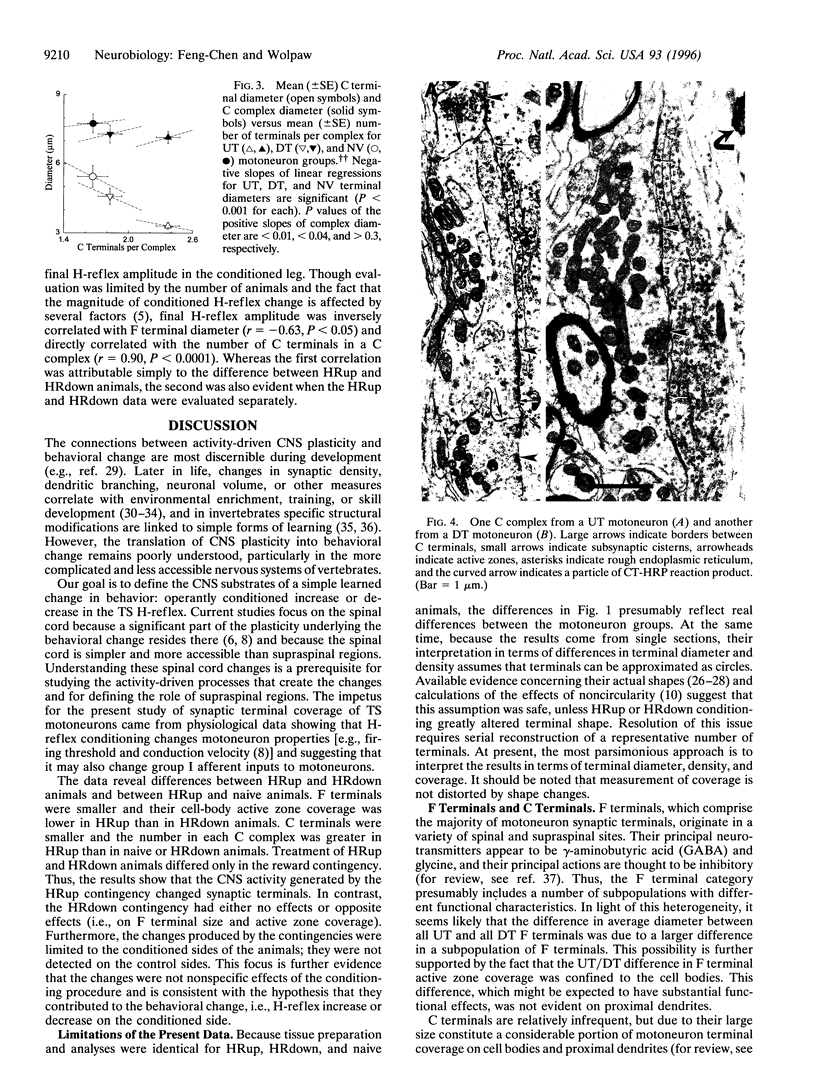
Abstract
Operant conditioning of the primate triceps surae H-reflex, the electrical analog of the spinal stretch reflex, creates a memory trace that includes changes in the spinal cord. To define the morphological correlates of this plasticity, we analyzed the synaptic terminal coverage of triceps surae motoneurons from animals in which the triceps surae H-reflex in one leg had been increased (HRup mode) or decreased (HRdown mode) by conditioning and compared them to each other and to motoneurons from unconditioned animals. Motoneurons were labeled by intramuscular injection of cholera toxin-horseradish peroxidase. A total of 5055 terminals on the cell bodies and proximal dendrites of 114 motoneurons from 14 animals were studied by electron microscopy. Significant differences were found between HRup and HRdown animals and between HRup and naive (i.e., unconditioned) animals. F terminals (i.e., putative inhibitory terminals) were smaller and their active zone coverage on the cell body was lower on motoneurons from the conditioned side of HRup animals than on motoneurons from the conditioned side of HRdown animals. C terminals (i.e., terminals associated with postsynaptic cisterns and rough endoplasmic reticulum) were smaller and the number of C terminals in each C complex (i.e., a group of contiguous C terminals) was larger on motoneurons from the conditioned side of HRup animals than on motoneurons either from the conditioned side of HRdown animals or from naive animals. Because the treatment of HRup and HRdown animals differed only in the reward contingency, the results imply that the two contingencies had different effects on motoneuron synaptic terminals. In combination with other recent data, they show that H-reflex conditioning produces a complex pattern of spinal cord plasticity that includes changes in motoneuron physiological properties as well as in synaptic terminals. Further delineation of this pattern should reveal the contribution of the structural changes described here to the learned change in behavior.
Full text
PDF

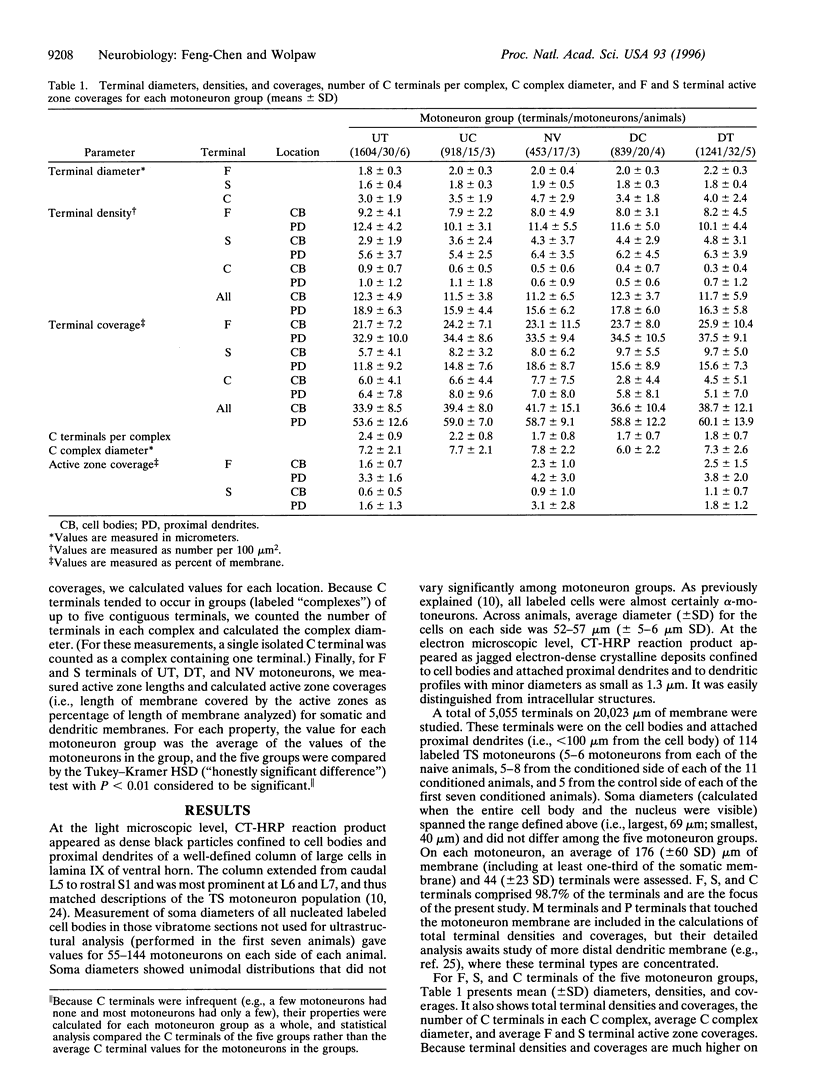
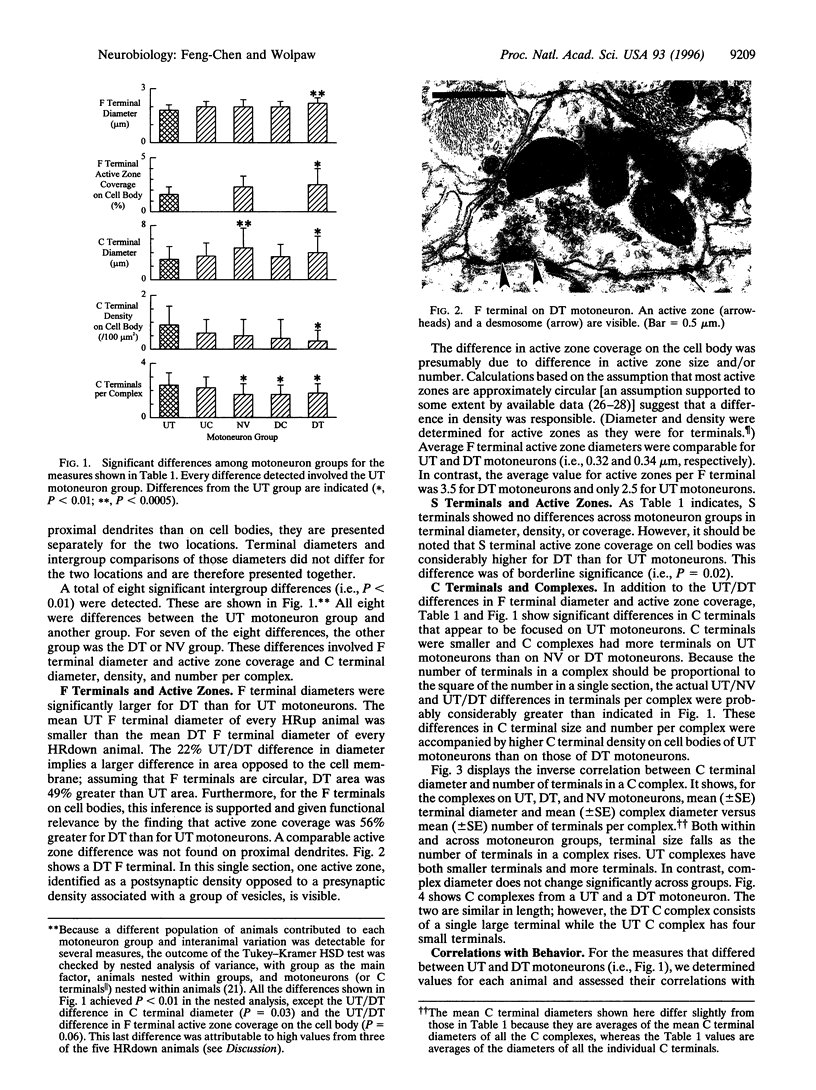

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Ikeno H., Dworkin J., McPhie D. L., Olds J. L., Lederhendler I., Matzel L., Schreurs B. G., Kuzirian A., Collin C. Contraction of neuronal branching volume: an anatomic correlate of Pavlovian conditioning. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1611–1614. doi: 10.1073/pnas.87.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Morphological aspects of synaptic plasticity in Aplysia. An anatomical substrate for long-term memory. Ann N Y Acad Sci. 1991;627:181–196. doi: 10.1111/j.1749-6632.1991.tb25924.x. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Black J. E., Isaacs K. R., Anderson B. J., Alcantara A. A., Greenough W. T. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard D. M., Brontë-Stewart H. M., Lisberger S. G. Expression of motor learning in the response of the primate vestibuloocular reflex pathway to electrical stimulation. J Neurophysiol. 1992 Jun;67(6):1493–1508. doi: 10.1152/jn.1992.67.6.1493. [DOI] [PubMed] [Google Scholar]
- Carp J. S. Physiological properties of primate lumbar motoneurons. J Neurophysiol. 1992 Oct;68(4):1121–1132. doi: 10.1152/jn.1992.68.4.1121. [DOI] [PubMed] [Google Scholar]
- Carp J. S., Wolpaw J. R. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994 Jul;72(1):431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Carp J. S., Wolpaw J. R. Motoneuron properties after operantly conditioned increase in primate H-reflex. J Neurophysiol. 1995 Apr;73(4):1365–1373. doi: 10.1152/jn.1995.73.4.1365. [DOI] [PubMed] [Google Scholar]
- Chen X. Y., Wolpaw J. R. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol. 1995 Jan;73(1):411–415. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- Conradi S. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand Suppl. 1969;332:5–48. [PubMed] [Google Scholar]
- Frost W. N., Clark G. A., Kandel E. R. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988 Jun;19(4):297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Frost W. N., Kandel E. R. Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J Neurophysiol. 1995 Jun;73(6):2413–2427. doi: 10.1152/jn.1995.73.6.2413. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Juraska J. M., Volkmar F. R. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav Neural Biol. 1979 Jul;26(3):287–297. doi: 10.1016/s0163-1047(79)91278-0. [DOI] [PubMed] [Google Scholar]
- Halter J. A., Carp J. S., Wolpaw J. R. Operantly conditioned motoneuron plasticity: possible role of sodium channels. J Neurophysiol. 1995 Feb;73(2):867–871. doi: 10.1152/jn.1995.73.2.867. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J. The fine structure of neurons and synapses in the motor nuclei of the cat spinal cord. J Comp Neurol. 1972 Apr;144(4):429–460. doi: 10.1002/cne.901440404. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Reassessing the mechanisms and origins of vocal learning in birds. Trends Neurosci. 1991 May;14(5):206–211. doi: 10.1016/0166-2236(91)90107-6. [DOI] [PubMed] [Google Scholar]
- Olucha F., Martínez-García F., López-García C. A new stabilizing agent for the tetramethyl benzidine (TMB) reaction product in the histochemical detection of horseradish peroxidase (HRP). J Neurosci Methods. 1985 Apr;13(2):131–138. doi: 10.1016/0165-0270(85)90025-1. [DOI] [PubMed] [Google Scholar]
- Perrett S. P., Ruiz B. P., Mauk M. D. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993 Apr;13(4):1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. P., Lewin G. R. An ultrastructural size principle. Neuroscience. 1994 Feb;58(3):441–446. doi: 10.1016/0306-4522(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Pierce J. P., Mendell L. M. Quantitative ultrastructure of Ia boutons in the ventral horn: scaling and positional relationships. J Neurosci. 1993 Nov;13(11):4748–4763. doi: 10.1523/JNEUROSCI.13-11-04748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye D. B., Saper C. B., Wainer B. H. Stabilization of the tetramethylbenzidine (TMB) reaction product: application for retrograde and anterograde tracing, and combination with immunohistochemistry. J Histochem Cytochem. 1984 Nov;32(11):1145–1153. doi: 10.1177/32.11.6548485. [DOI] [PubMed] [Google Scholar]
- Starr K. A., Wolpaw J. R. Synaptic terminal coverage of primate triceps surae motoneurons. J Comp Neurol. 1994 Jul 15;345(3):345–358. doi: 10.1002/cne.903450303. [DOI] [PubMed] [Google Scholar]
- Wolpaw J. R., Carp J. S. Adaptive plasticity in spinal cord. Adv Neurol. 1993;59:163–174. [PubMed] [Google Scholar]
- Wolpaw J. R., Herchenroder P. A., Carp J. S. Operant conditioning of the primate H-reflex: factors affecting the magnitude of change. Exp Brain Res. 1993;97(1):31–39. doi: 10.1007/BF00228815. [DOI] [PubMed] [Google Scholar]
- Wolpaw J. R., Herchenroder P. A. Operant conditioning of H-reflex in freely moving monkeys. J Neurosci Methods. 1990 Feb;31(2):145–152. doi: 10.1016/0165-0270(90)90159-d. [DOI] [PubMed] [Google Scholar]
- Wolpaw J. R., Lee C. L. Memory traces in primate spinal cord produced by operant conditioning of H-reflex. J Neurophysiol. 1989 Mar;61(3):563–572. doi: 10.1152/jn.1989.61.3.563. [DOI] [PubMed] [Google Scholar]
- Wolpaw J. R. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol. 1987 Feb;57(2):443–459. doi: 10.1152/jn.1987.57.2.443. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hertzberg E. L., Nagy J. I. Subsurface cisterns in alpha-motoneurons of the rat and cat: immunohistochemical detection with antibodies against connexin32. Synapse. 1991 Jun;8(2):119–136. doi: 10.1002/syn.890080206. [DOI] [PubMed] [Google Scholar]
- Yeow M. B., Peterson E. H. Active zone organization and vesicle content scale with bouton size at a vertebrate central synapse. J Comp Neurol. 1991 May 15;307(3):475–486. doi: 10.1002/cne.903070310. [DOI] [PubMed] [Google Scholar]