Abstract
Purpose
To determine tolerability and for the first time explore efficacy of bendamustine plus rituximab (BR) in multiply relapsed/refractory hairy cell leukemia (HCL), using 2 different dose levels of bendamustine.
Experimental design
HCL patients with ≥2 prior therapies requiring treatment received rituximab 375 mg/m2 days 1 and 15, plus bendamustine 70 (n=6) or 90 (n=6) mg/m2, days 1 and 2, for 6 cycles at 4-week intervals.
Results
At 70 and 90 mg/m2/dose of bendamustine, overall response rate was 100%, with 3 (50%) and 4 (67%) complete remissions (CR) in each respective group. Minimal residual disease (MRD) was absent in 67% and 100% of CRs, respectively. All 6 without MRD remain in CR at 30–35 (median 31) months of follow-up. Soluble CD22 and CD25 levels decreased with all responses, with median values decreasing from 17.7 and 42 ng/ml at baseline to undetectable and 2 ng/ml after CR, respectively (p<0.001). Of 12 patients receiving 72 cycles of BR, the most common toxicities were hematologic, including thrombocytopenia (83%), lymphopenia (75%), leukopenia (58%) and neutropenia (42%). Grade 3–4 hematologic toxicity included lymphopenia and thrombocytopenia (each 75%), leukopenia (58%), and neutropenia (25%). No significant dose-related differences were detected in response or toxicity.
Conclusion
BR has significant activity in HCL. Bendamustine at either 70 or 90 mg/m2/dose was highly effective in multiply relapsed/refractory HCL, and could be considered for achieving durable CRs without MRD in patients after failure of standard therapies. Since it was not dose-limiting, 90 mg/m2/dose was chosen for future testing.
INTRODUCTION
Hairy cell leukemia (HCL), a B cell malignancy comprising 2% of all leukemias, is effectively treated with purine analogs cladribine or pentostatin, producing complete remission (CR) rates of 75–90%, most lasting >10 years (1, 2). Although median time to relapse is 16 years (3), disease-free and relapse-free survival curves have not reached a plateau, suggesting that most patients who live long enough will eventually relapse (1–3). A high percentage of patients in CR have minimal residual disease (MRD) (4) even if assayed a median of 16 years later (5). While HCL cells comprising MRD are brightly CD20+ (6), CR rate to the anti-CD20 MAb rituximab as a single agent was 13% in the largest trial reported where all patients had prior purine analog and needed treatment because of HCL-related cytopenias (7, 8). Results of rituximab combined with purine analogs are more encouraging (3, 9, 10), but patients with multiple prior purine analogs may respond less well.
Bendamustine, approved for chronic lymphocytic leukemia (CLL) and relapsed B-cell non-Hodgkin’s lymphoma (NHL), has features of both alkylator and a purine analog (11). Lacking cross resistance with other alkylating agents (12), its multiple mechanisms of action include 1) activation of DNA-damage stress responses and apoptosis, 2) inhibition of mitotic checkpoints, 3) induction of mitotic catastrophe, 4) activation of a DNA repair pathway involving base excisions, 5) p53-dependent stress pathway initiation leading to apoptosis, and 6) down-regulation of genes needed for mitotic checkpoint regulation (12). Bendamustine alone was reported to achieve a temporary partial response (PR) in an HCL patient with multiply relapsed and transfusion dependent disease (13), but otherwise its activity in HCL is unreported. Bendamustine and rituximab (BR) are synergistic in vitro, with rituximab increasing malignant cell sensitivity to bendamustine (14–16). BR has been used effectively for untreated or relapsed and refractory CLL (17–19), indolent NHL and mantle cell lymphoma (20, 21), and diffuse large B-cell lymphoma (22). To determine the tolerability of BR in HCL, we performed a pilot trial using 2 different dose levels of bendamustine, 70 and 90 mg/m2, given on days 1 and 2 of 6 cycles with rituximab, and studied both toxicity and response. This 12-patient tolerability study constituted a separate cohort required prior to a larger and longer-term randomized cohort comparing BR and pentostatin-rituximab in multiply relapsed HCL, where determination of a safe dose of BR was required prior to randomization. The clinical data with BR in these 12 patients, while not statistically comparable with respect to dose level, to our knowledge constitutes the first evidence of its efficacy in HCL.
PATIENTS AND METHODS
Eligibility
Patients were diagnosed with classic or variant HCL, with ≥1 indication for treatment, including neutrophil count <1/nL, hemoglobin <10 g/dL, platelets <100/nL, lymphocytes > 5/nL, symptomatic splenomegaly, enlarged nodes, or repeated infections. Patients required ≥2 purine analog courses, or if purine analog-refractory (<1 year response to first course), ≥1 course of rituximab in addition. Patients required adequate organ function and lack of hepatitis or other serious infection as described (23), and signed informed consent as approved by the NCI Institutional Review Board (IRB).
Study Design
Six patients received bendamustine at 70 mg/m2, followed by another 6 at 90 mg/m2, on days 1 and 2 of 6 cycles, given 4 weeks apart. All patients received rituximab 375 mg/m2 days 1 and 15. Patients could be delayed up to 4 weeks, but could not be dose-reduced. Dose-limiting toxicity (DLT), which would require going off treatment, was defined as drug-related grade 3–4 toxicity by NCI Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0), except for 1) hematologic toxicity resolving in 4 weeks, 2) hematologic toxicity managed with transfusions and/or growth factor support, 3) grade 3 fever, 4) grade 3 gastrointestinal toxicity, and 5) grade 3–4 aspartate aminotransferase (AST), alanine aminotransferase (ALT) or gamma glutamyl transferase (GGT) elevation for <5 days. These 12 patients comprised the tolerability component of a larger and longer-term randomized trial of BR versus pentostatin-rituximab in multiply relapsed HCL which is currently ongoing (NCT01059786). While the goal of this non-randomized tolerability study was to determine a safe dose of bendamustine to use in the randomized component, its efficacy data was separate from the randomized trial and will not be used to compare BR to pentostatin-rituximab.
Response Criteria
CR required absence of HCL in blood and bone marrow by Wright or hematoxyllin/eosin (H/E) stains, no abnormal (palpable) hepatosplenomegaly or abnormal (>2cm) adenopathy by physical examination or imaging, and resolution of blood counts to neutrophils ≥1.5/nL, platelets ≥100/nL and hemoglobin (Hgb) ≥11 g/dL without growth factors or transfusions for ≥4 weeks. Bone marrow was obtained after 4 and 6 cycles, then yearly for CRs. Criteria for CR with MRD in the bone marrow biopsy by immunohistochemistry (IHC) were previously defined (4). MRD in blood or bone marrow aspirate (BMA) was defined as HCL cells detectable by flow cytometry, since PCR was less sensitive than flow cytometry (6). PR required ≥50% reduction in 1) circulating HCL count, 2) abnormal lymphadenopathy, 3) abnormal hepatosplenomegaly by computed tomography (CT) or physical examination, and achievement of normal blood counts as required for CR or a ≥50% improvement over baseline, including Hgb ≥9g/dL if transfusion dependent before enrollment. Progressive disease (PD) required a ≥50% increase in adenopathy or new lymph nodes, a ≥50% increase in liver or spleen size measured below the costal margin, or a ≥50% increase in circulating HCL cells. Stable disease was defined as lack of CR, PR, or PD. Patients meeting criteria for PR with respect to normal blood counts, but meeting all other criteria for CR, were considered CR only if MRD-negative by all tests of bone marrow and blood. Relapse from CR or PR was established when any one of the tests needed for the achieved response was no longer consistent with that response, whether bone marrow, blood count, or imaging. Statistical comparisons used Fisher’s exact test or Wilcoxon rank sum test using exact p-values, and all p-values were 2-sided.
RESULTS
Patient characteristics
Six patients each received 2 dose levels of bendamustine, 70 and 90 mg/m2 on days 1 and 2 with rituximab on days 1 and 15 of 6 cycles 4 weeks apart (Table 1). All but one had classic HCL cells, which were bright-positive for CD20, CD22, and CD11c, and positive for CD19, CD103, CD25 and CD123. One patient who received 70 mg/m2 of bendamustine had HCL variant (HCLv), and had similar flow cytometry except negative for CD25 and CD123, as expected (24, 25). One patient in each group (RB04 and RB07) was previously reported as wild-type for BRAF (26), expressing IGHV3–23 and IGHV3–48 immunoglobulin rearrangements, respectively, with 96.3% and 94.2% homology to germline. The IGHV4–34 rearrangement was not detected in any patient. HCLv patient RB06 was also wild-type for BRAF. Ages at enrollment ranged from 55 to 70 (median 62) years, with those at 70 mg/m2 slightly older (median 68 vs. 60 years, respectively, p=0.004). Patients in each group were previously treated with a median of 3 courses of purine analog, the last being pentostatin for 2 in the 70 mg/m2 group, and cladribine for all others. Four and five out of 6 patients at 70 and 90 mg/m2, respectively, responded to their last purine analog and durations of response to last purine analog were not significantly different among the 2 groups (p=0.18). In the 2 respective groups, 5 and 3 patients had prior rituximab monotherapy, 3 and 2 had prior splenectomy, and 6 and 3 had prior recombinant immunotoxins. The recombinant immunotoxins used for these 6 and 3 patients respectively included LMB-2 (27) (n=1), BL22 (28) (n=2), moxetumomab pasudotox (23) (n=2), BL22 followed by LMB-2 (n=1), and 1 each in the higher-dose group had prior LMB-2, BL22 or moxetumomab pasudotox. Thus HCL patients receiving BR were heavily pretreated and multiply relapsed. While characteristics differed between the 2 groups, no significant differences were noted except for a slight difference in age, although the patients receiving the lower dose tended to have somewhat more advanced disease.
Table 1.
Patient Demographics, clinical characteristics and dose of bendamustine.
| Bendamustine Dose | 70 mg/m2 | 90 mg/m2 | Total |
|---|---|---|---|
| No. of patients | 6 | 6 | 12 |
| Age (years) | |||
| Median | 68 | 60 | 62 |
| Range | 62–70 | 55–62 | 55–70 |
| Prior purine analog courses | |||
| Median | 3 | 3 | 3 |
| Range | 1–4 | 2–6 | 1–6 |
| Response to last purine analog | |||
| Number responding | 4 | 5 | 9 |
| % | 67 | 83 | 75 |
| Median response durations (mo) | 12 | 22 | 21 |
| Range (mo) | 2–43 | 18–116 | 2–116 |
| Previous exposure to Rituximab | |||
| No. of patients | 5 | 3 | 8 |
| % | 83 | 50 | 67 |
| Prior splenectomy | |||
| No. of patients | 3 | 2 | 5 |
| % | 50 | 33 | 43 |
Toxicity
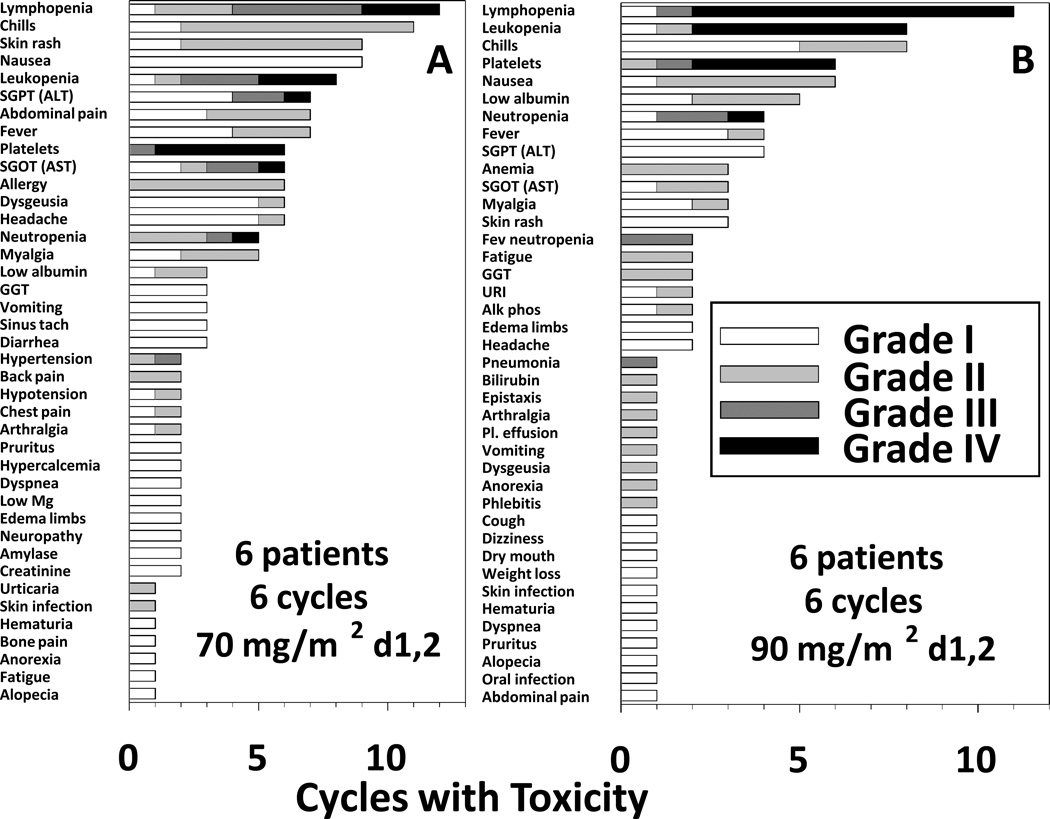
Seventy-two cycles were administered, including 36 at each bendamustine dose level. In this 12 patient pilot trial, we did not see DLT, with all 72 cycles administered without excessive delay. The main toxicities were hematologic. The most common grade 1–4 toxicities at 70 and 90 mg/m2 of bendamustine, respectively, were thrombocytopenia (67% and 100%), lymphopenia (67% and 83%), neutropenia (33% and 50%), and leukopenia (67% and 50%). The most common grade 3–4 hematologic toxicities included lymphopenia (67% and 83%), thrombocytopenia (67% and 83%), leukopenia (67% and 50%), and neutropenia (17% and 33%). Common non-hematologic toxicities, often related to rituximab infusion, included chills (67% and 67%), nausea (67% and 33%), ALT elevation (67% and 50%), and fever (33% and 67%). Some of these toxicities were also related to bendamustine, including taste disturbance (dysgeusia, 33% and 17%). Figure 1 displays the number of total cycles and grades in which toxicity was observed. The most common toxicities per cycle included lymphopenia, chills, leukopenia, nausea, skin rash and thrombocytopenia (platelets). Grade 3–4 hematologic toxicities in both groups included lymphopenia, leukopenia, thrombocytopenia, and neutropenia. Other grade 3–4 events included transaminase elevation, hypertension, febrile-neutropenia in 2 cycles (separate patients), and non-neutropenic pneumonia. All toxicities were reversible and only 1 patient, in the 90 mg/m2 arm, required a delay in treatment. This delay was due to prolonged neutropenia and thrombocytopenia, but occurred only between cycles 1 and 2; subsequent cycles were better tolerated after achieving response to treatment. Granulocyte Colony Stimulating Factor (G-CSF) was used both to treat neutropenia associated with fever and prevent recurrent neutropenia in these patients. Three of 6 patients at each dose level received G-CSF. Two patients had dermatomal cutaneous Zoster infections which resolved with antiretroviral therapy, and no Zoster infections were observed in patients taking antiretroviral prophylaxis, which instituted after the 2nd case.
Figure 1. Toxicity of BR at 2 dose levels of bendamustine.
A) 70 mg/m2 dose, B) 90 mg/m2 dose. For each dose level of bendamustine, the incidence of toxicity by cycle is shown by grade for all 36 cycles in 6 patients. SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; GGT, gamma glutamyl transferase; Mg, Magnesium, URI, upper respiratory infection; Alk phos, alkaline phosphatase; Pl. effusion, pleural effusion.
Decreases in CD4 and CD8+ T-cells
Since purine analogs in HCL are known to result in long-term reductions of CD4+ lymphocytes and to a lesser extent CD8+ lymphocytes (29, 30), we quantified these subsets in patients receiving BR. As shown in Table 2, CD4 and CD8 counts decreased to a median of 19% and 30% of baseline, recovering to 70% and 114% of baseline, respectively. Thus, although differences between the dose levels were not statistically different due to overlap, there were dose-dependent decreases in both CD4+ and CD8+ T-cells, which have significantly recovered even at the higher dose level, at a median observation time of 19 months from beginning BR. The higher frequency of grade 4 lymphopenia at 90 vs 70 mg/m2 (Figure 1), though not statistically significant, was probably because at the higher dose, there were both greater reductions in CD4 and CD8+ lymphocytes, and also lower baseline values. To prevent infections, those with CD4 counts under 200/mm3 received prophylaxis for both Herpes Zoster reactivation and Pneumocystis jiroveci pneumonia (PJP). No cases of PJP were observed. Of the 2 patients with Herpes Zoster reactivation after cycle 6, one at 70 mg/m2 had CD4 and CD8 counts (respectively) changing from 405 and 405 to 207 and 568 by cycle 6 day 251, and then to 506 and 214, by cycle 6 day 559. The other, at 90 mg/m2, had CD4 and CD8 counts (respectively) nadir from 126 and 140 to 37 and 52 by cycle 2 day 1, and then to 177 and 207, by cycle 6 day 441. Antiviral prophylaxis was instituted for all patients after this 2nd case.
Table 2.
Decreases in T-cell subsets and platelets.
| Bendamustine Dose | 70 mg/m2 | 90 mg/m2 | Total |
|---|---|---|---|
| No. of patients | 6 | 6 | 12 |
| Baseline CD4+ values (cells/mm3) | |||
| Median | 390 | 321 | 360 |
| Range | 73–5390 | 126–3792 | 73–5390 |
| Nadir CD4+ T-cells (% of baseline) | |||
| Median | 32% | 13% | 19% |
| Range | 22–56% | 1–29% | 1–56% |
| Recovery of CD4+ T-cells (% of baseline) | |||
| Median | 97% | 58% | 70% |
| Range | 11–274% | 71–140 | 11–274% |
| Baseline CD8 values (cells/mm3) | |||
| Median | 314 | 143 | 214 |
| Range | 77–4869 | 78–1795 | 77–4869 |
| Nadir CD8+ T-cells (% of baseline) | |||
| Median | 72% | 12% | 30% |
| Range | 1–140% | 4–41% | 1–140% |
| Recovery of CD8+ T-cells (% of baseline) | |||
| Median | 235% | 79% | 114% |
| Range | 14–529% | 32–148% | 14–529% |
| Decrease in platelets (nadir, absolute numbers, per nL) | |||
| Median | 18 | 25 | 25 |
| Range | 1–50 | 11–90 | 1–90 |
| Decrease in platelets (nadir, % of baseline) | |||
| No. of patients | 31% | 49% | 46% |
| % | 3–83% | 14–97% | 3–97% |
Platelet decrease after the first dose of BR
We observed a rapid but transient decrease in platelet count with just the first dose of bendamustine followed immediately by rituximab. As shown in Table 2, platelets fell in the first 1–2 days after beginning BR, with median decreases of 18 and 25/nL in the groups receiving 70 and 90 mg/m2 of bendamustine, resolving in several days. No hemorrhagic complications were observed but platelet transfusions were given for platelets <10/nL. Six patients with baseline spleen height >14 cm, compared to 6 with smaller or absent spleens, had significantly greater reductions in platelets (median 42 vs 7/nL, p=0.015) and percent reductions in platelets (median 76% vs 11%, p=0.009), suggesting that the transient thrombocytopenia was proportional to tumor burden. There was no significant difference in platelet reduction with bendamustine dose. Taken together, BR demonstrated an acceptable safety profile at either dose level, supporting the use of the higher dose if needed for response.
Patient response
Response was evaluated according to previously defined response criteria for HCL (23, 28). As shown in Table 3, of the 12 patients, all (100%) responded; 7 (58%) achieved CR, 3 at 70 and 4 at 90 mg/m2/dose of bendamustine, and the 5 others achieved PR. Time to respond was similar in the 2 groups (medians 94 vs 85 days, p=0.9), but time to CR showed a trend for shorter time at 90 than at 70 mg/m2 (n=4, 3, median 111 vs 223 days, p=0.057). With regard to MRD, 7 (58%) of 12 patients achieved negative bone marrow biopsy IHC. This included RB08, treated at 90/m2 of bendamustine, who had platelet recovery to < 100/nl (95/nL from a baseline of 35/nL) but was considered CR by protocol because of MRD negativity not only by IHC but also by blood and BMA flow cytometry. This patient would be considered CR with incomplete recovery (CRi). Lack of MRD indicated that incomplete platelet recovery was due to toxicity of BR and/or prior therapy, rather than inability to eradicate his HCL cells. Comparing the 70 vs 90 mg/m2 groups respectively, 67% vs 83% became MRD-negative in blood, and 33% vs 67% in BMA (Table 3). Thus detection of MRD was highest in BMA, lowest in blood, and intermediate by bone marrow IHC. Furthermore, all but one of the 7 CRs to BR achieved absence of MRD by all tests. Time to MRD-negative BMA, shown in Table 3 as time to MRD-free, was similar in 5 of the 6 MRD-negative patients, 82–112 days, and RB01 achieved an MRD-negative BMA at day 223 after restaging at 105 days was negative except for a positive BMA by flow cytometry. Statistical comparison of the 2 groups for response was not an anticipated endpoint of the trial, and no major differences were observed which were unexpected given the heterogeneity between the 2 groups.
Table 3.
Patient response vs dose of bendamustine
| Bendamustine Dose | 70 mg/m2 | 90 mg/m2 | Total |
|---|---|---|---|
| No. of patients | 6 | 6 | 12 |
| Standard criteria | |||
| CR | 3 (50%) | 4 (67%)* | 7 (58%) |
| PR | 3 (50%) | 2 (33%) | 5 (42%) |
| ORR | 6 (100%) | 6 (100%) | 12 (100%) |
| Time to CR (Median) | 223 days | 111 days | 162 days |
| Time to CR (range) | 213–328 days | 56–112 days | 56–328 days |
| Time to MRD-free (median) | 153 days | 112 days | 112 days |
| Time to MRD-free (range) | 82–223 | 110–112 | 82–223 |
| Negative for minimal residual disease (MRD) | |||
| Bone marrow IHC | 3 (50%) | 4 (66%) | 7 (58%) |
| Blood flow cytometry | 4 (67%) | 5 (83%) | 9 (75%) |
| Marrow flow cytometry | 2 (33%) | 4 (67%) | 6 (50%) |
One of the 4 CRs had incomplete recovery of platelets (CRi).
Patient response related to soluble receptors
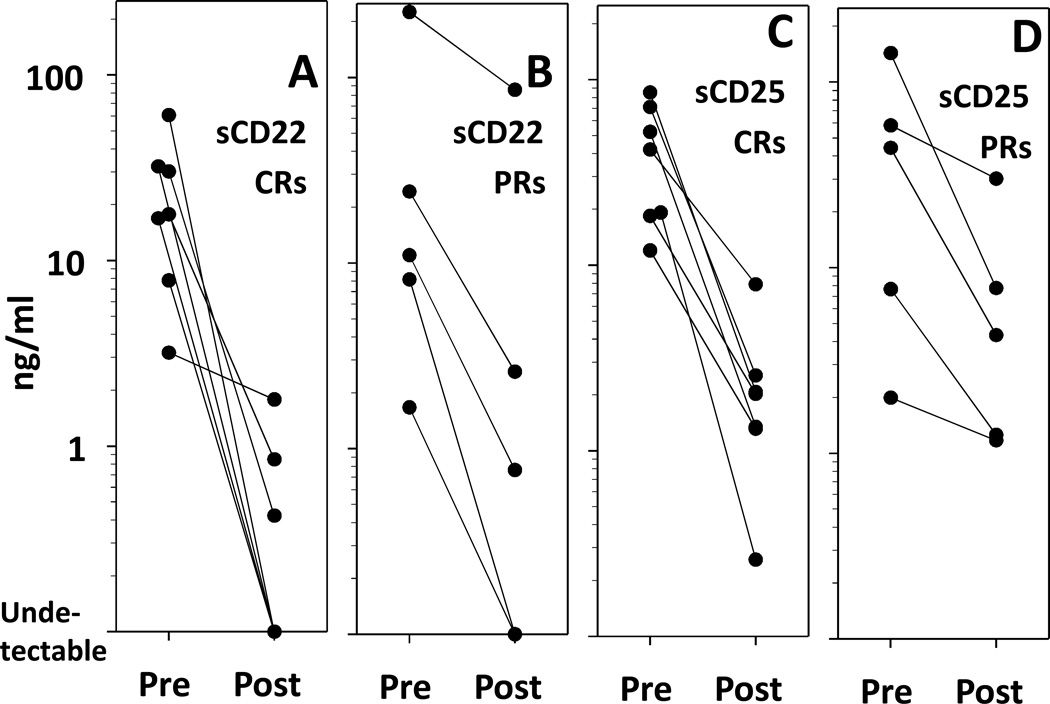
To determine the relationship of response to BR and HCL tumor markers, patients were followed for serum levels of soluble forms of CD22 (sCD22) and CD25 (sCD25), which were previously reported to correlate with tumor burden and response in HCL (31, 32). As shown in Figure 2, both sCD22 and sCD25 significantly decreased with response to BR. Median sCD22 decreased from 17.7 to 0 ng/ml (p=0.0015 Fig. 2A) and sCD25 from 42 to 2 ng/ml (p=0.0017, Fig. 2C) for CRs (n=7). We previously reported that sCD22 levels below 2 ng/ml are associated with CR, while higher ones are associated with PR or higher HCL disease burden (31). As shown in Figures 2B and 2D, sCD22 and sCD25 each decreased in patients achieving PR, but pre-treatment and post-treatment values overlapped for some patients. As expected, the sCD25 levels in patient RB06 with CD25-negative HCLv were low before and after BR (Fig. 2D, 1.99 to 1.17 ng/ml), and the small difference was possibly due to decrease in normal CD25+ lymphocytes. In this patient, sCD22 decreased from 225 to 86 ng/ml with PR (Fig. 2B).
Figure 2. Tumor markers in HCL patients after BR.
Serum levels of soluble CD22 (sCD22) (A, B) and soluble CD25 (sCD25) (C, D) are shown for patients achieving clearance of HCL in the marrow (A, C) and for patients with partial HCL clearing after BR (B, D).
Duration of response
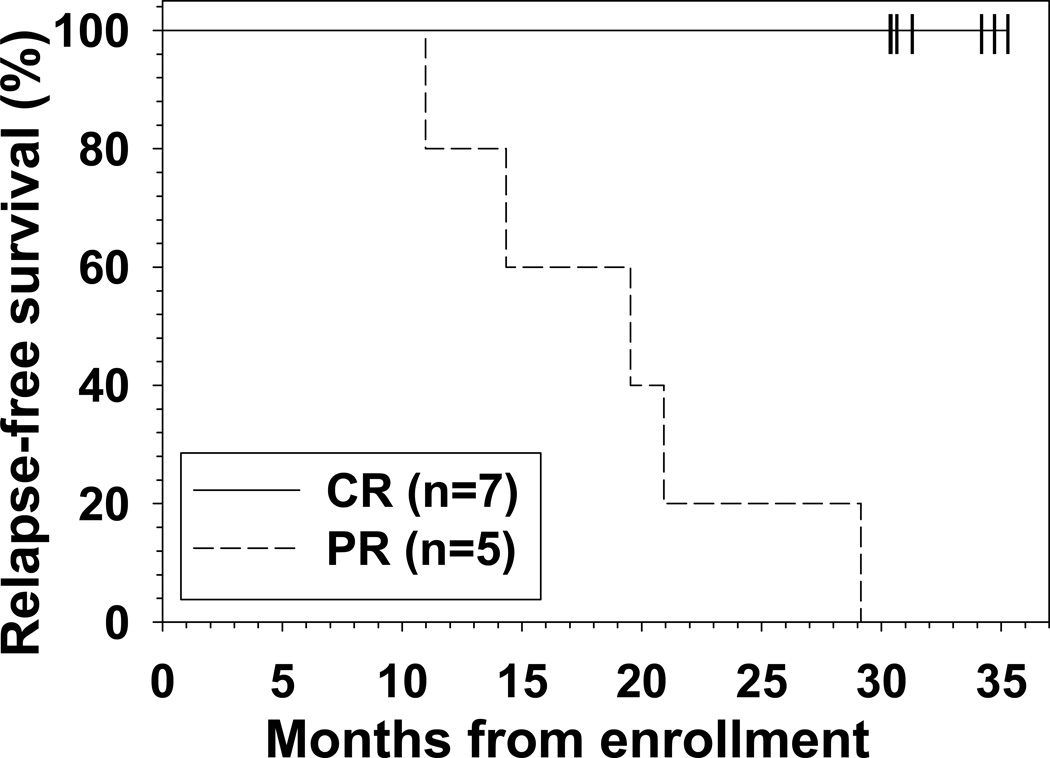
To determine CR duration, patients were followed with regular blood counts and bone marrow biopsies. The median disease-free survival has not been reached. Only 1 (RB04) of the 7 CRs, the patient who remained MRD+ by BMA, relapsed from CR with HCL cells in the bone marrow biopsy, after 32 months on protocol including 21 months in continuous CR. However, this patient has maintained all other criteria for CR, including resolved blood counts. The other 6 patients remain in CR at 30–35 (median 31) months of follow-up, including 27–31 (median 28) months in continuous CR. As shown in Figure 3, all 5 of the patients who achieved PR relapsed, including 3 at 70 and 2 at 90 mg/m2 of bendamustine. PRs lasted for 4 months in each of 2 patients (11–14 months on protocol), 11–15 months in 2 patients (20–21 months on protocol), and 27 months in 1 patient (29 months on protocol). In contrast, all 7 of the patients achieving CR as best response are still responding at 30–35 (median 31) months of follow-up, including 28–32 (median 30) months of continuous response. With respect to maintaining overall response as an endpoint, patients achieving CR fared better than those achieving PR as best response (p<0.001). Thus while CRs after BR are durable, durability of PR is suboptimal, indicating that achievement of CR should be the goal of treatment with BR in multiply relapsed HCL patients. Of the 5 patients who achieved PR, 4 have required retreatment. Each of these 4 patients received pentostatin and rituximab, and one of these 4 achieved a PR ongoing at 12 months (after a 14.5 month response to BR). One patient subsequently received vemurafenib without response. Additional follow-up and patients will be needed to determine whether eradication of MRD after BR by all tests including BMA flow cytometry is a significant predictor of response durability in CRs.
Figure 3. Duration of overall response after BR.
Patients achieving CR as best response (solid line) remain in major response (6 in CR, 1 relapsing to PR), compared to all of 5 with best response of PR (dashed line) relapsing at 11–29 (median 20) months (p<0.001).
DISCUSSION
Bendamustine with or without rituximab has demonstrated efficacy in a wide variety of hematologic malignancies (11), including both treated and untreated CLL (17–19, 33), indolent B-cell NHL and mantle cell lymphoma (MCL) (11, 20, 21, 34–36), aggressive B-cell NHL (37, 38), T-cell lymphomas (35), acute myeloid and lymphocytic leukemia and myelodysplastic syndrome (39), Hodgkin’s lymphoma (40), and multiple myeloma (41–43). In many of these settings it has demonstrated safety and efficacy in heavily pretreated patients and in older populations without major toxicities. The activity of bendamustine in HCL is previously unreported except for a case report using bendamustine alone, which achieved a PR in a multiply relapsed patient with advanced disease (13). The goal of this study was to examine the tolerability and explore the efficacy of 2 dose levels of bendamustine combined with rituximab, 70 and 90 mg/m2, days 1–2 for 6 cycles at 4 week intervals. We found that at either dose level, patients with multiply relapsed/refractory HCL tolerated BR without DLT or excessive dose delays, with ORR 100%, CR rate 58%, and CRs were usually without MRD and durable.
Choosing a dose level for bendamustine
Doses of bendamustine (given on days 1 and 2 of cycles) for clinical trials reported over the past several years have ranged from 70 mg/m2 every 4 weeks for 6 cycles for relapsed CLL (17) to 120 mg/m2 every 3 weeks for 6–8 cycles used for relapsed NHL and MCL (34, 36, 44) and aggressive NHL (38). Although detailed accounts of excessive toxicity with the higher dose level have not been published, more recently reported trials have employed the intermediate dose level of 90 mg/m2/dose every 4 weeks for 6 cycles for untreated CLL (18) and untreated or relapsed aggressive NHL (37). This dose and schedule in combination with bortezomib was also used for relapsed indolent NHL and MCL (21) and for multiple myeloma (41). Fischer et al. reported 26% of patients with relapsed CLL (17) having grade 4 hematologic toxicity at 70 mg/m2, compared with 26% of untreated CLL (18) at 90 mg/m2. In HCL, response is related to assessment of hematologic toxicity, since patients have pre-existing cytopenias which must respond to treatment. While 67 and 100% of our patients had grade 4 hematologic toxicity at 70 and 90 mg/m2, respectively, median time to respond was only 94 and 85 days, respectively, and this likely allowed toxicity to resolve, avoiding excessive delays. The median time to CR showed a trend favoring the higher dose group (median 111 vs 223 days, p=0.05), despite the requirement for cytopenias to improve before consideration of CR. However, statistical comparison of these 2 sequentially enrolled groups, particularly with respect to response, was not a goal of this trial, and the lower dose group was somewhat more advanced with respect to age, response to last purine analog, prior courses of rituximab, prior splenectomy, diagnosis of HCLv, and prior courses of immunotoxin. Thus the 90 mg/m2 dose level of bendamustine with rituximab appears appropriate for continued testing of patients with multiply relapsed HCL, but patients who are slower to respond may need dose reductions or delays.
Elimination of MRD with BR
MRD assessable by flow cytometry or IHC is reported to be common after CR to single-agent purine analog therapy (2, 4–6). Inability to eliminate MRD is probably related to emergence of purine analog-resistant populations of cells. Since HCL cells comprising MRD are bright-positive for CD20, the use of both purine analog and rituximab may allow the eradication of MRD, as has been observed clinically (3, 9, 10, 45, 46). Our data indicate that this is also possible with BR. We originally chose to administer 4 cycles of BR, but since only 1 of the 1st 6 patients had elimination of MRD by that point, and toxicity was minimal, we decided to use 6 cycles in all patients. We then did not deviate from 6 cycles since we needed to establish this dose as safe for the randomized trial which is comparing to 6 cycles of pentostatin and rituximab. In a non-protocol setting, it might be reasonable to discontinue treatment after achievement of CR without MRD, or perhaps a 1–2 cycles after this point. Based on published and unpublished observations of bendamustine alone (13), we believe it unlikely that bendamustine alone could eliminate MRD sufficiently to justify a trial of BR vs bendamustine alone to determine if the rituximab is necessary. With respect to whether these responses could have been achieved by rituximab alone, the 58% CRs and 100% ORR with BR compares favorably to 13% CRs (p=0.007) and 26% ORR (p<0.0001) achieved by rituximab alone in the largest trial using patients who would be eligible for this study (7). Despite the ability of BR to eliminate MRD, it is still premature to conclude that eradication of MRD will improve clinical outcome. Moreover, when MRD becomes undetectable, it may return and lead to relapse. Finally, standard tests of MRD, even BMA flow cytometry which was most sensitive in our experience (Table 3), may miss MRD which might be detectable through molecular methods (47) and then lead to relapse. Thus, additional patients, sufficient follow-up times, and both standard and molecular MRD assays will be required to determine how useful BR will be as a regimen to achieve long-term disease-free survival in HCL.
Prospective trials of rituximab with purine analog in HCL
This is the first prospective evaluation of bendamustine in HCL. While rituximab combined with either pentostatin or cladribine has been reported from retrospective studies with excellent results (3, 9), prospective studies of purine analogs combined with rituximab are few, particularly for multiply relapsed HCL. Ravandi et al. reported a total of 2 once-relapsed and 31 newly diagnosed classic HCL patients achieving CR, most without MRD, with rituximab following cladribine by 1 month (10, 45). Obviously, results of BR in multiply relapsed HCL are difficult to compare to these studies. Simultaneous administration of purine analog with rituximab might take better advantage of synergy, based on ex vivo assays showing that rituximab enhances sensitivity to cladribine or bendamustine (15, 16). It will be important to compare the BR regimen to that of pentostatin-rituximab to better understand the relative toxicity and efficacy of BR in HCL. This long-term study is now underway at NIH.
BR vs other treatments for multiply relapsed HCL
The ability of BR and other combinations of purine analogs and rituximab to eliminate MRD may give them a potential advantage over less myelosuppressive options such as BRAF inhibitors, which have not been shown to consistently eliminate MRD in HCL patients. Combinations of purine analog with rituximab can also be useful in HCL which is wild type for BRAF. The BTK inhibitor Ibrutinib has just begun multicenter testing in HCL and it is too early to predict whether the excellent responses seen in CLL and MCL will be achieved with this agent in HCL. The anti-CD22 recombinant immunotoxin moxetumomab pasudotox, now in multicenter phase III testing, may be advantageous over BR and other purine analog-rituximab combinations in that it can eliminate MRD without myelosuppression (23). However, the BR vs pentostatin-rituximab randomized trial has been used for patients who were ineligible for or relapsed after the immunotoxin because of anti-toxin antibodies. It will be important to further develop these new options for multiply relapsed HCL, and to use data from prospective trials to more rationally choose the best regimens for patients.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Although purine analogs cladribine and pentostatin are excellent agents for hairy cell leukemia (HCL), multiple courses are associated with resistance, making the optimal treatment for multiply relapsed HCL undefined and in need of additional options. Bendamustine is a purine analog with alkylator activity and kills cells by mechanisms of actions not possible with other purine analogs. The bendamustine-rituximab (BR) combination has established synergy against malignant cells in vitro, but its use has not been reported in HCL. The current study aims to determine the tolerability of BR in patients with multiply relapsed HCL at 2 different dose levels of bendamustine, with a later goal of comparing the BR regimen with pentostatin and rituximab. It also explores the efficacy of BR in this setting, including its ability to clear minimal residual disease, and is the first study to show that BR can achieve complete remissions in HCL.
Acknowledgments
The authors recognize our clinical staff Elizabeth Maestri, Natasha Kormanik, Joan Aaron, Rita Mincemoyer, Sonya Duke, and Barbara Debrah. We recognize Hong Zhou for technical assistance, and thank the attendings and fellows who helped care for the patients. This work was supported by the intramural research program, NCI, NIH, and also in part by the Hairy Cell Leukemia Research Foundation. Bendamustine for this trial was provided by Cephalon (Teva) and rituximab by Genentech.
Footnotes
Statement of potential conflicts of interest: Dr. Kreitman’s laboratory receives research support from Genentech, and the rituximab and bendamustine for this trial was supplied to the NIH from Genentech and Teva (formally Cephalon), respectively.
REFERENCES
- 1.Grever MR, Lozanski G. Modern strategies for hairy cell leukemia. J Clin Oncol. 2011;29:583–590. doi: 10.1200/JCO.2010.31.7016. [DOI] [PubMed] [Google Scholar]
- 2.Gidron A, Tallman MS. 2-CdA in the treatment of hairy cell leukemia: a review of long-term follow-up. Leuk Lymphoma. 2006;47:2301–2307. doi: 10.1080/10428190600822052. [DOI] [PubMed] [Google Scholar]
- 3.Else M, Dearden CE, Matutes E, Garcia-Talavera J, Rohatiner AZ, Johnson SA, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145:733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 4.Tallman MS, Hakimian D, Kopecky KJ, Wheaton S, Wollins E, Foucar K, et al. Minimal residual disease in patients with hairy cell leukemia in complete remission treated with 2-chlorodeoxyadenosine or 2-deoxycoformycin and prediction of early relapse. Clin Cancer Res. 1999;5:1665–1670. [PubMed] [Google Scholar]
- 5.Sigal DS, Sharpe R, Burian C, Saven A. Very long-term eradication of minimal residual disease in patients with hairy cell leukemia after a single course of cladribine. Blood. 2010;115:1893–1896. doi: 10.1182/blood-2009-10-251645. [DOI] [PubMed] [Google Scholar]
- 6.Sausville JE, Salloum R, Sorbara L, Kingma DW, Raffeld M, Kreitman RJ, et al. Minimal residual disease detection in hairy cell leukemia. Comparison of flow cytometric immunophenotyping with clonal analysis using consensus primer polymerase chain reaction for the heavy chain gene. Am J Clin Pathol. 2003;119:213–217. doi: 10.1309/G629-9513-NGLC-UB1K. [DOI] [PubMed] [Google Scholar]
- 7.Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102:810–813. doi: 10.1182/blood-2003-01-0014. [DOI] [PubMed] [Google Scholar]
- 8.Angelopoulou MK, Pangalis GA, Sachanas S, Kokoris SI, Anargyrou K, Galani Z, et al. Outcome and toxicity in relapsed hairy cell leukemia patients treated with rituximab. Leuk Lymphoma. 2008;49:1817–1820. doi: 10.1080/10428190802163289. [DOI] [PubMed] [Google Scholar]
- 9.Forconi F, Toraldo F, Sozzi E, Amato T, Raspadori D, Lauria F. Complete molecular remission induced by concomitant cladribine--rituximab treatment in a case of multi-resistant hairy cell leukemia. Leuk Lymphoma. 2007;48:2441–2443. doi: 10.1080/10428190701647903. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi F, O'Brien S, Jorgensen J, Pierce S, Faderl S, Ferrajoli A, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood. 2011;118:3818–3823. doi: 10.1182/blood-2011-04-351502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rummel MJ, Gregory SA. Bendamustine's emerging role in the management of lymphoid malignancies. Semin Hematol. 2011;48(Suppl 1):S24–S36. doi: 10.1053/j.seminhematol.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Ujjani C, Cheson BD. Bendamustine in chronic lymphocytic leukemia and non-Hodgkin's lymphoma. Expert Rev Anticancer Ther. 2010;10:1353–1365. doi: 10.1586/era.10.116. [DOI] [PubMed] [Google Scholar]
- 13.Kreitman RJ, Arons E, Stetler-Stevenson M, Miller KB. Response of Hairy Cell Leukemia to Bendamustine. Leuk Lymphoma. 2011;52:1153–1156. doi: 10.3109/10428194.2011.562575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Rummel MJ. Bendamustine: rebirth of an old drug. J Clin Oncol. 2009;27:1492–1501. doi: 10.1200/JCO.2008.18.7252. [DOI] [PubMed] [Google Scholar]
- 15.Chow KU, Boehrer S, Geduldig K, Krapohl A, Hoelzer D, Mitrou PS, et al. In vitro induction of apoptosis of neoplastic cells in low-grade non-Hodgkin's lymphomas using combinations of established cytotoxic drugs with bendamustine. Haematologica. 2001;86:485–493. [PubMed] [Google Scholar]
- 16.Chow KU, Sommerlad WD, Boehrer S, Schneider B, Seipelt G, Rummel MJ, et al. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43. [PubMed] [Google Scholar]
- 17.Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 18.Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 19.Iannitto E, Morabito F, Mancuso S, Gentile M, Montanini A, Augello A, et al. Bendamustine with or without rituximab in the treatment of relapsed chronic lymphocytic leukaemia: an Italian retrospective study. Br J Haematol. 2011;153:351–357. doi: 10.1111/j.1365-2141.2011.08597.x. [DOI] [PubMed] [Google Scholar]
- 20.Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 21.Friedberg JW, Vose JM, Kelly JL, Young F, Bernstein SH, Peterson D, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–2812. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N, et al. Multicenter Phase II Study of Bendamustine Plus Rituximab in Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2013;31:2103–2109. doi: 10.1200/JCO.2012.46.5203. [DOI] [PubMed] [Google Scholar]
- 23.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matutes E. Immunophenotyping and differential diagnosis of hairy cell leukemia. Hematol Oncol Clin North Am. 2006;20:1051+. doi: 10.1016/j.hoc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Shao H, Calvo KR, Grönborg M, Tembhare PR, Kreitman RJ, Stetler-Stevenson M, et al. Distinguishing Hairy Cell Leukemia Variant from Hairy Cell Leukemia: Development and Validation of Diagnostic Criteria. Leuk Res. 2012;37:401–409. doi: 10.1016/j.leukres.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi L, Arons E, Navarro W, Calvo KR, Stetler-Stevenson M, Raffeld M, et al. Both variant and IGHV4–34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood. 2012;119:3330–3332. doi: 10.1182/blood-2011-09-379339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Waldmann TA, et al. Phase I trial of recombinant immunotoxin Anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J. Clin. Oncol. 2000;18:1614–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 28.Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, FitzGerald DJP, Wilson WH, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seymour JF, Kurzrock R, Freireich EJ, Estey EH. 2-chlorodeoxyadenosine induces durable remissions and prolonged suppression of CD4+ lymphocyte counts in patients with hairy cell leukemia. Blood. 1994;83:2906–2911. [PubMed] [Google Scholar]
- 30.Seymour JF, Talpaz M, Kurzrock R. Response duration and recovery of CD4+ lymphocytes following deoxycoformycin in interferon-alpha-resistant hairy cell leukemia: 7- year follow-up. Leukemia. 1997;11:42–47. doi: 10.1038/sj.leu.2400513. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Margulies I, Onda M, Nagata S, Stetler-Stevenson M, Kreitman RJ. Soluble CD22 as a Tumor Marker for Hairy Cell Leukemia. Blood. 2008;112:2272–2277. doi: 10.1182/blood-2008-01-131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steis RG, Marcon L, Clark J, Urba W, Longo DL, Nelson DL, et al. Serum soluble IL-2 receptor as a tumor marker in patients with hairy cell leukemia. Blood. 1988;71:1304–1309. [PubMed] [Google Scholar]
- 33.Knauf WU, Lissitchkov T, Aldaoud A, Liberati AM, Loscertales J, Herbrecht R, et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: updated results of a randomized phase III trial. Br J Haematol. 2012;159:67–77. doi: 10.1111/bjh.12000. [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Friedberg JW, Kahl BS, Van der Jagt RH, Tremmel L. Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10:452–457. doi: 10.3816/CLML.2010.n.079. [DOI] [PubMed] [Google Scholar]
- 35.Damaj G, Gressin R, Bouabdallah K, Cartron G, Choufi B, Gyan E, et al. Results From a Prospective, Open-Label, Phase II Trial of Bendamustine in Refractory or Relapsed T-Cell Lymphomas: The BENTLY Trial. J Clin Oncol. 2013;31:104–110. doi: 10.1200/JCO.2012.43.7285. [DOI] [PubMed] [Google Scholar]
- 36.Ohmachi K, Ando K, Ogura M, Uchida T, Itoh K, Kubota N, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2059–2064. doi: 10.1111/j.1349-7006.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horn J, Kleber M, Hieke S, Schmitt-Graff A, Wasch R, Engelhardt M. Treatment option of bendamustine in combination with rituximab in elderly and frail patients with aggressive B-non-Hodgkin lymphoma: rational, efficacy, and tolerance. Ann Hematol. 2012;91:1579–1586. doi: 10.1007/s00277-012-1503-5. [DOI] [PubMed] [Google Scholar]
- 38.Weidmann E, Neumann A, Fauth F, Atmaca A, Al-Batran SE, Pauligk C, et al. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. Ann Oncol. 2011;22:1839–1844. doi: 10.1093/annonc/mdq671. [DOI] [PubMed] [Google Scholar]
- 39.Chacar C, Jabbour E, Ravandi F, Borthakur G, Kadia T, Estrov Z, et al. Phase I-II study of bendamustine in patients with acute leukemia and high risk myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2012;12:197–200. doi: 10.1016/j.clml.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskowitz AJ, Hamlin PA, Jr, Perales MA, Gerecitano J, Horwitz SM, Matasar MJ, et al. Phase II Study of Bendamustine in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol. 2013;31:456–460. doi: 10.1200/JCO.2012.45.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berenson JR, Yellin O, Bessudo A, Boccia RV, Noga SJ, Gravenor DS, et al. Phase I/II trial assessing bendamustine plus bortezomib combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. Br J Haematol. 2012 doi: 10.1111/bjh.12129. in press. [DOI] [PubMed] [Google Scholar]
- 42.Ponisch W, Bourgeois M, Moll B, Heyn S, Jakel N, Wagner I, et al. Combined bendamustine, prednisone and bortezomib (BPV) in patients with relapsed or refractory multiple myeloma. J Cancer Res Clin Oncol. 2012 doi: 10.1007/s00432-012-1339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lentzsch S, O'Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119:4608–4613. doi: 10.1182/blood-2011-12-395715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahl BS, Bartlett NL, Leonard JP, Chen L, Ganjoo K, Williams ME, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–114. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravandi F, Jorgensen JL, O'Brien SM, Verstovsek S, Koller CA, Faderl S, et al. Eradication of minimal residual disease in hairy cell leukemia. Blood. 2006;107:4658–4662. doi: 10.1182/blood-2005-11-4590. [DOI] [PubMed] [Google Scholar]
- 46.Cervetti G, Galimberti S, Andreazzoli F, Fazzi R, Cecconi N, Caracciolo F, et al. Rituximab as treatment for minimal residual disease in hairy cell leukaemia. European Journal of Haematology. 2004;73:412–417. doi: 10.1111/j.1600-0609.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- 47.Arons E, Margulies I, Sorbara L, Raffeld M, Stetler-Stevenson M, Pastan I, et al. Minimal residual disease in hairy cell leukemia patients assessed by clone-specific polymerase chain reaction. Clin Cancer Res. 2006;12:2804–2811. doi: 10.1158/1078-0432.CCR-05-2315. [DOI] [PubMed] [Google Scholar]