Abstract
OBJECTIVE:
To assess the regeneration of osteochondral defects in the joint cartilage of the knee induced by autologous platelet-rich plasma (PRP).
METHODS:
Osteochondral defects produced in the trochlear groove of both knees of ten sheep; defects of the right knees were filled with autologous PRP and the left knees were left unfilled. Macroscopic and microscopic evaluation was carried out 12 week later. The results were evaluated by the total score of both macroscopic and microscopic evaluations comparing the two sides through the Wilcoxon paired test.
RESULTS:
Macroscopic appearance was not uniform among animals, nor was it different between the right and left knees (p=0.3125), and in no case the regenerated tissue was equal to the normal surrounding cartilage. At histological examination, apparently normal cartilage was not detected in any knee, but a poorly differentiated cartilage was present in 7 right knees, compared to 3 left knees. Fibrocartilaginous tissue was present in most of the remaining knees, with a significant difference in the overall score between right and left knees (p=0.0313).
CONCLUSION:
The PRP as used in this study has reparative properties of the joint cartilage of sheep knees, mostly by stimulating the formation of a fibrocartilaginous tissue. Laboratory investigation.
Keywords: Knee, Platelet-rich plasma, Articular cartilage, Sheep
INTRODUCTION
In the 18th century, in his classical article on articular cartilage disease, Hunter reported that an ulcer of the articular cartilage was universally recognized as a troublesome disease, since it was more difficult to cure than a bone lesion and, once destroyed, the articular cartilage would never recover spontaneously. 1
In spite of the considerable progress experienced by modern surgery, articular cartilage lesions still represent a problem that is difficult to solve. We now know that the surgical stimulation of articular cartilage regeneration through holes drilled in the subchondral bone produces only one type of fibrous or fibrocartilaginous tissue, with biochemical characteristics and biomechanical properties that are inferior to those of normal cartilage, tending to deteriorate faster when submitted to weight-bearing. 2 , 3 Based on this information, specialists have invested considerable effort in the development of procedures capable of inducing articular cartilage regeneration, with treatment options that included both conservative approaches and surgical interventions. 4 However, according to McCormick et al., 5 no attempt to treat chondral or subchondral defects without surgery has effectively produced articular cartilage reparation.
In a review article, O´Driscoll presented two strategies for the surgical treatment of chondral lesions, aiming to prevent the premature degeneration of the joint: 1) increase the intrinsic regenerative capacity of the cartilage by stimulating the subchondral bone (debridement, abrasion chondroplasty, microfracture chondroplasty, electrical stimulation, pharmacological agents and growth factors); and 2) transplantation of tissues such as autologous and heterologous chondrocytes, chondrogenic cells (mosaicplasty, perichondrial graft) or other tissues (periosteal graft) with the ability to promote new cartilage growth. 6 Later on, Mithoefer et al. 4 mentioned a third strategy of surgical treatment focused on the use of base cells, including the transplantation of autologous chondrocytes, the implantation of chondrocytes combined with a frame, the implantation of cartilage grafts, gene therapy and stem cells. With the advances in the areas of genetic and tissue engineering, new tissue treatment methods have emerged. These include the use of platelet-rich plasma (PRP), which is an autologous blood plasma with a high concentration of platelets, far higher than whole blood, obtained by centrifugation and whose use was referred to in clinical practice to stimulate the healing of soft tissues and of bone in a variety of situations, based on the allegation that it carries a high concentration of platelet-derived growth factor (PDGF) and of transforming growth factor β (TGF-β), both known to stimulate the growth and regeneration of different tissues. 7
PRP has already been used by medical specialties, such as cosmetic and reconstructive surgery and otolaryngology, and in dentistry, particularly to stimulate hemostasis, the healing of soft parts and of bone, the adhesion of skin grafts, the integration of bone grafts and the fixation of implants on the flat bones of the skull and face. 8 - 11 However, despite the evidence that PRP can be useful to stimulate spontaneous tissue repair, its role in articular cartilage repair is still unknown and requires specific investigation.
Therefore, the hypothesis that PRP stimulates articular cartilage regeneration was tested experimentally in a model of osteochondral defect of sheep knees, with the results being evaluated by macroscopic examination and by histological analysis using a system of semi-quantitative scores.
METHODS
The study was approved by the Committee of Ethics in Animal Use of the institution of origin of the authors. All the animals were handled according to the recommendations of the Committee on Care and Use of Laboratory Animals (National Research Council, 1996). The study subjects were ten healthy ewes aged between 1 and 2 years, weighing between 30 and 50kg, and both right and left knees were operated on to create an osteochondral defect in the trochlear groove.
Surgical procedure: The animals were fasted for 24 hours and atropine (1%, 0.05mg/kg, subcutaneous route) and xylazine (2%, 0.22mg/kg, intramuscular route) were administered as premedication about 15min before the operation. The jugular vein was catheterized to draw 40ml of blood used in the preparation of the PRP. Supportive fluid therapy was performed with a 0.9% saline solution, administered in the regime of 1 ml/min; 1.0g of cephalothin sodium was administered intravenously as prophylactic antibiotic therapy.
The animals were then anesthetized with a combination of xylazine (2%, 0.1 mg/kg) and ketamine (8 mg/kg) in a sufficient volume to put the animal to sleep. When necessary, half of the initial dose was repeated. Intubation was not necessary as the entire surgical procedure was carried out in less than 40 min, but pure oxygen was administered continuously through a catheter inserted in a nostril.
Both knees were routinely prepared (trichotomy, antisepsis with a solution of 20% iodized alcohol, placement of sterile drapes surrounding the operating field) and the operation was started in the left knee. The femoral trochlea was exposed through a medial parapatellar incision involving the skin, the subcutaneous tissue and the joint capsule and the patella was dislocated laterally. An osteochondral defect in a diameter of 8mm was then produced in the middle of the trochlear groove using a handheld drill, taking care not to deepen the defect beyond the subchondral bone, but only until the bleeding became evident. The residues were carefully removed with saline solution and the defect was left empty. The patella was reduced, the wound closed by planes and a slightly compression bandage was applied. Then the right knee was submitted to an identical procedure, but the defect was filled with the PRP gel prepared immediately beforehand.
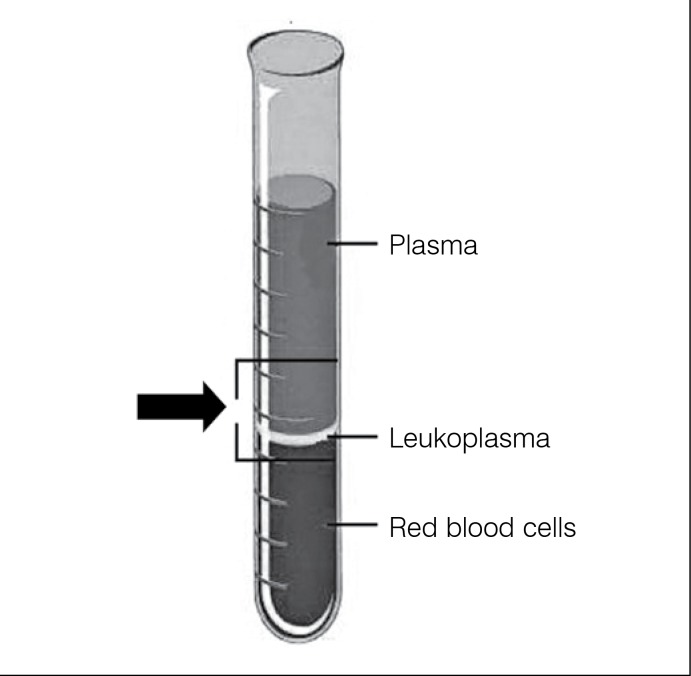
Preparation of the PRP: The PRP gel was prepared immediately beforehand, as follows: the total blood was drawn from the jugular vein in two 20ml syringes, each containing 2.5ml of 10% sodium citrate. Then 1ml of this blood was poured into a 5ml test tube for total blood cell count using an automatic analyzer (Coulter STKS Analyzer, Coulter Corporation, Hialeah, FL, USA). The remaining blood was divided into six equal portions placed in six test tubes and centrifuged for 8 min at 1,800 rpm, after which each sample was divided into three layers: an upper layer, of a yellowish hue (plasma); a lower layer, of a reddish hue (red blood cells); and an intermediate layer, of a whitish hue (leukocytes). The material between the lower portion of the plasma layer and the upper portion of the layer of red blood cells was pipetted (Figure 1) and transferred to two other 5ml test tubes then centrifuged again for 8 min at 1,000 rpm, after which the plasma predominated in each tube, with only a small layer of red blood cells covered by a thin layer of leukocytes in the bottom; 2ml of the lower portion of the plasma were drawn from each tube with a pipette and mixed; the material thus obtained is PRP, by definition. The platelets were counted in 1ml of this material to make sure that the concentration was higher than in the total blood. The concentration ranged between 120,000 and 250,000/µl, with 1,000,000/µl being detected in only one animal, generally corresponding to an increase of 2.57 times the concentration in the total blood (variation: 1.56 - 4.34 times). These values were within the range of optimum variation for PRP. One ml of 10% calcium chloride was added to the remaining material to induce plasma coagulation, resulting in the so-called PRP gel, which was then used to completely fill the osteochondral defect of the right knee. (Figure 2) PRP gel has adhesive properties and adhered easily to the lesion. 12 , 13 Histological studies: The animals were euthanized with an overdose of anesthetic (2.5% sodium pentobarbital) administered intravenously, 12 weeks after the operation. The distal extremity of the femurs were exposed and dried, leaving the joint surfaces untouched, for the macroscopic examination and for the collection of material for the histological examination. The macroscopic examination was performed immediately, with the following analysis parameters: intra-articular adhesion, degree of restoration of the joint surface, erosion of the cartilage and appearance of the new tissue formed inside the defect. (Table 1) 14
Figure 1. Schematic drawing showing the arrangement of the blood cells in the test tube after the first centrifugation and the region from where the material was pipetted for the second centrifugation.
Figure 2. Image of the PRP inserted in the osteochondral defect.
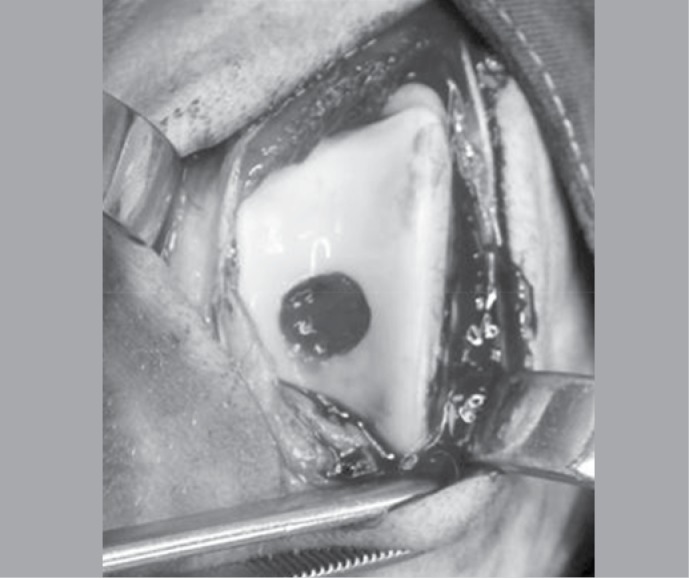
Table 1. Macroscopic evaluation scale.
| Description | Score |
|---|---|
| Intra-articular adhesion | |
| None | 2 |
| Minimum | 1 |
| Large | 0 |
| Restoration of the articular surface | |
| Complete | 2 |
| Partial | 1 |
| None | 0 |
| Erosion of cartilage | |
| None | 2 |
| Site of defect | 1 |
| Defect and normal adjacent cartilage | 0 |
| Appearance of the cartilage | |
| Translucent | 2 |
| Opaque | 1 |
| Discolored and/or irregular | 0 |
The specimens were identified, placed in separated containers containing a 10% formaldehyde aqueous solution for 48 hours for fixation and then individually decalcified in a 7.5% nitric acid aqueous solution for a further 48 hours. Each specimen was trimmed to leave just one block containing the entire lesion site, with a 5mm wide ring of surrounding normal cartilage, and with a 5mm thick layer of trabecular bone underneath the subchondral bone. All the blocks were left in a bath of running water for 2 hours to remove any acid residue, dehydrated by immersion in alcohol solutions of increasing concentrations (80, 85, 90 and 95%) for 1 hour each, then embedded in paraffin, according to the routine procedure. Histological sections with a thickness of 6 mm were then obtained, alternatively stained with Harris's hematoxylin (for analysis of the nucleus and of acid elements), eosin-phloxine (for analysis of basic elements) and Gomori trichome (for analysis of other elements). All the sections were examined under a light microscope by an experienced pathologist (JBN) on a double-blind basis, analyzing the following parameters: nature of the regenerated tissue (similarity to fibrous tissue, fibrocartilage or cartilage), surface regularity, structural integrity, union with the adjacent cartilage and leveling of the neoformed tissue. (Table 2) 15
Table 2. Microscopic evaluation scale.
| Description | Score |
|---|---|
| Nature of the predominant tissue | |
| Normal cartilage | 4 |
| Slightly differentiated cartilage | 2 |
| Fibrous tissue | 0 |
| Surface uniformity | |
| Smooth and intact | 2 |
| Partially broken | 1 |
| Completely broken | 0 |
| Structural integrity | |
| Intact | 2 |
| Partially separated | 1 |
| Completely separate | 0 |
| Union with the adjacent cartilage | |
| Complete | 2 |
| Partial | 1 |
| Absent | 0 |
| Level of neoformed tissue | |
| Same as adjacent cartilage | 2 |
| 50 to 100% of the normal cartilage | 1 |
| 0 to 50% of the normal cartilage | 0 |
Each parameter was given a score of 0, 1 or 2, corresponding to no restoration, to an intermediate restoration or to the complete restoration, respectively, in comparison to the normal cartilage; the nature of the predominant tissue received a score of 0, 2 or 4, corresponding to the fibrous tissue, to the slightly differentiated cartilage or to the normal cartilage, respectively. The results were evaluated by the total score of the macroscopic and histological evaluations and the data were analyzed by the descriptive statistic (median and variation) and in terms of absolute and relative frequency. The total scores involving both analyses were compared between the left knee (control group) and the right (study group) by the Wilcoxon paired test with two-tailed correction and significance level established at 5% (p<0.05).
RESULTS
The animals tolerated both the anesthesia and the surgical procedures very well and resumed walking as soon as they recovered from the anesthesia. The only postoperative complication was superficial infection and partial dehiscence of the cutaneous suture of the right knee observed in just one animal on the 17th postoperative day.
Macroscopic analysis: Intra-articular adhesion was not a problem, as it was not observed in any knee. Normal cartilage appearance was not observed in any right or left knee. Loss of coloration and irregularity of the surface of the normal surrounding cartilage was observed in eight (8/10) left knees, while opacity was evident in six (6/10). Apparent restoration of the articular surface of the defect (Figure 3) was only partial in seven (7/10) left knees (control), and completely absent in the other three; complete restoration was evident in two knees (2/10) on the right side (study group), but was only partial in four (4/10), while no sign of restoration was observed in the four remaining knees. Erosion of the normal surrounding cartilage was observed in eight (8/10) left knees, but was absent in the right knees, in which it appeared circumscribed to the defect in six knees (6/10). The general score referring to the macroscopic parameters was 4.2 (variation: 2 -6) for the left knees and 4.7 (variation: 2 - 7) for the right knees. (Table 3) The differences referring to the macroscopic findings between the right and left knees were not significant (p=0.3125) for any comparison. (Table 4)
Figure 3. Photograph showing the complete restoration on the articular surface of the defect.
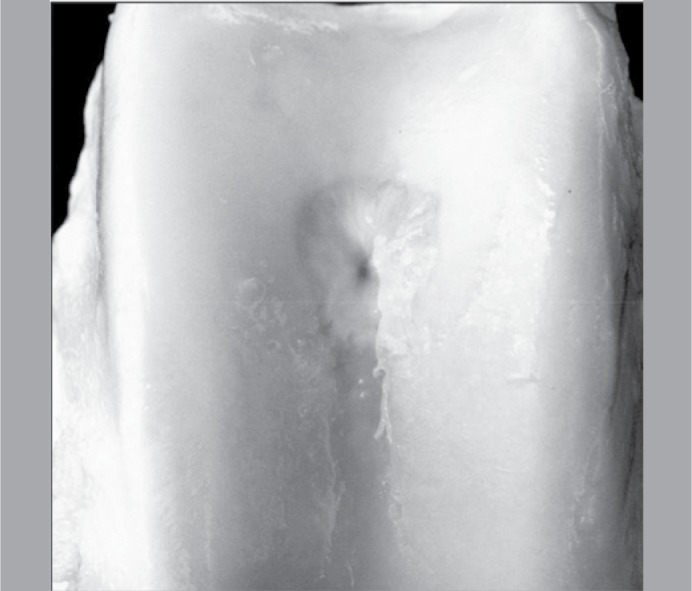
Table 3. General score of the macroscopic and histological parameters for the right and left knees.
| Knee | Right | left |
|---|---|---|
| Macro | 4.7 ± 1.7 | 4.2 ± 1.23 |
| (variation: 2 - 7) | (range: 2 - 6) | |
| Histo | 5.4 ± 2.32 | 3.6 ± 2.37 |
| (variation: 2 - 8) | (variation: 1 - 8) |
Table 4. Results of the Wilcoxon paired test applied to the general score of the macroscopic analysis (n=10).
| Knee | mean | minimum | maximum | p |
|---|---|---|---|---|
| Left | 4.2 | 2 | 6 | 0.3125* |
| right | 4.7 | 2 | 7 |
non-significant.
Microscopic analysis
Complete restoration to a cartilage of normal appearance was not observed in any knee. A slightly differentiated cartilage was observed in seven (7/10) right knees with clearly fibrous tissue in the three remaining knees (3/10). Exactly the opposite was observed in the left knees, with three showing slightly differentiated cartilage (3/10) and seven showing clearly fibrous tissue (7/10); details of the corresponding appearances are shown in Figures 4A and 4B. The surface of the regenerated tissue appeared smooth and regular, almost normal, in one left knee and in two right knees. In the remaining knees, the surface was interrupted, and was partially regular in five left knees and in six right knees. In its internal portion, the regenerated tissue exhibited large fissures in seven (7/10) left knees, but in only two right knees (2/10), with small fissures being observed in all the other knees (three left and eight right). The union between the regenerated tissue and the preexisting cartilage was similar in both groups, appearing complete in six (6/10) and partial in four (4/10) right and left knees. In all the knees, the surface of the regenerated tissue was uneven in relation to the adjacent normal cartilage, appearing superficially depressed. The unevenness was more accentuated in the left knees, in which the thickness of the regenerated tissue was less than 50% of the adjacent normal cartilage in the majority (6/10) of the specimens, while in the right knees this proportion ranged between 50% and 100%. (Figure 5) The general score referring to the histological parameters was 3.6 (variation: 1 - 8) for the left knees and 5.4 (variation: 2 - 8) for the right knees (Table 3). The differences between the right and left knees were significant (p=0.0313) for all the comparisons. (Table 5)
Figure 4. Light photomicrograph showing the repair tissue of the left knee (A) and of the right knee (B). Observe the presence of slightly differentiated cartilaginous tissue in a lesion filled with the PRP (B), in contrast with the clearly fibrous tissue in a knee that did not receive the PRP (A) (Gomori Trichrome, 40x).
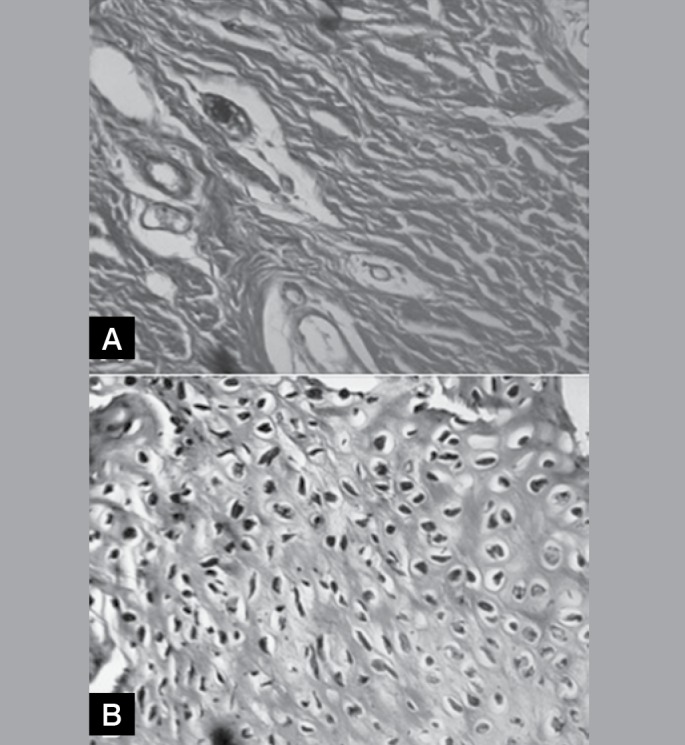
Figure 5A. Photomicrographs of slight increase of a histological section of the left knee. Note the fault and the separation of the neoformed tissue in relation to the adjacent cartilage (Hematoxylin-eosin, 1X).
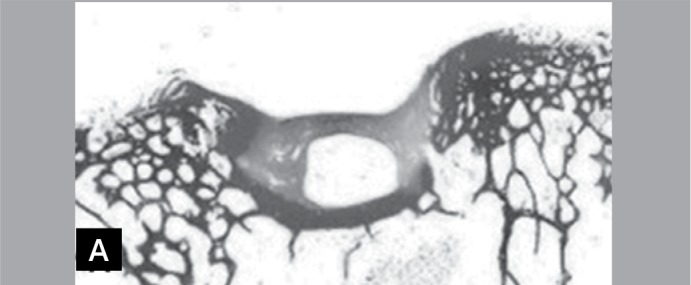
Table 5. Results of the Wilcoxon paired test applied to the general score of the macroscopic analysis (n=10).
| Knee | mean | minimum | maximum | p |
|---|---|---|---|---|
| Left | 3.6 | 1 | 8 | 0.0313 |
| right | 5.4 | 2 | 8 |
Figure 5B. Photomicrographs of slight increase of a histological section of the right knee. Note the leveling and the almost complete integration between the tissues (Hematoxylin-eosin, 1X).
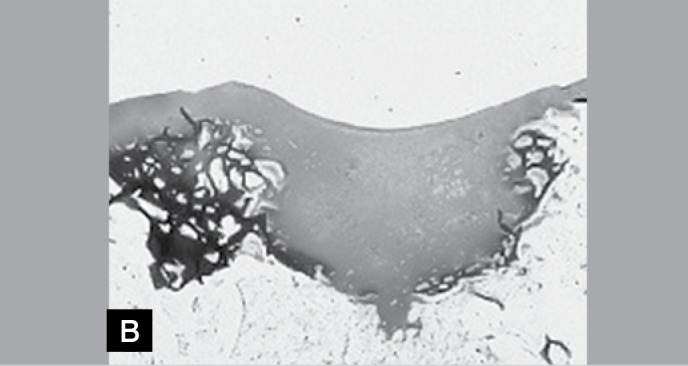
DISCUSSION
In spite of the major advances of modern orthopedic surgery, the surgical correction of osteochondral defects, mainly of the knee, represents a challenging problem to the specialist. Cartilage free grafts may be a possibility, but the sources of autogenous grafts are not appropriate, since they are not always available in the desired shape and size, while homogeneous grafts are still just a theoretical option because of the unresolved immunological problems. Therefore, the search for new treatment alternatives to promote articular cartilage regeneration has become imperative, giving rise to a variety of options, most of which involve some kind of surgical procedure. Ideally, the treatment should involve a minimally invasive procedure and the regenerated tissue should present histological and biochemical characteristics and biomechanical properties that are similar to those of the normal cartilage, including adherence to the surrounding normal cartilage and to the subchondral bone. 16 The first aspect is already possible through arthroscopy, but the second is far from ideal, as the regenerated tissue induced by most methods developed so far is invariably inferior to normal cartilage, by any comparison parameter.
Indeed, the stimulation of the subchondral bone by multiple drillings or scarification is frequently used to try to stimulate cartilage regeneration, seeing as it results in bleeding, blood clot formation, an inflammatory response and local release of growth factors, thus resulting in a granulation tissue that originates from the depths with regeneration potential. 17 However, the tissue formed by means of this sequence of events depends on the location, age and dimension of the defect and almost invariably appears as a fibrous or fibrocartilaginous tissue without any resemblance to the normal surrounding cartilage; 18 this tissue also tends to deteriorate quickly when submitted to repetitive weight-bearing. 2 , 3 In spite of some controversial findings, PRP has been used to repair defects in different tissues, especially in maxillofacial surgery and dentistry, but also in the cortical bone of the musculoskeletal system 19 and, theoretically, the articular cartilage could benefit from its regeneration stimulation properties. This was the main motivation of the present investigation, in which the participants tested the hypothesis that PRP applied locally to a deep cartilaginous lesion would produce at least partial regeneration of the lost cartilage, compared to an identical lesion left empty to regenerate spontaneously.
The experiment was conducted on a model of osteochondral defect of the articular cartilage of the trochlear groove of adult sheep knees, the dimensions of which, equivalent to about 50% of the human knee, 20 would greatly facilitate the surgical procedures and would probably correspond more closely to a similar situation in humans. 20 - 22 The osteochondral defect was produced in the trochlear groove for the simple reason that the degree of maintenance of the implant at this site is higher 18 due to the concavity of the articular surface, especially at the vertex. 23 The defect was created so as to reach the subchondral bone to cause some bleeding and consequent blood clot formation and was deep enough to retain the PRP gel in the bottom, facilitated by the normal properties of adhesion of PRP and as it is not disturbed by the sliding of the patella in the trochlear groove. The procedure did not appear to cause long-lasting discomfort to the animals, which were able of resume walking with total weight-bearing on the operated limbs early on in the second post-operative week. The only complication observed was one case of superficial infection, which was completely cured after antibiotic treatment, apparently without interfering in the outcome.
The complex process of morphogenesis whereby the different tissues are formed and repaired occurs through the differentiation of pluripotent mesenchymal cells stimulated by the different growth factors. These are peptides with the potential ability to promote differentiation and cell and tissue growth through the mediation of mitosis, chemotaxis and metabolism, thus stimulating and regulating the repair process of the various tissues, 24 with each one of the growth factors stimulating the development of a specific cell or tissue. The platelet-derived growth factors (PDGFs) have been related to the mitotic activity of the blood cells and of several tissues in serum-dependent cultures 25 and are known to initiate the spontaneous reparation of the connective issues, including those of the bone, increasing the mitoses (cell repair), angiogenesis (formation of new blood vessels) and macrophage activity (debridement). The natural repair process of any tissue is mediated by several growth factors and usually starts with the formation of the blood clot, characterized by the accumulation of platelets, which participate both in the blood coagulation process and in the initial inflammatory response through the release of cytokines. 26 , 27 This process can also occur in the articular cartilage provided the subchondral bone is injured, causing some bleeding and clot formation followed by the release of the growth factors, including PDGFs. 28 As PDGFs are highly unstable and do not last long after being injected in the bloodstream or in the tissues, PRP theoretically is an adequate vehicle for increasing their concentration inside the injured tissues. 12
PRP is defined as a concentrate containing from four to six times the quantity of platelets present in the normal total blood. It is obtained by centrifugation and its appearance and consistency are that of a gel, in which there is complete inversion of the nucleated cells/platelets ratio. 13 , 29 In reality, in addition to the concentration of platelets, the centrifugation should also eliminate the white cells, which irreversibly inhibit the synthesis of the cartilaginous matrix, injuring the cartilage structure. 30 The protocol used to obtain the PRP 7 , 31 used in this study is based on centrifugation, with separation of the plasma and of the leucocytes from the red blood cells, in a first step, and with separation of the platelet-rich from the platelet-poor plasma, in a second step. However, based on a pilot test, separation of the blood components was performed in a different manner from that suggested by preceding authors. In fact, after the first allegation, the bottom part of the centrifuged material was collected and the precipitate containing the red blood cells and the leukocytes was removed. This made it possible to obtain the inversion of the cell ratio and to concentrate the platelets above the total blood baseline level. Furthermore, the PRP obtained contained the least possible quantity of leukocytes.
Marx 7 reported that the concentration of platelets in PRP should be above the baseline value of total blood, while the ideal concentration is 1,000,000/µl in humans. Other authors 13 , 31 , 32 reported that the ideal concentration would be from 2 to 3 times higher than that of total blood. These definitions are based on the normal concentration of platelets that ranges between 150,000 and 350,000/µl. 33 According to data from the literature, the mean concentration of platelets in the total blood of sheep is 100,000/µl, 34 but in this study it was 125,000/µl (variation: 34,100 - 512,000/µl), resulting in a concentration of platelets in the PRP obtained ranging between 1.56 and 4.34 times (mean: 2.6 times) the normal values. Therefore, the PRP obtained was within very good limits of concentration and certainly presented stimulation properties.
There are very few studies on the use of PRP to repair the articular cartilage of the knee. Brehm et al. compared four different types of mold (scaffold), including PRP, for the implantation of autologous chondrocytes inside the defect, and demonstrated a very low PRP retention index after 4 weeks, in spite of its adhesion properties. 12 , 13 Our results indicate exactly the opposite, with a high index of PRP retention inside the defect, due to its accentuated adhesion. However, there is evidence that repair stimulation occurs very early on, during the first week, in response to the release of the growth factors through the decomposition of PRP within the first hours after implantation, as demonstrated by Marx. 7 Therefore, it is not imperative for PRP to remain inside the defect for much longer than a few days, or even hours.
Osteochondral defects measuring more than 3 mm in dia-meter in sheep knees do not heal spontaneously, as reported by Jackson et al. 21 and, later, by Saw et al. 35 for defects with a diameter of 4mm in the femoral trochlea. In this study, the defects with a diameter of 8mm were purposely used to prevent any spontaneous repair in the control group and to determine whether PRP can effectively increase the quantity and improve the quality of tissue repair in the experimental group. This hypothesis was confirmed, since the complete filling of the defect did not occur in any knee from the control group, while two knees from the study group presented complete articular surface restoration. However, no statistically significant difference was observed between the groups in relation to the macroscopic parameters studied. In fact, erosions and changes in appearance of the normal surrounding cartilage were observed in all the knees from the two groups, probably as a consequence of the surgical approach used (medial arthrotomy), which predisposes to the development of chondral abnormalities of the joint between the patella and the femoral trochlea, according to Lane et al. 20
According to the microscopic analysis, the thickness of the repair tissue was less than that of the surrounding cartilage in all the knees, thus leading to unevenness between both. However, the unevenness was more pronounced in the left untreated knees, in which the thickness of the neoformed tissue was lower than 50% of the normal value in most of the specimens. On the other hand, for the right knees the thickness was between 50 and 100% of the normal value in the majority of specimens (60%), resulting in a less pronounced unevenness. Moreover, the histological appearance of the regenerated tissue was closer to the normal cartilage for most of the right knees (7) than for most of the left knees, in which the tissue appeared clearly fibrous or, at the very most, fibrocartilaginous. Perhaps because of this, the structural integrity was more complete for most of the right knees, although the union with the surrounding normal cartilage was similar for both groups. There was no significant difference between the groups with regard to the individual evaluation of each macroscopic and histological parameter, but the general scores of the histological evaluation were significantly different between the groups. There is no reasonable explanation for the formation of fibrous tissue instead of joint cartilage after the stimulation of the subchondral bone in the tissue repair process. But paracrine factors of the local microenvironment of the osteochondral defect are possible responsible for the formation of fibrous tissue or for the inhibition of the formation of normal cartilage. 35 However, our results indicate that PRP somehow interferes positively with this mechanism, since the quality of the regenerated tissue was evidently better in the right knee.
In a study on the response of degenerative arthritis of the knee in humans to the intra-articular injection of PRP, Saito et al. 36 observed a significant improvement of clinical parameters (pain, range of motion), indicating that there are prospects of the clinical use of PRP in humans and our results seem to point in the same direction.
CONCLUSION
The authors conclude that PRP has sheep knee articular cartilage repair properties, particularly as it stimulates the formation of fibrocartilaginous tissue and may play a role of stimulating the repair of osteochondral defects of the human knee, with the advantage of being an autologous product whose preparation is a simple procedure.
Footnotes
Study conducted in the Department of Department of Biomechanics, Medicine and Rehabilitation of the Musculoskeletal System, Faculdade de Medicina de Ribeirão Preto da Universidade de Ribeirão Preto, SP, Brazil.
Citation: Carneiro MO, Barbieri CH, Barbieri Neto J. Platelet-rich plasma gel promotes regeneration of articular cartilage in knees of sheeps. Acta Ortop Bras. [online]. 2013;21(2):80-6. Available from URL: http://www.scielo.br/aob.
REFERENCES
- 1.Hunter W. Of the structure and diseases of articulating cartilages. Philos Trans. 1743 174-42:514–521. [Google Scholar]
- 2.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 3.Smith GD, Knutsen G, Richardson JB. A clinical review of cartilage repair techniques. J Bone Joint Surg Br. 2005;87(4):445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 4.Mithoefer K, McAdams TR, Scopp JM, Mandelbaum BR. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28(1):25–40. doi: 10.1016/j.csm.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.McCormick F, Yanke A, Provencher MT, Cole BJ. Minced articular cartilage-basic science, surgical technique, and clinical application. Sports Med Arthrosc. 2008;16(4):217–220. doi: 10.1097/JSA.0b013e31818e0e4a. [DOI] [PubMed] [Google Scholar]
- 6.O'Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80(12):1795–1812. [PubMed] [Google Scholar]
- 7.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Becker W, Lynch SE, Lekholm U, Becker BE, Caffesse R, Donath K, et al. A comparison of ePTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. J Periodontol. 1992;63(11):929–940. doi: 10.1902/jop.1992.63.11.929. [DOI] [PubMed] [Google Scholar]
- 9.Buckley RC, Breazeale EE, Edmond JA, Brzezienski MA. A simple preparation of autologous fibrin glue for skin-graft fixation. Plast Reconstr Surg. 1999;103(1):202–206. doi: 10.1097/00006534-199901000-00033. [DOI] [PubMed] [Google Scholar]
- 10.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 11.Zechner W, Tangl S, Tepper G, Fürst G, Bernhart T, Haas R, et al. Influence of platelet-rich plasma on osseous healing of dental implants: a histologic and histomorphometric study in minipigs. Int J Oral Maxillofac Implants. 2003;18(1):15–22. [PubMed] [Google Scholar]
- 12.Freymiller EG, Aghaloo TL. Platelet-rich plasma: ready or not? J Oral Maxillofac Surg. 2004;62(4):484–488. doi: 10.1016/j.joms.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet-rich plasma: a literature review. Implant Dent. 2009;18(4):303–310. doi: 10.1097/ID.0b013e31819e8ec6. [DOI] [PubMed] [Google Scholar]
- 14.Cook SD, Patron LP, Salkeld SL, Rueger DC. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003;85(Suppl 3):116–123. doi: 10.2106/00004623-200300003-00018. [DOI] [PubMed] [Google Scholar]
- 15.O'Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68(7):1017–1035. [PubMed] [Google Scholar]
- 16.Stone KR, Walgenbach AW, Freyer A, Turek TJ, Speer DP. Articular cartilage paste grafting to full-thickness articular cartilage knee joint lesions: a 2- to 12year follow-up. Arthroscopy. 2006 Mar;22(3):291–299. doi: 10.1016/j.arthro.2005.12.051. Erratum in: Arthroscopy. 2006;22(4):A16. [DOI] [PubMed] [Google Scholar]
- 17.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 18.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Wilson EMK, Barbieri CH, Mazzer N. Bone healing stimulation by platelet-rich plasma. An experimental study in rabbits. Acta Ortop Bras. 2006;14(4):208–212. [Google Scholar]
- 20.Lane JG, Massie JB, Ball ST, Amiel ME, Chen AC, Bae WC, et al. Follow-up of osteochondral plug transfers in a goat model: a 6-month study. Am J Sports Med. 2004;32(6):1440–1450. doi: 10.1177/0363546504263945. [DOI] [PubMed] [Google Scholar]
- 21.Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of fullthickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am. 2001;83(1):53–64. doi: 10.2106/00004623-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Brehm W, Aklin B, Yamashita T, Rieser F, Trüb T, Jakob RP, et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006;14(12):1214–1226. doi: 10.1016/j.joca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Huibregtse BA, Samuels JA, O'Callaghan MW. Development of a cartilage defect model of the knee in the goat for autologous chondrocyte implantation research. Trans Orthop Res Soc. 1999;24:797–797. [Google Scholar]
- 24.The potential role of growth and differentiation factors in periodontal regeneration. J Periodontol. 1996;67(5):545–553. [PubMed] [Google Scholar]
- 25.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 26.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 27.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Plateletrich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg WB, van der Kraan PM, Scharstuhl A, van Beuningen HM. Growth factors and cartilage repair. Clin Orthop Relat Res. 2001;(391 Suppl):S244–S250. doi: 10.1097/00003086-200110001-00023. [DOI] [PubMed] [Google Scholar]
- 29.Carlson NE, Roach RB Jr. Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc. 2002;133(10):1383–1386. doi: 10.14219/jada.archive.2002.0054. [DOI] [PubMed] [Google Scholar]
- 30.Roosendaal G, Vianen ME, Marx JJ, van den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: a human in vitro study. Arthritis Rheum. 1999;42(5):1025–1032. doi: 10.1002/1529-0131(199905)42:5<1025::AID-ANR23>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297–300. doi: 10.1016/s0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 32.Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55(11):1294–1294. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 33.George JN. Platelets. Lancet. 2000 Apr 29;355(9214):1531–1539. doi: 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- 34.Jakse N, Tangl S, Gilli R, Berghold A, Lorenzoni M, Eskici A, et al. Influence of PRP on autogenous sinus grafts. An experimental study on sheep. Clin Oral Implants Res. 2003;14(5):578–583. doi: 10.1034/j.1600-0501.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 35.Saw KY, Hussin P, Loke SC, Azam M, Chen HC, Tay YG, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic Acid: an experimental study in a goat model. Arthroscopy. 2009;25(12):1391–1400. doi: 10.1016/j.arthro.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Saito M, Takahashi KA, Arai Y, Inoue A, Sakao K, Tonomura H, et al. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol. 2009;27:201–207. [PubMed] [Google Scholar]