Abstract
OBJECTIVE:
To analyze the possible effects of low-intensity ultrasound on induced tibia fracture of rats in a dose commonly used in physical therapy treatments.
METHODS:
Twenty male Wistar rats were divided into two groups with 10 animals each. In the ultrasound group (USG), the animals were submitted to bone fracture and treatment with therapeutic ultrasound (TUS). Ultrasonic parameters are: frequency of 1.0 MHz, intensity of 0.2 W/cm2, pulsed mode at 20%, applied in stationary form during 10 minutes on the fracture region, for five weeks. The control group (CG) was submitted to bone fracture but not treated with ultrasound.
RESULTS:
The radiographies showed better consolidation in USG compared to CG. The statistical tests for alkaline phosphatase and serum calcium did not show significant difference between groups.
CONCLUSION:
According to this study, TUS, applied with these parameters (not commonly used for bone therapy) accelerates bone healing, confirmed by radiography, yet the biochemical analysis was not conclusive. One reason for this inconsistency may have been some inadequacy of the biochemical protocol, currently under investigation. Level of Evidence II, Prospective comparative study.
Keywords: Fracture healing, Ultrasonic therapy, Physical therapy, Tibia, Rats
INTRODUCTION
Fracture healing is a complex process that involves cell proliferation and differentiation, chemotaxis and synthesis of extracellular matrix, 1 responsible for the reestablishment of the mechanical, and consequently functional, integrity of the bone tissue. 1 , 2 However, this bone repair process may occur slowly (retarded healing) or even fail to develop (pseudoarthrosis), resulting in the impairment or disability of individuals. 3
The electrical properties of bone tissue, particularly piezoelectricity, motivated several researchers to develop techniques that would have a repercussion on the alteration of the bone metabolism, if the consolidation did not occur in the expected time or even to accelerate this metabolism in recent fractures. 1 , 4 - 7
The acceleration of the fracture healing process by low-intensity pulsed ultrasound (US), is well documented in scientific literature. 1 , 4 , 5 , 8 However, the cellular and molecular mechanisms, triggered in treatment with therapeutic ultrasound (TUS), are still not fully understood, and neither are the parameters used in the equipment during the treatment. 1 , 3 , 7 , 9 , 10
Low-intensity pulsed TUS, in propagating as mechanical energy, reaches the bone surface and generates electrical signals that stimulate the bone metabolism. 1 , 9 , 10 The direct action of low-intensity TUS on mechanoreceptors is probable, but it can also act through the release of agonists of osteoblast, inducing their proliferation and differentiation 1 and the release of prostaglandins, through activation of the P2X7 receptor in bone cells, allowing the inflow of ions, such as calcium. 1
Specific low-intensity (30mW/cm2) pulsed ultrasound equipment, which costs around 4 times more than therapeutic ultrasound equipment, 4 , 7 is used frequently in studies on animals.
Thus, the aim of this study is to ascertain the possible effects of low-intensity US, used in physical therapy treatments, on induced fracture of rat tibiae. The originality of the present study lies in the investigation of an intensity that is common in commercial ultrasound equipment in Physiotherapy, since the specific equipment uses 30mW/cm2.
MATERIALS AND METHODS
Ethical regulations
The study was conducted, after approval by the Institutional Review Board of Universidade do Estado do Pará, under protocol n.13/2009, in compliance with the precepts of Colégio Brasileiro de Experimentação Animal (COBEA) and with the Brazilian National Animal Vivisection Legislation in force (Federal Law 11,794 of October 8, 2008).
Samples
The study subjects were twenty male Wistar rats (Rattus novergicus albinus) from the Vivarium of UFPa (Universidade Federal do Pará), weighing between 300 to 350g or minimum age of 90 days. After a 15-day period of adaptation to the Experimental Surgery Laboratory (LCE) of Universidade do Estado do Pará (UEPA), they were accommodated in cages measuring 45 x 15 x 30cm, with the bottom covered with autoclavable rice straw, changed on alternate days, in a controlled environment and receiving water and feed ad libitum. The animals were randomly distributed into 2 groups, with 10 animals each. (Figure 1) In the ultrasound group (USG), the animals were submitted to bone injury and treatment with low-intensity TUS. In the control group (CG), the animals were submitted to bone injury and were not treated with TUS.
Figure 1. Organization chart of the groups.
Establishment of the fracture
The 20 rats were weighed on digital scales then anesthetized with Ketamine(r) (0.9 mL/kg) and Xylazine(r) (0.5 mL/kg), administered intraperitoneally. After clinical confirmation of anesthesia, the animals were positioned in right lateral decubitus and had the right tibia fractured in the middle third, with the fracture equipment adapted 11 from the system described by Matheus et al. 12 , (Figure 2) which caused a closed fracture. There was no type of immobilization of the segment or pharmacological treatment afterwards. 13
Figure 2. Equipment for causing traumatic injury of small rodents, composed of a wooden base (A), 40 cm high metal rod (B), device for grading the energy to be released for the injury (C), 460g soft metal bar (D) and blunt tip (E).
Treatment with ultrasound
Before the start of the treatment, the TUS equipment was gauged and calibrated by the Electrical Engineering sector of Universidade Federal do Pará. The treatment with therapeutic ultrasound began 24 hours after the induction of the bone injury. The SONACEL PLUS(r) model BIOSET ultrasound apparatus was applied at the fracture site, with frequency of 1 MHz, intensity of 0.2W/cm2 (SATA), pulsed mode, duty cycle of 20%, and ERA (Effective Radiating Area) of 0.8cm2. Commercial water-soluble ultrasound gel was used as a contact material. The treatment was carried out for 10 minutes, once a day, over five consecutive days and two days of interval, until 25 sessions were completed, simulating the physical therapy treatment.
Post-treatment procedure
On the 34th day, the animals were once again anesthetized with Ketamine(r) (0.9 mL/kg) and Xylazine(r) (0.5 mL/kg), administered intraperitoneally. After the anesthesia, the animals were submitted to exsanguination through cardiac puncture (5 mL) and then decapitated. The hind limb of each animal was carefully removed and submitted to radiological and biochemical analysis.
Radiological analysis
The evaluation of the bone injury was performed using the same radiographic technique (40kV x 2mAs) and always at the same distance from the X-ray tube (1m).
Biochemical analysis
The levels of alkaline phosphatase (ALP) and serum calcium (SC) in the animals' blood were determined after 12 hours of fasting on the 34th day after establishment of the fracture. After coagulation, the serum was separated by centrifugation in Eppendorf tubes at 1000G.
The serum ALP dosing was performed with a laboratory kit (Labtest) and enzyme activity was estimated through absorbance at 590 nm. The SC was dosed using the laboratory kit (Labtest) and its concentration was estimated through absorbance at 570 nm. Both analyses were carried out in the automated Labtest system - VITALAB SELECTRA E(r) (Vital Scientific N.V.) and analyzed in the biochemistry laboratory of UEPA.
Statistical analysis
The statistical analysis consisted of the application of the Student's t-test, considering a significance level α=0.05, using Bioestat 5.0 software.
RESULTS
The study subjects were 20 male Wistar rats (Rattus novergicus albinus), with average age of 150 days and bodyweight of 284.95±48g, (Table 1), with 18 rats constituting the final sample: CG (n=8) and USG (n=10). The sudden death of 2 animals occurred in the CG prior to the start of treatment.
Table 1. Data on the weight of each rat from the sample (n=20), in grams.
| Rats | Weight (in grams) |
|---|---|
| 1 | 250 |
| 2 | 269 |
| 3 | 330 |
| 4 | 350 |
| 5 | 340 |
| 6 | 220 |
| 7 | 270 |
| 8 | 370 |
| 9 | 245 |
| 10 | 350 |
| 11 | 335 |
| 12 | 250 |
| 13 | 250 |
| 14 | 310 |
| 15 | 240 |
| 16 | 240 |
| 17 | 250 |
| 18 | 260 |
| 19 | 240 |
| 20 | 330 |
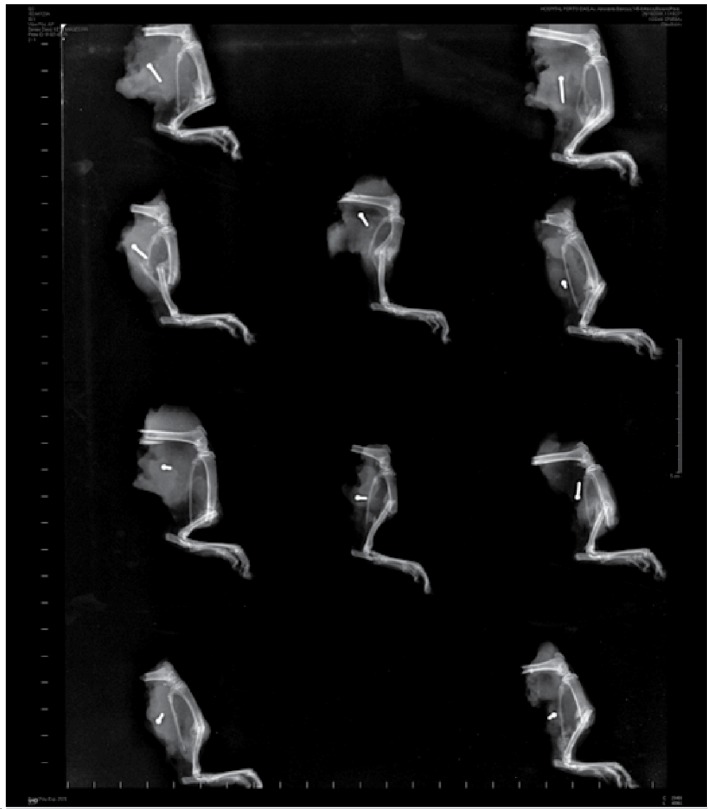
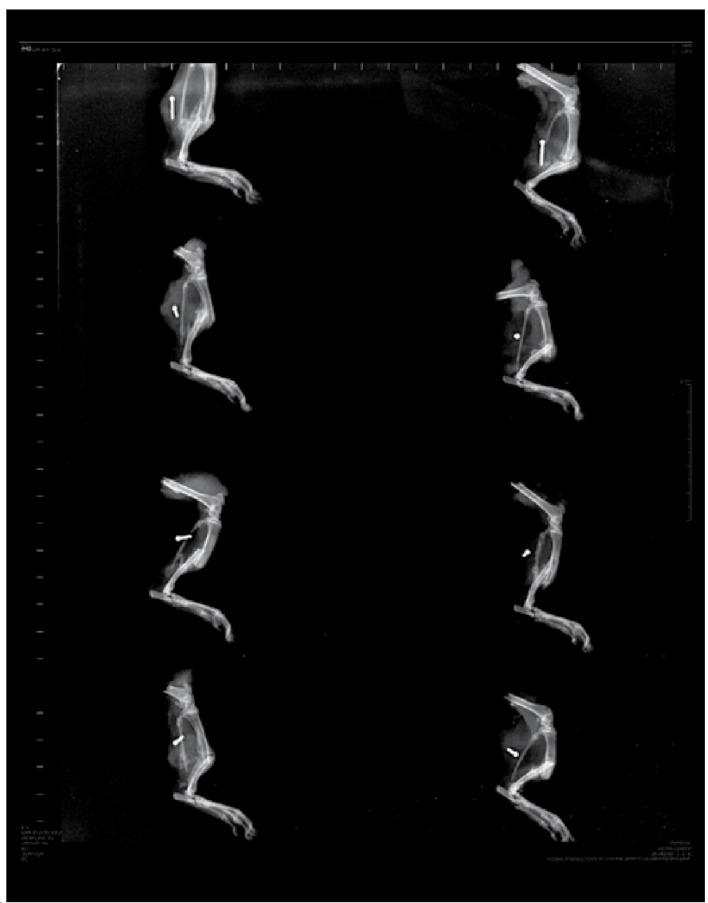
Transverse diaphyseal fracture of middle third of the tibia and absence of signs of osteomyelitis were observed in the radiographs in both groups. Bone callus in formation is observed in USG, (Figure 3) while interpenetration of fragments is exhibited in CG, characterizing them as impacted fractures. (Figure 4) This therefore defines the acceleration of consolidation in USG in comparison to CG. As regards the data from the biochemical analysis, it was possible to evaluate two markers for bone remodeling: ALP and SC. (Table 2) In relation to the analysis of ALP and SC, no statistically significant effect was evidenced. (Table 3)
Figure 3. Radiography of USG, evidencing transverse in diaphyseal fracture of the middle third of the tibia.
Figure 4. Radiography of CG, evidencing diaphyseal fracture in the middle third of the tibia.
Table 2. SC and ALP results of the sample, USG (n = 9), CC (n= 8).
| Group/rats | Serum calcium (mg/dL) | Alkaline phosphatase (U/L) |
|---|---|---|
| USG 1 | 14.59 | 353 |
| USG 3 | 14.93 | 335 |
| USG 4 | 16.73 | 419 |
| USG 5 | 14.41 | 630 |
| USG 6 | 14.20 | 775 |
| USG 7 | 14.39 | 343 |
| USG 8 | 14.53 | 306 |
| USG 9 | 14.73 | 354 |
| USG 10 | 14.31 | 312 |
| CG 1 | 13.74 | 290 |
| CG 2 | 14.39 | 649 |
| CG 3 | 14.34 | 322 |
| CG 4 | 14.71 | 228 |
| CG 5 | 15.08 | 220 |
| CG 6 | 15.13 | 468 |
| CG 7 | 14.90 | 612 |
| CG 8 | 14.98 | 533 |
Table 3. Comparative data of USG (n=9) and of CC (n=8), in relation to the variables SC and ALP.
| Biochemical analysis | USG | CG | p* | ||
|---|---|---|---|---|---|
| Mean | sd | Mean | sd | ||
| Serum calcium | 14.76 | 0.77 | 14.66 | 0.48 | 0.76 |
| Alkaline phosphatase | 425.22 | 164.52 | 415.25 | 172.20 | 0.91 |
| p ≥ 0.05 (Student's t-test). | |||||
p ≥ 0.05 (Student's t-test).
DISCUSSION
The treatment with ultrasound has been widely used in bone repair. However, there is still controversy over its biological potentials and its effects on tissue repair, and its use is often neglected or based on practical experience, which results in erroneous procedures. 3
The small temperature increase produced by TUS has a repercussion on the action of some enzymes, namely, matrix metalloproteinase-1 and collagenase. 1 , 14 Accordingly, TUS can serve to effectively reestablish or normalize metabolic temperatures in the tissue healing regions.
Moreover, the treatment with low-intensity pulsed TUS is a good stimulator of the different cells of the osseous system, 1 accelerating the healing of the clinical fracture and increasing bone formation through osteoblast activity. 1 , 15 In addition, it increases the activity of ALP and SC. 1 , 8 , 16 These actions thus enable the use of pulsed ultrasound in therapeutic applications. 1 In this study, the bone of choice was the tibia, as it is the most frequently fractured long bone and associated with a high incidence of fracture healing retardation and bone nonunion. 2 The fracture was initially executed in a pilot study, making a transverse cut in the tibia with a scalpel. Once the osteotomy was confirmed, the fracture site was reduced and the skin was sutured. After the surgery, the area was disinfected with bactericide agent.
Five days after the osteotomy, it was observed that the animals evolved to develop infection in the sectioned area and compartment syndrome, which was also described in other studies. 13 In an attempt to avoid this syndrome, a new study model was subsequently applied with immobilization using a plaster splint. However, the experiment was not successful, as the animals also developed compartment syndrome, corroborating the findings of other authors. 13 , 17 Another problem identified was the presence of infection at the site of the surgical incision, which would alter the bone healing process. 13 For this reason, the closed fracture model is justified as it greatly reduces the risks of infection, besides causing minimum damage to soft tissues. 13 , 18 Zacharias et al. 17 assert that models for the production of fractures by invasive means can lead to complications such as suture dehiscence and, consequently, deep infection, as was the case in their study.
Due to the controversy over the fracture method, it was proposed that equipment adapted 11 from the system described by Matheus et al. 12 be created, obtaining low-cost, easily reproducible handmade equipment, which standardizes the fracture. It was tested previously and successfully on 16 female and 18 male Wistar rats (Rattus novergicus albinus), with average age of 90 days and mean bodyweight of 250g. This equipment was used to obtain the fractures in this study.
Invasive fracture stabilization methods can also result in complications, which happened to Pelker and Friedlaender, 19 who used Kirschner wires and excluded 87 animals from the experiment, due to several complications, including deaths in the perioperative period and femoral fracture, during the assembly of the wires.
Complications can also occur when noninvasive methods are employed to stabilize fractures. 13 These facts, together with the different descriptions of fracture models that do not employ any type of immobilization, justify the decision of the investigators not to use them. 13 , 17 , 18
After the random distribution of the groups, USG was treated with TUS. According to the principles of Wolff's law, ultrasonic stimulation in bone repair transmits micromechanical forces and stress to the fracture site, resulting in bone formation. Consequently, several studies with TUS for the repair of injured bone tissues can be found in the literature. 1 , 2 , 12 Low-intensity pulsed therapeutic ultrasound was used as it stimulates bone metabolism in propagating as mechanical energy. 1 , 9 , 10
The intensity of 0.2W/cm2 was used as it is commonly found in commercial ultrasound equipment in Physiotherapy, since the specific equipment uses 30mW/cm2 and costs about 3 to 4 times more. The demonstration of the feasibility of using more accessible equipment would allow better diffusion of the treatment to accelerate bone healing.
Albertin 20 compared different treatment times (5, 10, 20 and 40 minutes) applying low-intensity ultrasound to the bone defects of rabbits and concluded that, with the exception of 5 minutes, the other times caused an increase in ossification. Due to this, and seeking similarities with the physical therapy treatment, the time of 10 minutes was chosen.
To elucidate the effect of the TUS, a biochemical analysis was conducted by means of the alkaline phosphatase activity (since this enzyme indicates osteoblast activity that determines bone formation or reabsorption 1 , 8 , 16 , 21 ) with an analysis of serum calcium as this indicates bone matrix synthesis process. 1 , 8 , 16 , 21 Radiographs were taken to ratify the recommendations indicated by the other exams, with an analysis of the basic parameters, such as bone callus, fracture type and location. 13
In the present study, the biochemical analysis did not reveal a statistically significant effect between the groups. This could have happened for three reasons: (I) the long period between the fracture and the biochemical analysis, since there is a tendency for both groups to present equivalent values, after a long post-fracture period; (II) the inadequacy of the biochemical protocol; or (III) the sample size was not sufficient to reveal significant difference.
The persistence of the fracture line in the radiographs up to the fifth week is consistent with data from the literature. Castro et al. 13 verified that, at 6 weeks after the fracture, the fracture line still remained clear. Utvag and Reikeras 22 verified that, 20 days after the fracture in rats, there was still a visible fracture line, while after 40 days, the line was hardly perceptible. The persistence of this continuity solution could be attributed to the absence of immobilization.
The presence of bone callus in a more accelerated process of formation in USG than in CG suggests that TUS can be used as adjuvant treatment in bone fractures. 1 A study described by Chan et al. 4 in tibia fractures in 17 rabbits (New Zealand) treated with TUS (30mW/cm2, 1.5MHz), for 20 minutes per day over 4 weeks also verified the efficacy of TUS in the experimental group, when being analyzed by radiography in the 2nd and 4th weeks, proving more effective in the initial phase of the treatment. According to the findings of the present study, a survey conducted by Takikawa et al. 5 with rats, inducing pseudoarthrosis with the promotion of low-intensity ultrasound treatment (30mW/cm2) for 20 minutes per day over 6 weeks, discovered a significant improvement in the radiographic analysis of the group treated with ultrasound.
No complications were observed in this survey, although it is known that several factors are capable of interfering in the bone healing process, including: irradiation, form of immobilization, type of fracture, fracture line, nerve section, presence of bone tumor, soft tissue interposition and infection at the fracture site. 13 New studies are necessary to expand knowledge of the effect of therapeutic ultrasound on the bone tissue. Hence it is suggested that the bone healing process evaluation analysis method be adopted throughout the treatment period, using consolidation process analysis methods such as scanning electron microscopy, histopathological analysis, ultrasound diagnosis and histomorphometry.
CONCLUSION
Intervention by means of low-intensity pulsed TUS (0.2 W/cm2), with a duty cycle of 20%, applied in stationary form during 10 minutes in the fracture region, for 5 weeks, accelerated healing, confirmed by radiography. The biochemical analysis did not reveal significant difference between the groups, but the levels of ALP and SC were higher in USG. Therefore, these data suggest that therapeutic ultrasound (in doses differing from those of the equipment normally used for this therapy) can accelerate the bone healing process.
Footnotes
Study conducted at the Laboratory of Experimental Surgery of Universidade do Estado do Pará - Belém, PA, Brazil.
Citation: Fontes-Pereira AJ, Teixeira RC, Oliveira AJB, Pontes RWF, Barros RSM, Negrão JNC. The effect of low-intensity therapeutic ultrasound in induced fracture of rat tibiae. Acta Ortop Bras. [online]. 2013;21(1):18-22. Available from URL: http://www.scielo.br/aob.
REFERENCES
- 1.Alvarenga EC, Rodrigues R, Caricati-Neto A, Silva-Filho FC, Paredes-Gamero EJ, Ferreira AT. Low-intensity pulsed ultrasound-dependent osteoblast proliferation occurs by via activation of the P2Y receptorrole of the P2Y1 receptor. Bone. 2010;46(2):355–362. doi: 10.1016/j.bone.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Lirani APR, Larzaretti-Castro M. Evidências da ação de agentes físicos sobre o metabolismo do tecido ósseo e seus potenciais usos clínicos. Arq Bras Endocrinol Metab. 2005;49(6):891–896. doi: 10.1590/s0004-27302005000600006. [DOI] [PubMed] [Google Scholar]
- 3.Evagelista AR, Furtado CS, Vilardi JR, Alves BMO. Estudo do efeito ultra-sônico na consolidação óssea. Fisioter Bras. 2003;4(2):139–143. [Google Scholar]
- 4.Chan CW, Qin L, Lee KM, Zhang M, Cheng JC, Leung KS. Low intensity pulsed ultrasound accelerated bone remodeling during consolidation stage of distraction osteogenesis. J Orthop Res. 2006;24(2):263–270. doi: 10.1002/jor.20015. [DOI] [PubMed] [Google Scholar]
- 5.Takikawa S, Matsui N, Kokubu T, Tsunoda M, Fujioka H, Mizuno K, et al. Low-intensity pulsed ultrasound initiates bone healing in rat nonunion fracture model. J Ultrasound Med. 2001;20(3):197–205. doi: 10.7863/jum.2001.20.3.197. [DOI] [PubMed] [Google Scholar]
- 6.Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1983;101(3):153–159. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 7.Busse JW, Bhandari M, Kulkarni AV, Tunks E. The effect of low-intensity pulsed ultrasound therapy on time to fracture healinga meta-analysis. CMAJ. 2002;166(4):437–441. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang RS, Lin WL, Chen YZ, Tang CH, Huang TH, Lu BY, et al. Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone. 2005;36(2):276–283. doi: 10.1016/j.bone.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Fréz AR, Ariza D, Ferreira JRL, Alves ÉPB, Breda GR, Centenaro LA, et al. Efeito do ultra-som terapêutico contínuo em placas epifisárias de coelhos. Rev Bras Med Esporte. 2006;12(3):150–152. [Google Scholar]
- 10.Sousa VL, Alvarenga J, Padilha JG, Filho, Canola JC, Ferrigno CRA, Alves JM, et al. Ultra-som pulsado de baixa intensidade em fraturas diafisáriasaplicação clínica em cães. Ciênc Rural. 2008;38(4):1030–1037. [Google Scholar]
- 11.Pereira AJF, Matusin DP, Oliveira AJB, Pontes RWF, Kruger MAV, Pereira WCA. Instrumento para produção de fratura transversa em ossos longos de pequenos roedores. XXII Congresso Brasileiro de Engenharia Biomédica ; Tiradentes, Minas Gerais. 2010. [Google Scholar]
- 12.Matheus JP, Oliveira FB, Gomide LB, Milani JG, Volpon JB, Shimano AC. Effects of therapeutic ultrasound on the mechanical properties of skeletal muscles after contusion. Rev Bras Fisioter. 2008;12(3):241–247. [Google Scholar]
- 13.Castro PCF, Hoshino A, Brito RB, Dias J, Leônidas B, Brito JAF, et al. Estudo do processo de consolidação óssea em ratos tratados com acetaminofenavaliações radiográfica e histológica. Rev Bras Ortop. 2005;40(10):614–620. [Google Scholar]
- 14.Khanna A, Nelmes RT, Gougoulias N, Maffulli N, Gray J. The effects of LIPUS on soft-tissue healinga review of literature. Br Med Bull. 2009;89:169–182. doi: 10.1093/bmb/ldn040. [DOI] [PubMed] [Google Scholar]
- 15.Rutten S, Nolte PA, Korstjens CM, van Duin MA, Klein-Nulend J. Low-intensity pulsed ultrasound increases bone volume, osteoid thickness and mineral apposition rate in the area of fracture healing in patients with a delayed union of the osteotomized fibula. Bone. 2008;43(2):348–354. doi: 10.1016/j.bone.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Korstjens CM, Nolte PA, Burger EH, Albers GH, Semeins CM, Aartman IH, et al. Stimulation of bone cell differentiation by low-intensity Ultrasounda histomorphometric in vitro study. J Orthop Res. 2004;22(3):495–500. doi: 10.1016/j.orthres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Zacharias DPM, Waitzberg DL, Bevilacqua LR, Gonçalves EL. Modelo experimental de traumatismo osteomuscular em ratos. Acta Cir Bras. 1990;5(1):13–16. [Google Scholar]
- 18.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;(355) Suppl:S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 19.Pelker RR, Friedlaender GE. The Nicolas Andry Award-1995Fracture healing. Radiation induced alterations. Clin Orthop Relat Res. 1997;(341):267–282. [PubMed] [Google Scholar]
- 20.Albertin LM. The effect of treatment with ultrasound on the repair process in bone. Rev Bras Fisioter. 2004;8(1):1–6. [Google Scholar]
- 21.Zhang ZJ, Huckle J, Francomano CA, Spencer RG. The effects of pulsed lowintensity ultrasound on chondrocyte viability, proliferation, gene expression and matrix production. Ultrasound Med Biol. 2003;29(11):1645–1651. doi: 10.1016/j.ultrasmedbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Utvåg SE, Reikerås O. Effects of nail rigidity on fracture healingStrength and mineralisation in rat femoral bone. Arch Orthop Trauma Surg. 1998;118(1-2):7–13. doi: 10.1007/s004020050301. [DOI] [PubMed] [Google Scholar]