNAs we amplified as indicated in Table 2, although for the present analysis if a neuron expressed a given peptide mRNA it was classified as such, even if it co-expressed other mRNAs.
Table 2.
Neuropeptide phenotyping using single cell RT-PCR revealed neurons that expressed single mRNA species and multiple mRNA species.
| Neuropeptide phenotype | Number of neurons |
|---|---|
| OT | 13 |
| VP | 10 |
| TRH | 4 |
| CRH | 5 |
| OT/VP | 29 |
| VP/TRH | 1 |
| OT/TRH | 2 |
| CRH/TRH | 2 |
| OT/VP/TRH | 10 |
| OT/VP/CRH | 2 |
Oxytocin Neurons
We obtained recordings from a total of 54 PVN neurons expressing mRNA for OT, of which 10 were further classified as MNC, 32 as NE and 12 as PA in accordance with their electrophysiological fingerprints. While NPW-23 responsive neurons were observed in all three of these groups of OT expressing PVN cells the PA group were unique in that all of the responsive cells (9/12) were hyperpolarized (mean 9.1 ± 2.5mV) by bath administration of NPW-23 (Chi-square test p< 0.05 compared to MNC and NE). In contrast, both MNC (2 depolarized, 5 hyperpolarized, 3 unaffected), and NE (9 depolarized, 7 hyperpolarized, 16 unaffected) OT neurons showed mixed responses to NPW-23 as illustrated in Figure 2.
Vasopressin Neurons
We also obtained recordings from 50 PVN neurons expressing mRNA for VP, of which 11 were classified as MNC, 30 as NE and 9 as PA in accordance with their electrophysiological fingerprints (Figure 3). While VP-expressing neurons also showed both depolarizing (mean 11.3 ± 3.9 mV), and hyperpolarizing (mean 8.8 ± 1.3 mV) responses to NPW-23 as illustrated in Figure 4, the predominant effect in PA (5/6 responders), and MNC (6/7) VP expressing neurons was hyperpolarization. In contrast, similar proportions of NPW-23 responsive VP expressing NE cells demonstrated either depolarization (5/13) or hyperpolarization (8/13), following bath administration of the peptide.
TRH Neurons
Recordings were obtained from a total of 19 PVN neurons expressing mRNA for TRH, of which 6 were electrophysiologically classified as MNC, 10 as NE and 3 as PA (Figure 4). The most common category of TRH expressing PVN neurons recorded were, as anticipated, NE TRH cells and the majority of responsive cells in this category (5/7) depolarized (mean 8.7 ± 3.7 mV) in response to NPW-23 as illustrated in Figure 4. In contrast, as for OT PA neurons the small number of TRH PA cells influenced by NPW-23 all hyperpolarized (2/2) in response to the peptide, as did 3/4 NPW-23 responsive MNCs.
CRH neurons
Neurons expressing mRNA for CRH (1 MNC, 6 NE, 1 PA) on the other hand, were largely unresponsive to NPW-23 (7/8), with the only responsive CRH NE cell showing a clear hyperpolarization as illustrated in Figure 5.
Discussion
In this study we have attempted to identify the cellular targets through which NPW exerts control over neuroendocrine and autonomic function by describing the effects of NPW-23 on the excitability of different subgroups of PVN neurons. Using a combination of electrophysiological and molecular techniques we have correlated the responsiveness of individual neurons to NPW-23 with electrophysiological, and neuropeptide phenotype. In accordance with many studies examining the ability of neuropeptides to influence membrane excitability, when examining the effects of NPW-23 on PVN neurons we observed heterogeneous responses with subpopulations of cells depolarized, hyperpolarized or unresponsive to NPW-23. As there has only been one shared receptor for NPB and NPW found in rodents, this heterogeneity likely arises as a result of differential ion channel expression between PVN neurons. However, we cannot rule out that an as yet unidentified receptor for NPW-23 may be responsible for either the depolarizing or hyperpolarizing responses. Future experiments examining the expression of the NPBWR1 receptor in depolarizing and hyperpolarizing PVN neurons are required to examine this issue further.
As with our previous study showing that NPW-23 predominantly depolarized somatostatin expressing neurons in the hypothalamic arcuate nucleus (16) it was only by using a combination of molecular and electrophysiological phenotyping techniques that we were able to isolate specific functional groups of PVN neurons that responded in a characteristic manner to NPW-23 exposure. Interestingly, a result of our molecular phenotyping revealed that most of our PVN neurons were positive for multiple neuropeptide mRNA’s, with OT/VP and OT/VP/TRH being the most common combinations. This finding mirrors previous studies in the supraoptic nucleus where most OT and VP neurons were found to co-express these two mRNA species in supraoptic nucleus MNC’s (17). An apparent limitation of PCR-based phenotyping of cells is the interpretation of results that may arise from the amplification of minor RNA species and the extent to which these species reflect protein expression. The use of post-hoc immunolabelling of cells could help to alleviate some of these concerns. Likewise, experiments using GFP-labeled mice would also be useful to clarify the interpretation of our results. However, in the PVN OT/VP MNC’s, identified with single cell RT-PCR, do appear to represent a distinct population of neurons characterized by showing no response to adiponectin exposure, while cells singly expressing OT and VP showed either depolarizing or hyperpolarizing responses (18).
We found that NPW-23 had varying effects on the excitability of PVN OT-expressing neurons. However, PA OT-expressing neurons responsive to NPW-23 were all hyperpolarized. This finding may have particular relevance to the NPW regulation of feeding behavior. Several studies have demonstrated that both NPB and NPW cause increased feeding and accompanying weight gain (3,5,10) (but see (4)). OT neurons projecting to the nucleus tractus solitarius (NTS) are believed to participate in the satiety inducing activities of OT (19). Thus NPW-23-induced inhibition of these OT neurons would promote prolonged feeding and therefore increases in weight. Consistent with this interpretation the satiety-promoting adipokine adiponectin has opposing effects to NPW-23 on this population of PVN neurons having been demonstrated to predominantly excite PA OT neurons (20). The effect of NPW-23 on OT neurons that possessed electrophysiological fingerprints identifying them as presumptive NE or MNC neurons, which both release OT into the circulation, was mixed with nearly half depolarized and half hyperpolarized. Interestingly, this is consistent with findings that icv injection of NPW or NPB has no effect on circulating levels of OT (3,5). Likewise, we also found slightly more NE and MNC VP neurons were hyperpolarized by NPW-23 than depolarized which again this supports previous findings that VP levels were not significantly altered by icv NPW-23 or NPB (3,5). There is an apparently contradictory report that both OT and VP plasma levels can be elevated by icv NPW (21), however, this can be reconciled if in this study the OT and VP neurons had low activity levels such that NPW-induced depolarization and therefore release would be the observed response to its introduction into the CNS.
Much like with OT and VP, both NPW-23-induced depolarizations and hyperpolarizations of TRH-expressing neurons were encountered. Interestingly, however, NE TRH neurons tended to be depolarized by NPW-23. While it is not known if NPW increases circulating thyroid stimulating hormone levels or enhances thermogenesis, intracerebroventricular NPW does elevate plasma prolactin levels (3). Prolactin release from the pituitary is under the control of a variety of factors, including TRH (22,23). Consequently, the selective excitation of PVN TRH neurons by NPW-23 may be partially responsible for NPW’s ability to stimulate prolactin release. Conversely, NPW-23 had almost no effect on neurons expressing mRNA for the neuropeptide CRH. This was a somewhat surprising result considering NPW’s ability to increase plasma ACTH concentrations (3,5) and suggests that NPW may be acting either upstream or downstream from the PVN in this regard. Based on our findings the NE cells that depolarized to NPW-23 in the study by Taylor et al (11) were likely TRH-expressing cells or other NE cells that expressed neuropeptide phenotypes that we did not test for, such as neurotensin. While CRH-expressing NE cells would have belonged to their population of cells that hyperpolarized or were found to be non-responsive, which account for nearly half of the cells they tested. Interestingly, the amygdala has high levels of NPBWR1 expression and may represent how intracerebroventricular NPW results in elevated plasma ACTH levels (15,24). Therefore, in a previous study of NE PVN neurons it is likely that those depolarized by NPW-23 were not CRH-expressing cells (11). However, the lack of activity at PVN CRH neurons is entirely in keeping with NPW’s role as a promoter of feeding, since CRH is generally known to act as a satiety factor and to increase metabolic activity (25,26).
In conclusion we have shown specific hyperpolarizing actions of NPW-23 on OT preautonomic neurons, depolarizing effects of TRH neuroendocrine cells, but no direct effects on CRH neurons. The observations support the concept that NPB fibers, which have been shown course through the PVN, play important roles in modulating the excitability of specific cell groups in this critical autonomic control center in the hypothalamus. These observations further emphasize the vital integrative roles of single neuropeptides in differentially regulating the excitability of functionally distinct subgroups of anatomically adjacent neurons in PVN to contribute to the integrated regulation of feeding and metabolism.
Acknowledgements
We would like to thank Ms. Christie Hopf for technical assistance during this study. This study was supported by NIH # HL66023 to WKS and AVF.
References
- 1.Shimomura Y, Harada M, Goto M, Sugo T, Matsumoto Y, Abe M, Watanabe T, Asami T, Kitada C, Mori M, Onda H, Fujino M. Identification of neuropeptide W as the endogenous ligand for orphan G-protein-coupled receptors GPR7 and GPR8. J Biol Chem. 2002;277:35826–35832. doi: 10.1074/jbc.M205337200. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, Yoshida T, Miyamoto N, Motoike T, Kurosu H, Shibata K, Yamanaka A, Williams SC, Richardson JA, Tsujino N, Garry MG, Lerner MR, King DS, O'Dowd BF, Sakurai T, Yanagisawa M. Characterization of a family of endogenous neuropeptide ligands for the G protein-coupled receptors GPR7 and GPR8. Proc Natl Acad Sci U S A. 2003;100:6251–6256. doi: 10.1073/pnas.0837789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker JR, Cardinal K, Bober C, Taylor MM, Samson WK. Neuropeptide W acts in brain to control prolactin, corticosterone, and growth hormone release. Endocrinology. 2003;144:2816–2821. doi: 10.1210/en.2002-0161. [DOI] [PubMed] [Google Scholar]
- 4.Mondal MS, Yamaguchi H, Date Y, Shimbara T, Toshinai K, Shimomura Y, Mori M, Nakazato M. A role for neuropeptide W in the regulation of feeding behavior. Endocrinology. 2003;144:4729–4733. doi: 10.1210/en.2003-0536. [DOI] [PubMed] [Google Scholar]
- 5.Samson WK, Baker JR, Samson CK, Samson HW, Taylor MM. Central neuropeptide B administration activates stress hormone secretion and stimulates feeding in male rats. J Neuroendocrinol. 2004;16:842–849. doi: 10.1111/j.1365-2826.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu N, Chu C, Kunitake T, Kato K, Nakazato M, Kannan H. Cardiovascular actions of central neuropeptide W in conscious rats. Regul Pept. 2007;138:82–86. doi: 10.1016/j.regpep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Hondo M, Ishii M, Sakurai T. The NPB/NPW neuropeptide system and its role in regulating energy homeostasis, pain, and emotion. Results Probl Cell Differ. 2008;46:239–256. doi: 10.1007/400_2007_056. [DOI] [PubMed] [Google Scholar]
- 8.Jackson VR, Lin SH, Wang Z, Nothacker HP, Civelli O. A study of the rat neuropeptide B/neuropeptide W system using in situ techniques. J Comp Neurol. 2006;497:367–383. doi: 10.1002/cne.20989. [DOI] [PubMed] [Google Scholar]
- 9.Schulz S, Stumm R, Hollt V. Immunofluorescent identification of neuropeptide B-containing nerve fibers and terminals in the rat hypothalamus. Neurosci Lett. 2007;411:67–71. doi: 10.1016/j.neulet.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Levine AS, Winsky-Sommerer R, Huitron-Resendiz S, Grace MK, de Lecea L. Injection of neuropeptide W into paraventricular nucleus of hypothalamus increases food intake. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1727–R1732. doi: 10.1152/ajpregu.00638.2003. [DOI] [PubMed] [Google Scholar]
- 11.Taylor MM, Yuill EA, Baker JR, Ferri CC, Ferguson AV, Samson WK. Actions of neuropeptide W in paraventricular hypothalamus: implications for the control of stress hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2005;288:R270–R275. doi: 10.1152/ajpregu.00396.2004. [DOI] [PubMed] [Google Scholar]
- 12.Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2000;523(Pt 1):193–209. doi: 10.1111/j.1469-7793.2000.t01-1-00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luther JA, Daftary SS, Boudaba C, Gould GC, Halmos KC, Tasker JG. Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J Neuroendocrinol. 2002;14:929–932. doi: 10.1046/j.1365-2826.2002.00867.x. [DOI] [PubMed] [Google Scholar]
- 14.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 15.Singh G, Maguire JJ, Kuc RE, Fidock M, Davenport AP. Identification and cellular localisation of NPW1 (GPR7) receptors for the novel neuropeptide W-23 by [125I]-NPW radioligand binding and immunocytochemistry. Brain Res. 2004;1017:222–226. doi: 10.1016/j.brainres.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 16.Price CJ, Samson WK, Ferguson AV. Neuropeptide W influences the excitability of neurons in the rat hypothalamic arcuate nucleus. Neuroendocrinology. 2008;88:88–94. doi: 10.1159/000117577. [DOI] [PubMed] [Google Scholar]
- 17.Xi D, Kusano K, Gainer H. Quantitative analysis of oxytocin and vasopressin messenger ribonucleic acids in single magnocellular neurons isolated from supraoptic nucleus of rat hypothalamus. Endocrinology. 1999;140:4677–4682. doi: 10.1210/endo.140.10.7054. [DOI] [PubMed] [Google Scholar]
- 18.Hoyda TD, Fry M, Ahima RS, Ferguson AV. Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol. 2007;585:805–816. doi: 10.1113/jphysiol.2007.144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hoyda TD, Samson WK, Ferguson AV. Adiponectin depolarizes parvocellular paraventricular nucleus neurons controlling neuroendocrine and autonomic function. Endocrinology. 2009;150:832–840. doi: 10.1210/en.2008-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki M, Onaka T, Nakazato M, Saito J, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y. Centrally administered neuropeptide W-30 activates magnocellular neurosecretory cells in the supraoptic and paraventricular nuclei with neurosecretion in rats. J Endocrinol. 2006;190:213–223. doi: 10.1677/joe.1.06636. [DOI] [PubMed] [Google Scholar]
- 22.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 23.Yu R, Ashworth R, Hinkle PM. Receptors for thyrotropin-releasing hormone on rat lactotropes and thyrotropes. Thyroid. 1998;8:887–894. doi: 10.1089/thy.1998.8.887. [DOI] [PubMed] [Google Scholar]
- 24.Singh G, Davenport AP. Neuropeptide B and W: neurotransmitters in an emerging G-protein-coupled receptor system. Br J Pharmacol. 2006;148:1033–1041. doi: 10.1038/sj.bjp.0706825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inui A. Feeding and body-weight regulation by hypothalamic neuropeptides--mediation of the actions of leptin. Trends Neurosci. 1999;22:62–67. doi: 10.1016/s0166-2236(98)01292-2. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]