Abstract
Objective
Although an increasing number of hypertension-associated genetic variants is being reported, replication of these findings in independent studies has been challenging. Several genes in a human chromosome 1q linkage region have been reported to be associated with hypertension. We examined polymorphisms in three of these genes (ATP1B1, RGS5 and SELE) in relation to hypertension and blood pressure in a cohort of African–Americans.
Methods
We genotyped 87 single nucleotide polymorphisms (SNPs) from the ATP1B1, RGS5 and SELE genes in a well characterized cohort of 968 African–Americans and performed a case–control study to identify susceptibility alleles for hypertension and blood pressure regulation. Single SNP and haplotype association testing was done under an additive genetic model with adjustment for age, sex, BMI and ancestry-by-genotype (principal components).
Results
A total of 12 SNPs showed nominal association with hypertension and/or blood pressure. The strongest signal for hypertension was for rs2815272 in the RGS5 gene (P = 9.3 × 10–3). For SBP, rs3917420 in the SELE gene (P = 9.0 × 10–4) and rs4657251 in the RGS5 gene (P = 9.7 × 10–3) were the top hits. Effect size for each of these variants was approximately 2–3 mmHg. A five-SNP haplotype in the SELE gene also showed significant association with SBP after correction for multiple testing (P < 0.01).
Conclusion
These findings provide additional support for the genetic role of ATP1B1, RGS5 and SELE in hypertension and blood pressure regulation.
Keywords: African–Americans, candidate gene, haplotype, hypertension, single nucleotide polymorphism
Introduction
Essential hypertension, characterized by sustained elevated blood pressure (BP) with no identifiable cause, is a public health burden worldwide [1]. The condition affects over 65 million adults [2], and its prevalence in the US population increased from 24.4 to 28.9% between 1988–1994 and 1999–2004 [3]. Compared with their European–American counterparts, African–Americans are disproportionately affected [2,4] and suffer from a greater burden of disease-associated complications [5,6]. Hypertension is a multifactorial disorder likely resulting from the effect of multiple genetic variants [7] interplaying with environmental factors [8]. Linkage scans and candidate gene-based association studies have identified more than 160 candidate genomic regions for SBP, DBP and clinically diagnosed hypertension [9]. Over the last 2 years, large-scale genome-wide association studies (GWASs) have identified several hypertension candidate loci [10–12].
A chromosome 1q locus for BP-related phenotypes was reported in two independent linkage studies [13,14]. These findings were supported by observation of mouse and rat BP-related quantitative trait loci in regions syntenic to the human 1q chromosomal locus [15,16]. Genome-wide linkage scan in the GenNet Network of the Family Blood Pressure Program (FBPP) replicated a locus on chromosome 1q25 linked to DBP in European–Americans [17]. Fine mapping of this locus by the authors showed association of three genes (ATP1B1, RGS5 and SELE) in the linked locus with hypertension [17]. In that study, ATP1B1 variants were associated with SBP in European–Americans and with DBP in both African–Americans and European–Americans, whereas variants in RGS5 and SELE were associated with SBP in African–Americans [17]. Other studies have produced supporting evidence for these observations. For example, association of a common haplotype in the ATP1B1 gene with an increased risk of hypertension was recently reported in a Chinese population [18]. Polymorphisms in the SELE gene have been reported to be associated with both SBP and DBP [19]. Haplotype-based analysis showed evidence of association of RGS5 gene polymorphisms with essential hypertension in Chinese [20]. In the present study, we conducted association studies of single nucleotide polymorphisms (SNPs) in the ATP1B1, RGS5 and SELE genes with hypertension and BP in African–Americans enrolled from the Washington, District of Columbia metropolitan area.
Methods
Study sample
The individuals studied were all participants in the Howard University Family Study (HUFS), a population-based family study of African–Americans in the Washington metropolitan area [11]. Participants were sought through door-to-door canvassing, advertisements in local print media and at health fairs and other community gatherings. The study was conducted in accordance with the Declaration of Helsinki. Ethical approval for the study was obtained from the Howard University Institutional Review Board. All individuals provided written informed consent for the collection of samples and subsequent analysis. The participants for this study comprised 968 unrelated participants in the HUFS. During a clinical examination, demographic information was collected by interview. Weight, height, waist and hip circumference were measured using standard methods as follows: weight was measured in light clothes on an electronic scale to the nearest 0.1 kg, and height was measured with a stadiometer to the nearest 0.1 cm. BMI was computed as weight in kilograms divided by the square of the height in meters. Waist circumference was measured to the nearest 0.1 cm at the narrowest part of the torso as seen from the anterior aspect. BP was measured in the sitting position using an oscillometric device (Omron Healthcare Inc, Vernon Hills, Illinois, USA). Three BP readings were taken with a 10-min interval between readings. The reported SBP and DBP readings were the average of the second and third readings. Pulse pressure was calculated as the difference between the SBP and DBP. Hypertension status was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg and/or treatment with antihypertensive medication.
Single nucleotide polymorphism selection and genotyping
To ensure optimal coverage of variations, SNPs in the three genes were selected using a haplotype tagging strategy. Using the International HapMap Project YRI data as a reference, tag SNPs at a pairwise r2 value at least 0.8 and with a minimum minor allele frequency (MAF) of 0.02 were selected for genotyping. We supplemented this set of SNPs with all known nonsynonymous coding SNP in the genes. This strategy yielded a total number of 87 SNPs from the three genes. Out of these, 29 SNPs were in the ATP1B1 gene, 33 SNPs in the RGS5 gene and 25 SNPs in the SELE gene (see Table, Supplemental Digital Content 1, http://links.lww.com/HJH/A117). Average intermarker distances were 992 bp for ATP1B1, 2017 bp for RGS5 and 828 bp for SELE gene. Genotyping was done as part of a 768-SNP custom panel on the Illumina GoldenGate assay. Four of the 87 SNPs had a MAF less than 0.01 and one SNP had a locus success rate of less than 90%. These five SNPs were excluded from further analyses. The remaining 82 SNPs were all in Hardy–Weinberg equilibrium (P > 0.001). For the overall experiment, genotype call rate was 99.32% and the concordance rate for blind duplicates was 99.98%.
Statistical analyses
Genotype and allele frequencies were estimated by gene counting. Fisher's exact test was performed to determine deviations of genotype distributions from the expected Hardy–Weinberg equilibrium. Linkage disequilibrium was visualized using Haploview [21]. Hypertension was analyzed as a binary trait (cases versus controls) using logistic regression under an additive genetic model with adjustment for age, sex, BMI and the first principal component of the genotypes derived from a set of 142 ancestry informative SNPs. Association with SBP and DBP was tested under an additive linear model with adjustment for age, sex, BMI, treatment and the first principal component of the genotypes. Given the strong prior information about the role of these genes in hypertension, we considered this a replication study and nominal P values of less than 0.05 were considered significant. Haplotype blocks were constructed using the method of Gabriel et al. [22] and haplotype association tests were conducted using the resulting haplotype blocks with the same phenotypes, covariates and models as for the single-locus analyses. We first performed an overall omnibus test followed by tests for each haplotype against the others within a block. All association analyses were performed using the PLINK software package, v1.06 [23]. Multiple testing was controlled for by adjusting for the number of SNPs within each gene using the Bonferroni method. Bonferroni-corrected P value thresholds were 1.7 × 10–3 for ATP1BI, 1.5 × 10–3 for RGS5 and 2 × 10–3 for SELE.
Results
The characteristics of the hypertensive (n = 532) and normotensive (n = 436) individuals are shown in Table 1. Mean weight, waist-to-hip ratio and DBP were higher in men compared with their female counterparts in both groups. Conversely, women had higher BMI and waist circumference than the men.
Table 1.
Characteristics of the study population (n = 968)
| Hypertensive individuals (n = 532) |
Normotensive individuals (n = 436) |
|||
|---|---|---|---|---|
| Characteristic | Male | Female | Male | Female |
| Number of participants | 217 | 315 | 184 | 252 |
| Age (years) | 52.8 (10.8) | 54.4 (12.2) | 44.6 (9.1) | 43.2 (9.9) |
| Weight (kg) | 92.5 (26.7) | 87.3 (24.0) | 84.6 (20.0) | 83.9 (24.0) |
| BMI (kg/m2) | 30.2 (8.2) | 33.2 (8.8) | 27.2 (6.4) | 31.2 (8.7) |
| Waist circumference (cm) | 99.8 (18.2) | 99.9 (16.3) | 92.1 (14.7) | 94.8 (17.60) |
| Waist-to-hip ratio | 0.92 (0.07) | 0.86 (0.09) | 0.89 (0.06) | 0.84 (0.07) |
| SBP (mmHg) | 143.4 (21.3) | 144.1 (21.9) | 120.1 (10.5) | 117.7 (11.1) |
| DBP (mmHg) | 91.1 (14.0) | 86.0 (13.1) | 75.6 (8.5) | 74.8 (7.7) |
Data are mean (SD).
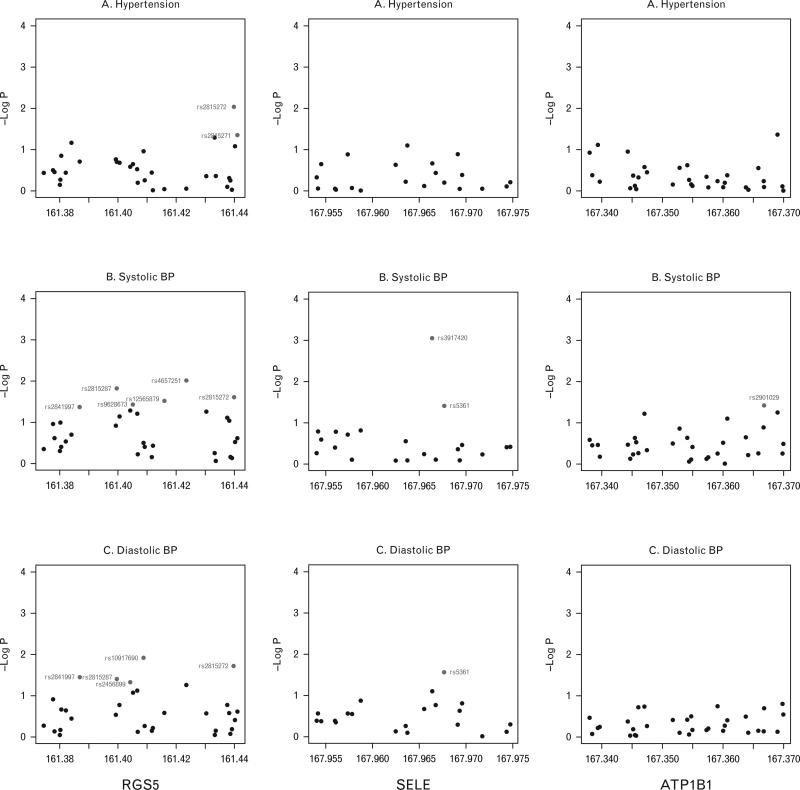
Significantly associated SNPs at a nominal replication P value of 0.05 or less from all three genes are shown in Table 2. Three SNPs from the RGS5 gene were associated with hypertension: rs2815272, rs2815271 and rs2999967 (Fig. 1). The strongest association with hypertension was for rs2815272 (P = 9.3 × 10–3); this SNP also showed significant nominal association with both SBP and DBP (Fig. 1). When SBP was examined as the phenotype, six intronic SNPs from RGS5 showed association with a P value less than 0.05 (Table 2, Fig. 1), rs3917420 being the strongest (P = 9.0 × 10–4). Five SNPs were associated with DBP (Table 2, Fig. 1), four of which are intronic (rs10917690, rs2841997, rs2815287 and rs2456899) and one (rs2815272) is located in the upstream of the RGS5 gene.
Table 2.
Single nucleotide polymorphisms showing nominal evidence of association (P<0.05) in the study
| SNP | Coordinate | Type | Gene | A1 | MAF | P value |
|---|---|---|---|---|---|---|
| HTN | ||||||
| rs2815272a | 163173247 | Upstream | RGS5 | G | 0.1872 | 9.3 × 10–3 |
| rs2815271 | 163174426 | Upstream | RGS5 | G | 0.3340 | 0.0425 |
| rs2999967 | 163166676 | Intronic | RGS5 | C | 0.3183 | 0.0500 |
| SBP | ||||||
| rs3917420 | 169699779 | Intronic | SELE | C | 0.0357 | 9.0 × 10–4 |
| rs4657251 | 163156864 | Intronic | RGS5 | C | 0.3107 | 9.7 × 10–3 |
| rs2815287b | 163132965 | Intronic | RGS5 | A | 0.3005 | 0.0150 |
| rs2815272a | 163173247 | Upstream | RGS5 | G | 0.1872 | 0.0246 |
| rs12565879 | 163149312 | Intronic | RGS5 | A | 0.2919 | 0.0304 |
| rs9628673 | 163138501 | Intronic | RGS5 | A | 3446 | 0.0369 |
| rs2901029 | 169100169 | Intronic | ATP1B1 | A | 0.4013 | 0.0388 |
| rs5361 | 169701060 | Coding | SELE | C | 0.0429 | 0.0398 |
| rs2841997b | 163120239 | Intronic | RGS5 | A | 0.0517 | 0.0423 |
| DBP | ||||||
| rs10917690 | 163142168 | Intronic | RGS5 | G | 0.1096 | 0.0123 |
| rs2815272a | 163173247 | Upstream | RGS5 | G | 0.1872 | 0.0194 |
| rs5361 | 169701060 | Coding | SELE | C | 0.0429 | 0.0276 |
| rs2841997b | 163120239 | Intronic | RGS5 | A | 0.0517 | 0.0366 |
| rs2815287a | 163132965 | Intronic | RGS5 | A | 0.3005 | 0.0401 |
| rs2456899 | 163137589 | Intronic | RGS5 | G | 0.0703 | 0.0483 |
Coordinate based on NCBI human assembly 37.1. A1, associated allele; HTN, hypertension; MAF, minor allele frequency.
Shows association with three phenotypes.
Shows association with two phenotypes.
Fig. 1.
Association plots of single nucleotide polymorphisms in the three genes with hypertension, SBP and DBP plotted as –log10(P) against the SNPs tested with corresponding physical position (Mb). Red circles represent SNPs with P < 0.05.
No SNP in the ATPB1 or the SELE gene was significantly associated with hypertension in our study. One intronic SNP, rs2901029 in the ATPB1 gene, showed association with SBP (Fig. 1, IIB). Two SNPs in the SELE gene appeared associated with SBP (Fig. 1, IIIB), rs3917420 bearing the stronger signal (P = 9.0 × 10–4). The second one, rs5361, is a nonsynonymous coding SNP (S149R) and was associated with both SBP (P = 0.0398) and DBP (P = 0.0276).
The effect sizes of SBP-associated and DBP-associated alleles adjusted for age, sex, BMI and ancestry are shown in Table 3. The effect sizes ranged from –2.244 [standard error of the mean (SEM) 0.866; confidence interval (CI) –3.94 to –0.547] to 7.233 mmHg (SEM 2.175; CI 2.971–11.5) for SBP and from –2.015 (SEM 0.804; CI –3.591 to –0.439) to 2.773 mmHg (SEM 1.256; CI 0.31–5.235) for DBP, for individual SNPs. The largest effect size for SBP was 7.233 mmHg for rs3917420, located in intron 4 of the SELE gene (P = 9 × 10–4).
Table 3.
Effect sizes of associated variants
| SNP | Gene | Phenotype | Effect size | SEM (95% CI) | P value |
|---|---|---|---|---|---|
| rs3917420 | SELE | SBP | 7.233 | 2.175 (2.971–11.5) | 0.0009 |
| rs4657251 | RGS5 | SBP | –2.244 | 0.866 (–3.94 to –0.547) | 0.0097 |
| rs2815287 | RGS5 | SBP | 2.152 | 0.883 (0.42–3.883) | 0.0150 |
| rs2815287 | RGS5 | DBP | 1.169 | 0.569 (0.054–2.284) | 0.0402 |
| rs2815272 | RGS5 | SBP | 2.297 | 1.02 (0.297–4.297) | 0.0246 |
| rs2815272 | RGS5 | DBP | 1.547 | 0.66 (0.253–2.841) | 0.0194 |
| rs12565879 | RGS5 | SBP | –1.894 | 0.874 (–3.606 to 0.181) | 0.0304 |
| rs9628673 | RGS5 | SBP | 1.789 | 0.857 (0.111–3.468) | 0.0369 |
| rs2901029 | ATP1B1 | SBP | 1.698 | 0.821 (0.089–3.306) | 0.0388 |
| rs5361 | SELE | SBP | 4.018 | 1.952 (0.192–7.845) | 0.0398 |
| rs5361 | SELE | DBP | 2.773 | 1.256 (0.31–5.235) | 0.0275 |
| rs2841997 | RGS5 | SBP | 3.614 | 1.777 (0.131–7.097) | 0.0423 |
| rs2841997 | RGS5 | DBP | 2.396 | 1.144 (0.153–4.639) | 0.0366 |
| rs10917690 | RGS5 | DBP | –2.015 | 0.804 (3.591 to –0.439) | 0.0124 |
| rs2456899 | RGS5 | DBP | 1.955 | 0.989 (0.0173–3.893) | 0.0483 |
Effect sizes in mmHg. Data were analyzed under an additive model. CI, confidence interval; SEM, standard error of the mean; SNP, single nucleotide polymorphism.
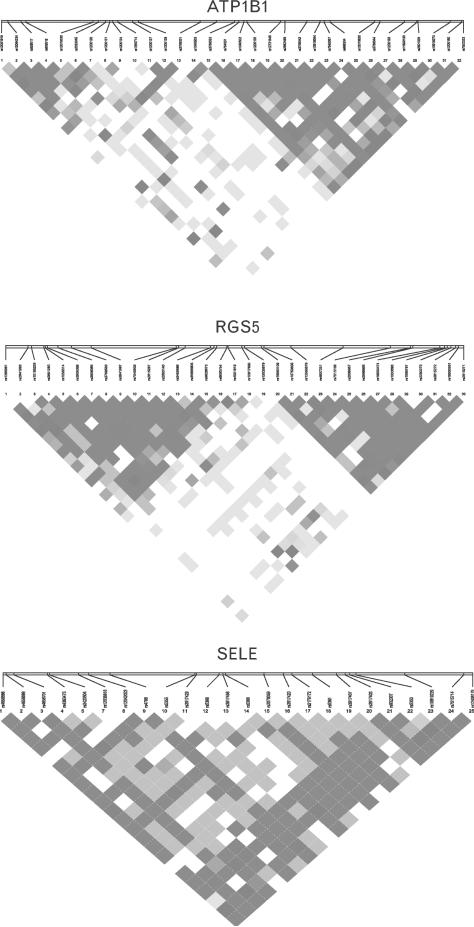
Linkage disequilibrium plots for the three genes in the study population are shown in Fig. 2. Two haplotype blocks could be defined in each of the RGS5 and the SELE gene (data not shown). One haplotype CCAGC (rs4656701–rs4363475–rs12038818–rs4786–rs5368) in the SELE gene showed a significant association with SBP (P = 6.5 × 10–3). The association remained significant after correction for multiple testing (P < 0.05/5 haplotypes in the block, P < 0.01).
Fig. 2.
Patterns of linkage disequilibrium in the three genes in our study samples. Red squares for strong linkage disequilibrium (LD), blue squares for nonsignificant LD and white squares for little or no LD.
Discussion
Identifying genes underlying human essential hypertension has proven challenging. The recent publications of several GWAS have shed insight into some of the genes that are important at the population level [10–12]. Although the list of genetic variants associated with hypertension has increased over the past few years, few have been replicated in independent studies. This is probably due to genetic heterogeneity as well as modulation of hypertension by low-risk variants with small effect and low penetrance. Additionally, there is the possibility of interpopulation variation given that several susceptibility variants for hypertension vary across geographic regions and/or populations. In the present study, we focused on three chromosome 1q positional candidate genes: ATP1B1, RGS5 and SELE in a sample of African–Americans. To maximize the utility of the study, we carefully characterized each study participant, adjusted for covariates in analysis, selected tag SNPs that covered the entire span of each gene and adjusted for ancestry (admixture), which could produce spurious association in population-based case–control study designs [24].
We found one ATP1B1 variant, rs2901029, to be significantly associated with SBP. Two recent reports investigated the relationship of BP with ATP1B1 genetic polymorphisms and haplotypes [17,18], but neither of these studies included this SNP. Among three SNPs (rs3766031, rs12079745 and rs1138486) reported to be associated with SBP previously [17], the only one in our SNP set – rs3766031 – did not show a significant association (data not shown). Our observed SBP effect size for rs2901029 was 1.698 mmHg, which is somewhat less than previously reported for ATP1B1 SNPs (3.5–5.1 mmHg) [17]. The ATP1B1 gene encodes for the β1 subunit of the Na,K-ATPase (the sodium pump), a plasma membrane bound oligomeric enzyme that catalyses one molecule of ATP to exchange three Na+ ions for two K+ ions across the cell membrane by a coupled active transport, thereby maintaining their normal physiological gradient [25]. Na,K-ATPase influences BP by regulating sodium reabsorption in renal tubules. In rats of the Milan hypertensive strain (MHS), a primary increase of renal tubular Na+ reabsorption is involved in the development of hypertension [26]. Particularly, the MHS rat showed an increased activity and expression of Na-K pump units per cell compared with their Milan normotensive strain controls [27]. Thus, the finding of association between ATP1B1 variants and hypertension in this study is consistent with the gene's biological function.
For the RGS5 gene, one SNP, rs2815272, was significantly associated with hypertension, SBP and DBP, whereas two SNPs (rs2815287 and rs2841997) were associated with both SBP and DBP. These three SNPs have been reported to be associated with SBP and/or DBP [17]. The estimated effect sizes on SBP in our study ranged from 2.2 to 3.6 mmHg, similar to the range of effect sizes (1.5–3.6 mmHg) reported in the literature [17]. RGS5 is one of the intracellular regulators of G protein signaling (RGS) proteins [28]. It is expressed in most major organs, including heart, lungs and kidneys, as well as in highly specialized cell types such as vascular smooth muscle cells and cardiac myocytes [29]. Recently, two independent studies demonstrated that Rgs5-deficient mice were hypotensive relative to the wild-type controls [30,31], further lending credence to its potential role in BP regulation.
E-selectin (SELE) is a member of the selectin family and is specifically expressed on the surface of stimulated endothelial cells [32]. Endothelium plays an important role in the regulation of BP, and endothelial dysfunction, defined as reduced vasodilating response to endothelial stimuli, is a hallmark of hypertensive patients [33,34]. Soluble E-selectin (sE-selectin) is released by the endothelial cells and serves as a plasma marker of endothelial dysfunction or damage [35]. Increased serum levels of sE-selectin have been reported in essential hypertension [36,37]. Two SNPs (rs3917420 and rs5361) in the SELE gene were associated with SBP in this study. We also found rs3917420 in complete linkage disequilibrium (r2 = 1) with a nonsynonymous coding SNP, rs5366. A G→C change at this SNP leads to a Glu→Gln change in position 421 of the protein. However, this mutation is classified as ‘possibly damaging’ by PolyPhen and ‘tolerated’ by the SIFT prediction tool, making it less likely to be the functional variant at this locus. We did not observe any association of rs5368 and rs2076059 with SBP in our study sample, as previously described [17].
An important issue in many association studies including ours is that significantly associated variants are often located in noncoding regions, raising the question of their functionality and possible role in disease predisposition. An intronic SNP may alter the function of a nearby regulatory element or be in linkage disequilibrium with another causative variant that is directly involved in hypertension susceptibility. Introns also contain several short sequences of cis-splicing motifs that are important for efficient splicing, such as acceptor and donor sites at either end of the intron, and a branch point site, which are required for proper splicing by the spliceosome. Additionally, 25% or more of the miRNAs genes that are short noncoding RNAs and known to regulate gene expression by binding to sequences on the target mRNA [38] and are often embedded within introns [39,40]. Similarly, upstream sequences as well as introns are known to contain promoters that control gene transcription.
Appropriate correction of statistical significance for multiple testing is required in genetic association studies to reduce false-positive findings. These corrections range from the most conservative Bonferroni correction, to False Discovery Rate, to weighted correction of combined data, to no correction at all [41,42]. We have reported our findings as replication of previous reports and, thus, relied on a significance threshold with a P value of 0.05. We also tested for Bonferroni correction of our positive findings. One intronic SNP rs3917420, and a five SNP haplotype, CCAGC, in the SELE gene (rs4656701–rs4363475–rs12038818–rs4786–rs5368) showed association with SBP after Bonferroni correction for multiple testing.
Consistency and accuracy of phenotypic characterization are essential for genetic association studies. BP readings show heterogeneity depending on the method, environment and time of measurement that may increase type II error and, thus, reduce the chance of identifying all the genetic loci-influencing BP [43]. In one such study, a majority of the best associated signals were seen with ambulatory SBP and DBP compared to BP measured at home or clinic [43]. Therefore, the reliance on clinic measurements in the present study is a potential limitation. Also, although we adjusted for prior treatment in our analyses, not being able to measure ‘true’ SBP and DBP values may have weakened potential associations.
In conclusion, we have systematically investigated variants in the ATP1B1, RGS5 and SELE genes on chromosome 1q for their association with hypertension and related traits. We have extended previous reports of association of these genes in an independent African–Americans population from Washington, District of Columbia metropolitan area. Although we have replicated some of the previous findings, we are also reporting new BP-associated genetic variants. On the basis of the physiological roles of these three genes in BP homeostasis, follow-up functional studies of these variants may decipher their underlying pathophysiological implications. The current wave of sequencing studies will facilitate the identification of rare and less common hypertension susceptibility variants.
Supplementary Material
Acknowledgements
The Howard University Family Study (HUFS) was supported by grants S06GM008016-380111 to A.A.A. and S06GM008016–320107 to C.N.R., both from the MBRS/SCORE Program of the National Institute of General Medical Sciences, National Institutes of Health (NIH). Participant enrollment for the HUFS was carried out at the Howard University General Clinical Research Center, which is supported by grant 2M01RR010284 from the National Center for Research Resources (NCRR), NIH. This research was supported in part by the RCMI Program at Howard University funded by the Division of Research Infrastructure, NCRR, NIH (RR003048) and by the Intramural Research Program of the Center for Research on Genomics and Global Health (CRGGH). The CRGGH is supported by the National Human Genome Research Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the Center for Information Technology, and the Office of the Director at the NIH (Z01HG200362).
The authors would like to thank participants, physicians, and investigators in the HUFS.
Abbreviations
- ATP1B1
ATPase, Na+/K+ transporting, beta 1 polypeptide (gene)
- DBP
diastolic blood pressure
- RGS5
regulator of G-protein signaling 5 (gene)
- SBP
systolic blood pressure
- SELE
selectin E (gene)
- SNP
single nucleotide polymorphism
Footnotes
This work was previously presented at the 60th Annual Meeting of the American Society of Human Genetics, 2010 and at the 12th RCMI International Symposium on Health Disparities, 2010.
Conflicts of interest
The authors have declared that no conflicts of interest exist.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004. 44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 3.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008. 525:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 4.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 2007. 49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 5.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African American and white men: 16-year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 6.Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among US adults: the third National Health and Nutrition Examination Survey mortality follow-up study. Ann Epidemiol. 2008;18:302–309. doi: 10.1016/j.annepidem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Williams SM, Ritchie MD, Phillips JA, 3rd, Dawson E, Prince M, Dzhura E, et al. Multilocus analysis of hypertension: a hierarchical approach. Hum Hered. 2004;57:28–38. doi: 10.1159/000077387. [DOI] [PubMed] [Google Scholar]
- 8.Klimentidis YC, Dulin-Keita A, Casazza K, Willig AL, Allison DB, Fernandez JR. Genetic admixture, social-behavioral factors and body composition are associated with blood pressure differently by racial-ethnic group among children. J Hum Hypertens. 2011 doi: 10.1038/jhh.2010.130. [Epub ahead of print]. doi:10.1038/ jhh.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samani NJ. Genome scans for hypertension and blood pressure regulation. Am J Hypertens. 2003;16:167–171. doi: 10.1016/s0895-7061(02)03244-2. [DOI] [PubMed] [Google Scholar]
- 10.Wellcome Trust Case Control Consortium Genome-wide association study of 14 000 cases of seven common diseases and 3 000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009;5:e1000564. doi: 10.1371/journal.pgen.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt SC, Ellison RC, Atwood LD, Pankow JS, Province MA, Leppert MF. Genome scans for blood pressure and hypertension: the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. 2002;40:1–6. doi: 10.1161/01.hyp.0000022660.28915.b1. [DOI] [PubMed] [Google Scholar]
- 14.James K, Weitzel LR, Engelman CD, Zerbe G, Norris JM. Framingham Heart Study. Genome scan linkage results for longitudinal systolic blood pressure phenotypes in subjects from the Framingham Heart Study. BMC Genet. 2003;4(Suppl 1):S83. doi: 10.1186/1471-2156-4-S1-S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPetrillo K, Tsaih SW, Sheehan S, Johns C, Kelmenson P, Gavras H, et al. Genetic analysis of blood pressure in C3H/HeJ and SWR/J mice. Physiol Genomics. 2004;17:215–220. doi: 10.1152/physiolgenomics.00212.2003. [DOI] [PubMed] [Google Scholar]
- 16.Stoll M, Kwitek-Black AE, Cowley AW, Jr, Harris EL, Harrap SB, Krieger JE, et al. New target regions for human hypertension via comparative genomics. Genome Res. 2000;10:473–482. doi: 10.1101/gr.10.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YP, Liu X, Kim JD, Ikeda MA, Layton MR, Weder AB, et al. Multiple genes for essential-hypertension susceptibility on chromosome 1q. Am J Hum Genet. 2007;80:253–264. doi: 10.1086/510918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao B, Zhang Y, Niu W, Gao P, Zhu D. Association of ATP1B1 single-nucleotide polymorphisms with blood pressure and hypertension in a Chinese population. Clin Chim Acta. 2009;407:47–50. doi: 10.1016/j.cca.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Sass C, Pallaud C, Zannad F, Visvikis S. Relationship between E-selectin L/F554 polymorphism and blood pressure in the Stanislas cohort. Hum Genet. 2000;107:58–61. doi: 10.1007/s004390000325. [DOI] [PubMed] [Google Scholar]
- 20.Xiao B, Zhang Y, Niu WQ, Gao PJ, Zhu DL. Haplotype-based association of regulator of G-protein signaling 5 gene polymorphisms with essential hypertension and metabolic parameters in Chinese. Clin Chem Lab Med. 2009;47:1483–1488. doi: 10.1515/CCLM.2009.344. [DOI] [PubMed] [Google Scholar]
- 21.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 23.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 26.Barber BR, Ferrari P, Bianchi G. The Milan hypertensive strain: a description of the model. In: Ganten D, de Jong W, editors. Handbook of hypertension. Vol. 16. Elsevier; Amsterdam: 1994. pp. 316–345. [Google Scholar]
- 27.Ferrandi M, Tripodi G, Salardi S, Florio M, Modica R, Barassi P, et al. Renal Na,K-ATPase in genetic hypertension. Hypertension. 1996;28:1018–1025. doi: 10.1161/01.hyp.28.6.1018. [DOI] [PubMed] [Google Scholar]
- 28.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signaling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Seki N, Sugano S, Suzuki Y, Nakagawara A, Ohira M, Muramatsu M, et al. Isolation, tissue expression, and chromosomal assignment of human RGS5, a novel G-protein signaling regulator gene. J Hum Genet. 1998;43:202–205. doi: 10.1007/s100380050071. [DOI] [PubMed] [Google Scholar]
- 30.Cho H, Park C, Hwang IY, Han SB, Schimel D, Despres D, Kehrl JH. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol. 2008;28:2590–2597. doi: 10.1128/MCB.01889-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nisancioglu MH, Mahoney WM, Jr, Kimmel DD, Schwartz SM, Betsholtz C, Genové G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol. 2008;28:2324–2331. doi: 10.1128/MCB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 33.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 34.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, et al. Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Blann A, Seigneur M. Soluble markers of endothelial cell function. Clin Hemorheol Microcirc. 1997;17:3–11. [PubMed] [Google Scholar]
- 36.Blann AD, Tse W, Maxwell SJ, Waite MA. Increased levels of the soluble adhesion molecule E-selectin in essential hypertension. J Hypertens. 1994;12:925–928. [PubMed] [Google Scholar]
- 37.De Caterina R, Ghiadoni L, Taddei S, Virdis A, Almerigogna F, Basta G, et al. Soluble E-selectin in essential hypertension: a correlate of vascular structural changes. Am J Hypertens. 2001;14:259–266. doi: 10.1016/s0895-7061(00)01276-0. [DOI] [PubMed] [Google Scholar]
- 38.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berezikov E, van Tetering G, Verheul M, van de Belt J, van Laake L, Vos J, et al. Many novel mammalian microRNA candidates identified by extensive cloning and RAKE analysis. Genome Res. 2006;16:1289–1298. doi: 10.1101/gr.5159906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 42.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–213. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- 43.Padmanabhan S, Menni C, Lee WK, Laing S, Brambilla P, Sega R, et al. The effects of sex and method of blood pressure measurement on genetic associations with blood pressure in the PAMELA study. J Hypertens. 2010;28:465–477. doi: 10.1097/HJH.0b013e32833594d7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.