Abstract
OBJECTIVES:
To identify the prevalence of erbB-2 and vascular endothelial growth factor (VEGF) in osteosarcoma biopsies and to correlate them with possible prognosis factors.
METHODS:
Retrospective study conducted at the Hospital do Câncer de Barretos-SP including 27 osteosarcoma biopsies immunohistochemically stained for VEGF and erbB-2. The pathological characteristics were collected from medical records of patients to correlate with markers.
RESULTS:
In 27 biopsies, four overexpressed VEGF and three overexpressed erbB-2. Two thirds of patients had no metastases. Almost all patients with overexpression of VEGF showed metastases. Overexpression of erbB-2 was inversely related to the presence of metastases. There was no significant association between markers and prognosis.
CONCLUSION:
We identified a low prevalence of erbB-2 and VEGF in the sample. There was no significant association between overexpression of markers and pathological features. A larger sample and a longer follow-up, in addition to using new laboratory techniques can determine the real expression of VEGF and erbB-2 and its role in osteosarcoma. Level of Evidence III, Case-Control Study.
Keywords: Vascular endothelial growth factor A; Genes, erbB-2; Osteosarcoma; Immunohistochemistry
INTRODUCTION
Osteosarcoma is a type of aggressive bone cancer of mesenchymal origin generally found in youths aged between 10 and 25 years. Any bone of the human body can be affected by this neoplasia and the general survival at five years is approximately 65 to 75%. The main causes of death are pulmonary metastases diagnosed by computed tomography (CT) in 35 to 45% of the patients. 1 Several protocols have been developed in recent decades, including chemotherapy in high doses and new techniques for resections with oncological margin and reconstructions to preserve extremities. Despite the evolution in the field of oncology, the search for better results in survival curves is still a challenge, especially in patients with metastases. 2 - 5
Tumor growth depends on the proliferation of new blood vessels in their interior, which are directly and indirectly related to the release of several growth factors both by the primary tumor as by the resulting metastatic lesions. The identification of tumor markers has been a motive for research with the objective of identifying and stratifying patients with high or low risk of developing metastases. Overexpression of Vascular Endothelial Growth Factor (VEGF) and of Human Epidermal Growth Factor Receptor 2 (HER-2) has evidenced conflicting results with regard to prognosis in individuals with osteosarcoma. 4 - 10
Vascular endothelial growth factor (VEGF) is a homodimeric protein that increases vascular endothelial permeability and stimulates the proliferation of endothelial cells. This protein is directly involved in the angiogenesis process, which is responsible for neovascularization, tumor growth and dissemination of metastases. Neovascularization is evident in histological samples of classical osteosarcomas, which reflects the aggressiveness and the metastatic potential of this type of cancer. 4 , 9 , 11
In tumors of the gastrointestinal tract such as gastric carcinoma, esophageal tumors and colorectal carcinomas, overexpression of VEGF suggests a direct relationship with a worse prognosis. However, clinical evidence is not yet well established between expression of this angiogenesis marker and survival in osteosarcoma patients. 2 , 4 , 5
The HER-2 oncogene (ErbB-2 or neu) is responsible for the encoding of a transmembrane glycoprotein called HER-2, which is overexpressed in 20 to 40% of breast cancer patients, being associated with a worse prognosis. 12 This biological marker has also been related to lower survival, poor response to chemotherapy and the presence of metastases in some studies involving osteosarcoma. 6 , 8 - 10
This historical cohort has the goal of identifying the prevalence of VEGF and HER-2 in osteosarcoma biopsies prior to chemotherapy and of correlating the overexpression of these markers with clinical and pathological characteristics involved in the prognosis.
PATIENTS AND METHODS
Patients and histological samples
In this retrospective study 27 patients with osteosarcoma had their medical records reviewed and their biopsies stained for VEGF and HER-2. The samples belonged to patients diagnosed between 2005 and 2009. All the samples were fixed in 10% formalin, embedded in paraffin blocks and stored in the pathology department of Hospital de Câncer de Barretos, Brazil. The criteria for inclusion were patients of both sexes, aged between 5 and 30 years, with a diagnosis of osteosarcoma without previous treatment, besides presence or absence of metastases. Treatment protocol violation was considered a study exclusion criterion. Clinical and pathological characteristics such as age, sex, histological subtype, end of protocol, response to neoadjuvant chemotherapy and the presence of metastases were analyzed and tabulated. This study was approved by the Institutional Review Board (CEP #195/2009).
Immunohistochemical technique
All the samples were embedded in paraffin blocks and sectioned in thicknesses of 2 to 3 micra in a standardized microtome then immunohistochemically stained using the avidin-biotin-peroxidase complex method. The VEGF primary antibody was a rat anti-human monoclonal antibody diluted at 1:50 (DakoCitomation, Denmark, A/S), and for HER-2, the rabbit antihuman polyclonal antibody diluted at 1:200 (DakoCitomation, Denmark, A/S). Antigen recovery was performed through incubation in humid heat in a citrate buffer (Dako - 10 mM, pH 9 and 90°C). The reaction amplification was carried out in the Dakoautostainer (Universal Staining System - Dako). The positive and negative controls were used for each test.
Immunohistochemical evaluation
Two pathologists (SM and CRV) analyzed the osteosarcoma samples without any information about the patients' history and used a microscope of two binocular heads simultaneously. Stained cell percentage frequencies that were almost similar were found on the slides by both professionals with a kappa index below 5%. VEGF was considered positive when more than 30% both of the cytoplasm and of the membrane of the tumor cells were stained. 4 HER-2 was evaluated through a semi-quantitative scale. The samples were graded from 0 (zero) to +3 according to the proportion of stained cells both in the cytoplasm and in the membrane. Results from zero to +1 were considered as up to 30% of the stained cells, and from +2 up to +3, as above 30%. Only immunohistochemical staining above 30% was classified as HER-2 overexpression. 8 , 13
Statistical analysis
All the variables were described by absolute and relative frequencies, except for age which was described by mean and standard deviation. To compare the groups we applied the student's t-test (age) and Fisher's exact test (other variables). To estimate the survival curves, the Kaplan-Meier method was applied and to compare them we used the log-rank test. The sample size was calculated in PEPI (Programs for Epidemiologists) version 4.0 and based on the study by Kaya et al. 4 For a significance level of 5% (p ≤ 0.05), a power of 90%, a survival proportion of 90% in the VEGF negative group and a proportion of 20% in the VEGF positive group, we obtained a minimum total of 22 patients. The analyses were performed in the SPSS (Statistical Package for the Social Sciences) program, version 18.0.
RESULTS
The results of this study are summarized in Tables 1 and 2.
Table 1. Significance of the variables compared with the expression of VEGF.
| Variables | Total sample (n=27) | VEGF negative(n=23) | VEGF positive(n=4) | p-value |
| n (%) | n (%) | n (%) | ||
| Age bracket | 0.545** | |||
| = 14 | 6 (22.2) | 6 (26.1) | 0 (0.0) | |
| > 14 | 21 (77.8) | 17 (73.9) | 4 (100) | |
| Sex | 0.106** | |||
| Male | 15 (55.6) | 11 (47.8) | 4 (100) | |
| Female | 12 (44.4) | 12 (52.2) | 0 (0.0) | |
| Histological subtype | 0.554* | |||
| Osteoblastic | 13 (48.1) | 11 (47.8) | 2 (50.0) | |
| Teleangiectatic | 4 (14.8) | 4 (17.4) | 0 (0.0) | |
| Chondroblastic | 3 (11.1) | 2 (8.7) | 1 (25.0) | |
| Fibroblastic | 3 (11.1) | 2 (8.7) | 1 (25.0) | |
| Undifferentiated | 4 (13.8) | 4 (17.4) | 0 (0.0) | |
| Metastasis | 0.065** | |||
| Yes | 8 (29.6) | 5 (21.7) | 3 (75.0) | |
| No | 19 (70.4) | 18 (78.3) | 1 (25.0) | |
| Response to Chemo (Huvos-Ayala) | 0.053* | |||
| Poor (I-II) | 15 (55.6) | 15 (65.2) | 0 (0.0) | |
| Good (III-IV) | 6 (22.2) | 4 (17.4) | 2 (50.0) | |
| N.D. | 6 (22.2) | 4 (17.4) | 2 (50.0) | |
| Protocol complete | 0.531* | |||
| Yes | 13 (48.1) | 11 (47.8) | 2 (50.0) | |
| No | 9 (33.3) | 7 (30.4) | 2 (50.0) | |
| N.D. | 5 (18.5) | 5 (21.7) | 0 (0.0) |
N.D. = Not determined; VEGF = vascular endothelial growth factor. Pearson's chi-square test
Fisher's exact test
Table 2. Significance of the variables compared with the expression of HER-2.
| Variables | Total sample (n=27) | HER-2 negative (n=24) | HER-2 positive (n=3) | p-value |
| n (%) | n (%) | n (%) | ||
| Age bracket | 0.545** | |||
| = 14 | 6 (22.2) | 5 (20.8) | 1 (33.3) | |
| > 14 | 21 (77.8) | 19 (79.2) | 2 (66.7) | |
| Sex | 0.231** | |||
| Male | 15 (55.6) | 12 (50.0) | 3 (100) | |
| Female | 12 (44.4) | 12 (50.0) | 0 (0.0) | |
| Histological subtype | 0.686* | |||
| Osteoblastic | 13 (48.1) | 11 (45.8) | 2 (66.7) | |
| Teleangiectatic | 4 (14.8) | 4 (16.7) | 0 (0.0) | |
| Chondroblastic | 3 (11.1) | 3 (12.5) | 0 (0.0) | |
| Fibroblastic | 3 (11.1) | 3 (12.5) | 0 (0.0) | |
| Undifferentiated | 4 (13.8) | 3 (12.5) | 1 (33.3) | |
| Metastasis | 1.000** | |||
| Yes | 8 (29.6) | 7 (29.2) | 1 (33.3) | |
| No | 19 (70.4) | 17 (70.8) | 2 (66.7) | |
| Response to Chemo (Huvos-Ayala) | 0.003* | |||
| Poor (I-II) | 15 (55.6) | 15 (62.5) | 0 (0.0) | |
| Good (III-IV) | 6 (22.2) | 6 (25.0) | 0 (0.0) | |
| N.D. | 6 (22.2) | 3 (12.5) | 3 (100) | |
| Protocol complete | 0.758* | |||
| Yes | 13 (48.1) | 12 (50.0) | 1 (33.3) | |
| No | 9 (33.3) | 8 (33.3) | 1 (33.3) | |
| N.D. | 5 (18.5) | 4 (16.7) | 1 (33.3) |
N.D. = Not determined; HER-2 = Epidermal growth factor type 2. Pearson's chi-square test
Fisher's exact test
Fifteen patients were male and 12 female, with average age of 13 years (seven to 27 years). The cases were divided by age into over (n=21) and under (n=6) 14, considering a greater or lesser risk of tumor aggressiveness. Thirteen individuals concluded the Brazilian protocol (GBTO), nine remained in treatment during this study 3 and five did not have data in their medical records. All osteosarcomas were staged as IIB and III according to Enneking's Staging System. 14 Eight patients (30%) presented pulmonary metastases upon diagnosis and were classified as stage III. None of the patients presented a description of non-pulmonary metastases.
The histological subtypes were described according to the WHO - Classification of Bone Tumors, 2002. 15 The classification of Huvos-Ayala was used to describe the response to neoadjuvant chemotherapy as poor (I-II) and good (III-IV). 16 More than half of the patients (56%) presented poor response to neoadjuvant chemotherapy treatment, while 22% presented good response and another 22% did not present records of anatomopathological examinations. Table 1 describes all the clinical and pathological variables analyzed. The follow-up interval was recorded from initial biopsy until July 2009. The minimum follow-up period was six months.
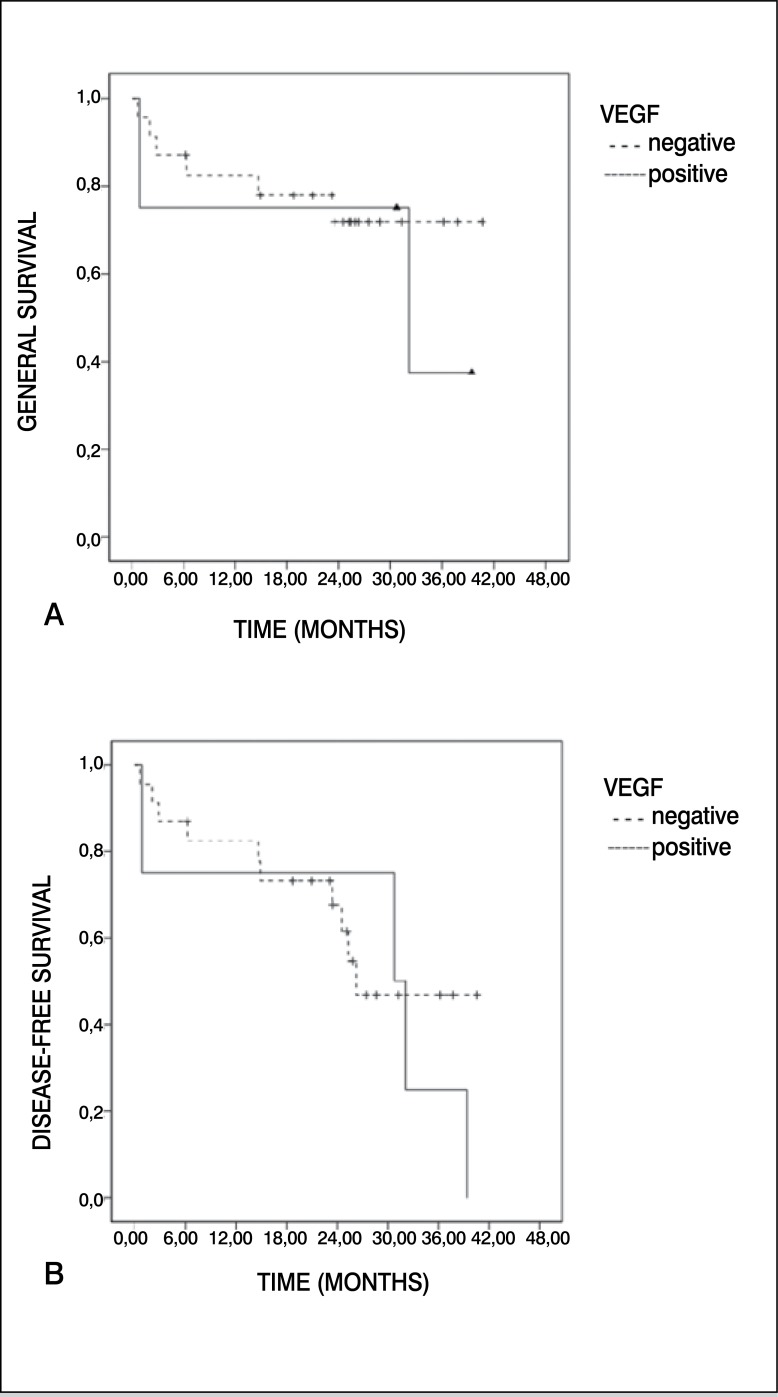
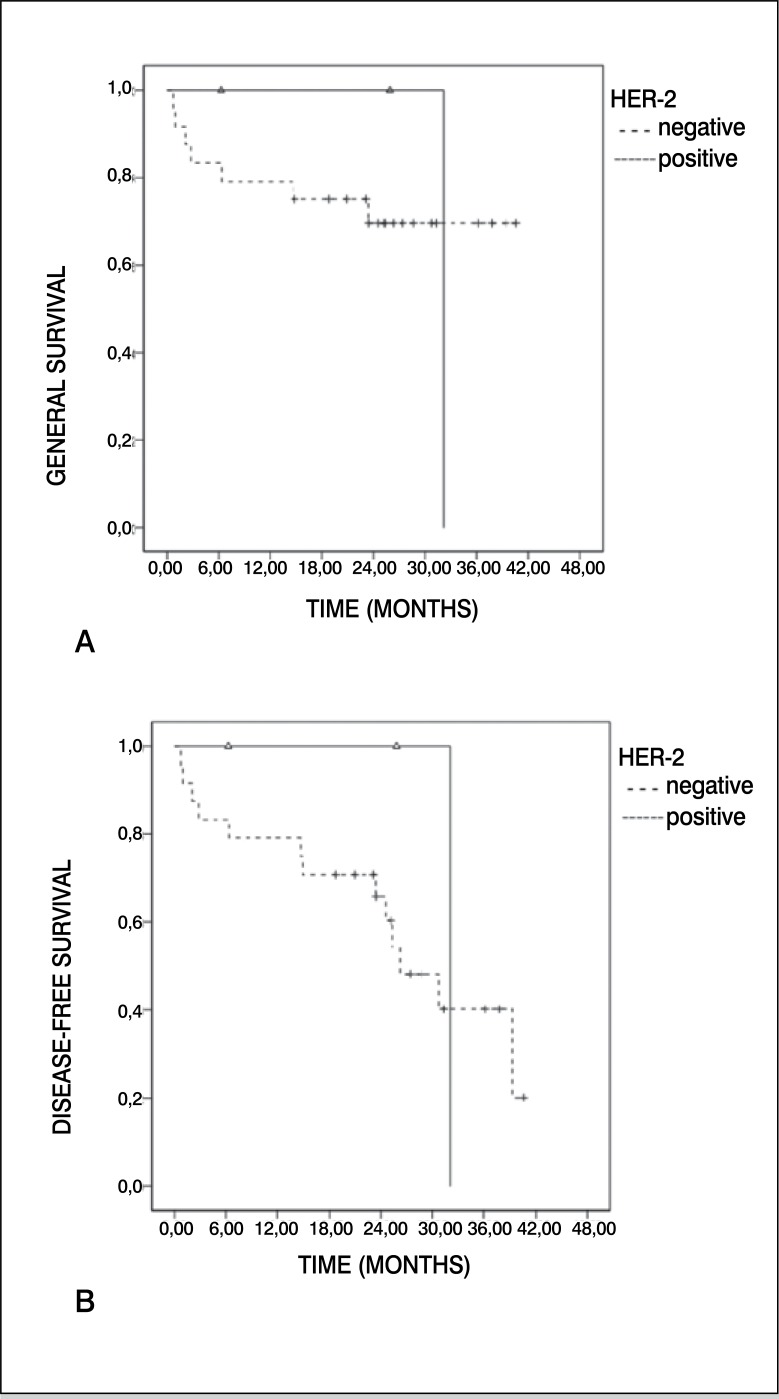
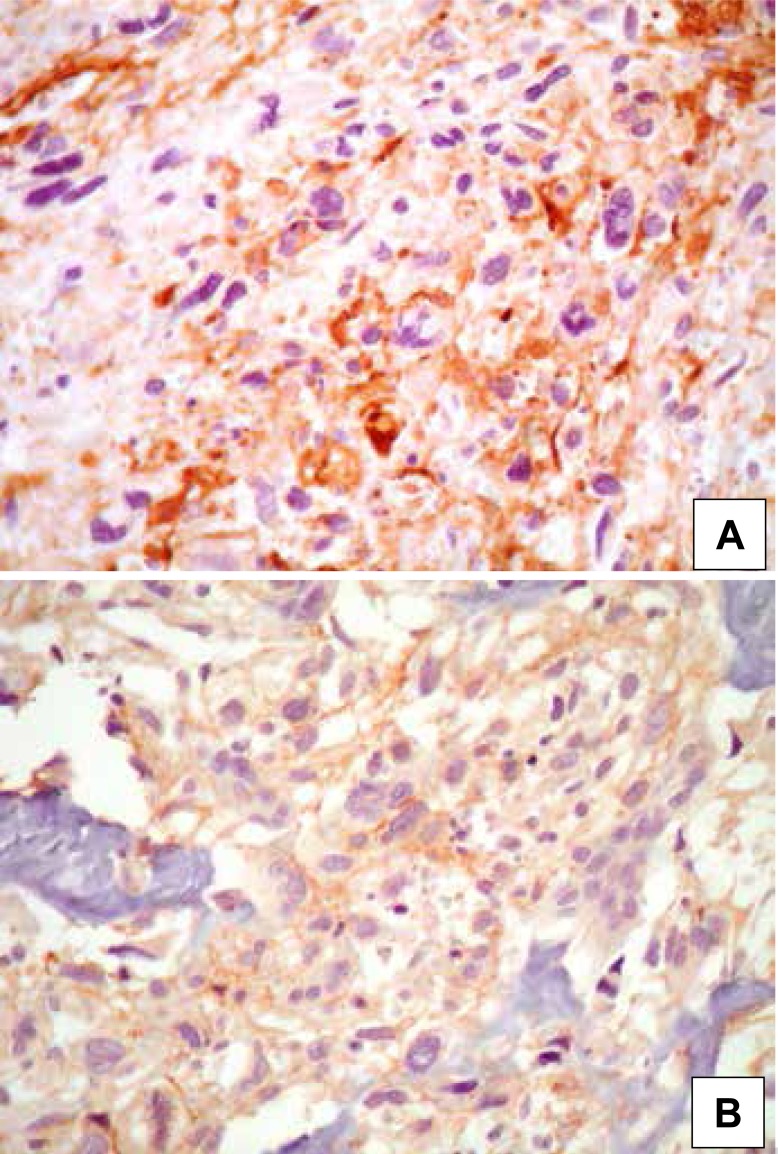
Only four samples (15%) presented overexpression of VEGF. All the samples positive for VEGF were found in the male sex, and over 14 years. Three quarters (75%) of the patients who overexpressed VEGF presented pulmonary metastases, inferring a theoretical risk for worse prognosis already described in previous publications. 4 , 7 Table 1 summarizes the correlation between VEGF and the variables analyzed. We did not find significant correlation when conducting descriptive and univariate analyses. Kaplan-Meier survival curves for VEGF are described in Figure 1. Overexpression of HER-2 was observed in three cases (11%), all male and two (67%) over 14 years of age. Pulmonary metastases were identified in only one third of the patients with HER-2 considered positive (>1+). The samples positively-stained for HER-2 did not present a description of response to chemotherapy in our medical files. We did not find significant correlation when conducting descriptive and univariate analyses. Kaplan-Meier survival curves for HER-2 are described in Figure 2. Immunohistochemistry of VEGF is demonstrated in Figure 3A, while immunohistochemistry of HER-2 is demonstrated in Figure 3B.
Figure 1. Kaplan-Meier curve for (A) general survival and (B) disease-free survival for positive cases of VEGF compared with negative.
Figure 2. Kaplan-Meier curve for (A) general survival and (B) disease-free survival for positive cases of HER-2 (ErbB-2) compared with negative.
Figure 3. Photomicrography of (A) osteosarcoma with cytoplasmic positivity for VEGF (400X) and photomicrography of (B) osteosarcoma with membrane positivity for HER-2 (400X).
DISCUSSION
The low prevalence of VEGF (15%) and HER-2 (11%) in osteosarcoma biopsies analyzed through immunohistochemistry was demonstrated in this study. Different methods to quantify the presence of biological markers theoretically involved in the proliferation and dissemination of tumors have been described in recent decades. Laboratory tests such as immunohisto-chemistry, DNA and RNA testing are among the best known. Differences between research protocols including a wide variety of antibodies and of techniques without standardization for gene identification are responsible for the discrepancy in studies around the world. 15 , 17
Since HER-2 complementary DNA was isolated approximately 25 years ago, important discoveries have come to light in the mechanism of action of the receptor tyrosine kinases (RTK), which, when mutated or altered, become potent oncoproteins. In 1985, the Ullrich of Genentech group described the complete primary structure of supposed RTK that demonstrated a high degree of homology with the human Epidermal Growth Factor Receptor (EGFR), having been denominated human EGFR-related 2, or HER2 (17). Two years later, Slamon et al. 12 reported that HER2 was amplified in 30% of the invasive breast cancer cases and, for the first time, demonstrated significant correlation between overexpression of HER2, reduced survival and increased tumor recurrence.
In the late 1990s, two studies suggested that overexpression of HER-2 in osteosarcoma could be associated with reduced survival and the development of metastases. In one of these studies, 26 osteosarcoma samples were analyzed for HER-2 through immunohistochemistry, and overexpression of HER-2 was identified in 42% of the samples. However, the metastatic patients were included in the same sample and the chemotherapy protocols were not adequately described. Finally, patients in stages IIB and III should have been analyzed separately for HER-2, as it is common knowledge that the prognosis of metastatic individuals (III) is inferior to that of non-metastatic individuals (IIB). 6 In another study, 53 osteosarcoma samples were analyzed, while all patients with metastases (stage III) were excluded. Neither data regarding general survival, nor any description of uni- or multivariate analyses were described. 10
In 2002, Akatsuka et al. 8 proposed a different explanation for the association between overexpression of HER-2 and the clinical findings in osteosarcoma, comparing HER-2 levels between samples of biopsies, of tumors resected after neoadjuvant chemotherapy and of pulmonary metastases in 19 patients not metastatic upon diagnosis. The disappearance of HER-2 was visible during the treatment with chemotherapy in 14 of 19 patients (74%) as they became metastatic. These findings suggest that overexpression of HER-2 does not play an important role in the development of metastases, and that tumors where HER-2 is overexpressed will benefit more from chemotherapy in relation to general, disease-free survival when compared to those with negative HER-2.
Expression of VEGF has been used as a more objective marker to assess the importance of angiogenesis in solid tumors such as osteosarcoma. A study with 30 patients identified that VEGF mRNA was expressed in all the tumor samples, and in 80% in VEGF165 isoform. Only 20% expressed VEGF121 isoform. 83% of those testing VEGF165-positive developed metastases, while this occurred in only 16% of those testing VEGF165-negative. In the same groups, VEGFR-1 and 2 (type 1 and 2 VEGF receptors) were expressed in more than half the patients, yet the expression was not correlated with the prognosis. The individuals of this study had not been undergone previous treatment and did not present metastases upon diagnosis, and VEGF was analyzed through reverse transcriptase polymerase chain reaction (RT-PCR). 7 , 18
In another study, 63% of 27 patients expressed VEGF through immunohistochemistry. Eighty-two percent of the samples where VEGF was positive developed metastases, while only 10% in the samples where VEGF was negative. As expected, the survival of the VEGF-positive individuals was inferior to that of the individuals testing negative. 4 In 2004 a North American research group described the possible relationship between the expression of VEGF in osteosarcoma biopsies and the level of vascular permeability through nuclear magnetic resonance (NMR) in 15 patients. Ten samples stained positive to immunohistochemistry, four with 1+, another four with 2+, and two more with 3+. Any positivity both in cytoplasm and in membrane was considered. The only significant finding between overexpressed VEGF and high permeability coefficient was possible when the samples were stratified, i.e., evaluated separately (0, 1+, 2+, 3+) according to the number of crosses. 19
A meta-analysis including 387 patients in 11 publications identified a rate of risk of 2.84 for VEGF-positive in relation to the risk of death when compared with the VEGF-negative. These findings, despite the heterogeneity of the publications included, suggest worse prognosis for biopsies where VEGF appeared overexpressed. 20
In our study, besides the low prevalence of immunological markers, we presented in a descriptive manner a tendency for the presence of metastases in osteosarcoma patients testing VEGF-positive and HER-2-negative. No significant correlation was found between HER-2 and VEGF with regards to general survival, free of disease and of the clinical and pathological variables. Although there is not yet any consensus in the literature, VEGF positivity and HER-2 negativity have suggested worse results in the evolution of this disease.
CONCLUSION
Overexpression of the VEGF and HER-2 proteins demonstrated low prevalence, of only 11% and 15%, respectively. In spite of the adequate methodology employed, these results are at the lower limit of current publications. Our findings, in spite of the limited significance, put up for discussion the true prevalence of VEGF and HER-2 and their possible association with the prognosis. The increase in sample size and in the follow-up period may provide more information about the relevance of these markers in osteosarcoma patients.
Footnotes
Acta Ortop Bras. [online]. 2013;21(4):233-8. Available from URL: http://www.scielo.br/aob.
Work performed at Hospital de Câncer de Barretos - Fundação Pio XII and at Hospital de Clínicas de Porto Alegre - UFRGS - Porto Alegre, RS, Brazil.
REFERENCES
- 1.Unni KK. Osteosarcoma. In: Unni KK, editor. Dahlin's bone tumors. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 122–157. [Google Scholar]
- 2. Kaya M, Wada T, Kawaguchi S, Nagoya S, Yamashita T, Abe Y, et al. Increased pre-therapeutic serum vascular endothelial growth factor in patients with early clinical relapse of osteosarcoma. Br J Cancer. 2002;86(6):864–869. doi: 10.1038/sj.bjc.6600201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petrilli AS, de Camargo B, Filho VO, Bruniera P, Brunetto AL, Jesus-Garcia R, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161–1168. doi: 10.1200/JCO.2005.03.5352. [DOI] [PubMed] [Google Scholar]
- 4. Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6(2):572–577. [PubMed] [Google Scholar]
- 5. DuBois S, Demetri G. Markers of angiogenesis and clinical features in patients with sarcoma. Cancer. 2007;109(5):813–819. doi: 10.1002/cncr.22455. [DOI] [PubMed] [Google Scholar]
- 6. Onda M, Matsuda S, Higaki S, Iijima T, Fukushima J, Yokokura A, et al. ErbB-2 expression is correlated with poor prognosis for patients with osteosarcoma. Cancer. 1996;77(1):71–78. doi: 10.1002/(SICI)1097-0142(19960101)77:1<71::AID-CNCR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7. Lee YH, Tokunaga T, Oshika Y, Suto R, Yanagisawa K, Tomisawa M, et al. Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur J Cancer. 1999;35(7):1089–1093. doi: 10.1016/s0959-8049(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 8. Akatsuka T, Wada T, Kokai Y, Kawaguchi S, Isu K, Yamashiro K, et al. ErbB2 expression is correlated with increased survival of patients with osteosarcoma. Cancer. 2002;94(5):1397–1404. doi: 10.1002/cncr.10360. [DOI] [PubMed] [Google Scholar]
- 9. Akatsuka T, Wada T, Kokai Y, Sawada N, Yamawaki S, Ishii S. Loss of ErbB2 expression in pulmonary metastatic lesions in osteosarcoma. Oncology. 2001;60(4):361–366. doi: 10.1159/000058533. [DOI] [PubMed] [Google Scholar]
- 10. Gorlick R, Huvos AG, Heller G, Aledo A, Beardsley GP, Healey JH, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol. . 1999;17(9):2781–2788. doi: 10.1200/JCO.1999.17.9.2781. [DOI] [PubMed] [Google Scholar]
- 11. Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. . 1998;77(6):956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 13. Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, et al. c-erbB-2 expression and response to adjuvanttherapy in women with node-positive early breast cancer. N Engl J Med. 1994;330(18):1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- 14. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;(153):106–120. [PubMed] [Google Scholar]
- 15.Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press; 2002. [Google Scholar]
- 16.Huvos AG. Bone tumors: diagnosis, treatment, and prognosis. 2nd ed. Philadelphia: W.B. Saunders; 1991. [Google Scholar]
- 17. Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985; 230(4730):1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 18. McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5 (Suppl 1):3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 19. Hoang BH, Dyke JP, Koutcher JA, Huvos AG, Mizobuchi H, Mazza BA, et al. VEGF expression in osteosarcoma correlates with vascular permeability by dynamic MRI. Clin Orthop Relat Res. 2004;(426):32–38. doi: 10.1097/01.blo.0000141492.52166.20. [DOI] [PubMed] [Google Scholar]
- 20. Qu JT, Wang M, He HL, Tang Y, Ye XJ. The prognostic value of elevated vascular endothelial growth factor in patients with osteosarcoma: a meta-analysis and systemic review. J Cancer Res Clin Oncol. 2012;138(5):819–825. doi: 10.1007/s00432-012-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]