Abstract
Finding mechanisms of viral resistance and new ways to tackle chronic hepatitis will help find a cure for this disease. In ‘Bench to Bedside’, Christopher Walker and Benoît Callendret highlight studies showing that overcoming immune exhaustion during chronic infection by blocking several inhibitory pathways of T cells may restore an adequate immune response. In ‘Bedside to Bench’, Lawrence Corey, Joshua Schiffer and John Scott discuss recent advances in antiviral therapy with protease inhibitors and the findings of a mathematical model that predicts possible single and double mutations prior to antiviral therapy.
About 600 million people are at risk for progressive liver diseases because of infection with the hepatitis B (HBV) or hepatitis C virus (HCV). Therapies to control these viruses have improved over the past decade, but limitations still remain. Pegylated type I interferon provides a sustained clinical cure in only a subset of people with chronic hepatitis B1. A combination of pegylated type I interferon and the synthetic nucleoside ribavirin is the standard of care for eradication of HCV from the liver, but it too often fails because of HCV and host genetic factors that remain poorly understood2. Remarkably, the mechanisms of antiviral activity for type I interferon and ribavirin are still not known. Direct inhibition of virus replication is important, but modulation of host immunity cannot be excluded.
HBV and HCV replication can also be suppressed with direct-acting antivirals, small molecules that inhibit viral enzymes crucial for replication1,3. They include nucleoside or nucleotide analogs that impair HBV polymerase activity and new inhibitors of the HCV protease and polymerase that are nearing licensure or completion of late-phase clinical testing.
But nucleotide analogs in HBV infection have shown the potential for emergence of resistant variants or a rebound in replication when therapy is discontinued1, and therapy of HCV infection will face similar challenges unless multiple antivirals can be combined to erect a high barrier to resistance mutations4. For now, direct antivirals such as telaprevir will be used with the current standard-of-care regimen to prevent replication of variants resistant to this inhibitor of the HCV protease4.
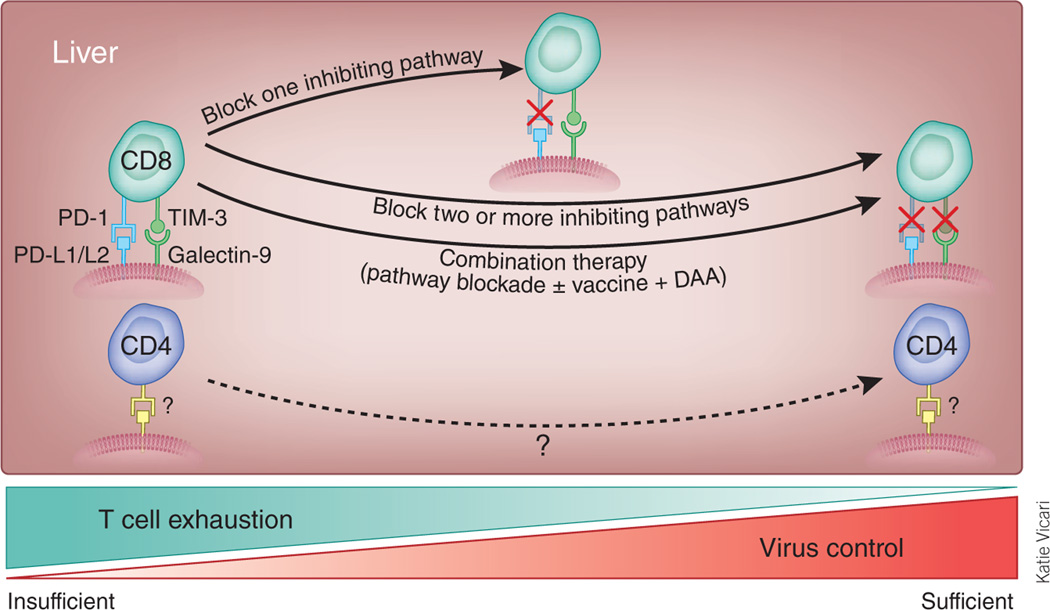
Control of highly mutable hepatotropic viruses might be enhanced by new approaches to immune modulation (Fig. 1). Persistent virus replication is facilitated by exhaustion of CD8+ T cells, a process that involves sequential loss of cytotoxicity, production of interleukin-2, tumor necrosis factor-α and finally interferon-γ5.
Figure 1.
Multiple inhibitory pathways may be activated in the exhausted CD8+ T cells in the persistently infected liver, including PD-1 and TIM-3. Blockade of one inhibitory pathway may partially restore effector functions. Blocking antibodies may be directed against the PD-1 and TIM-3 receptors or possibly their ligands (PD-L1 and PD-L2 (PD-L1/L2) and galectin-9, respectively). Full rescue of CD8+ T cell exhaustion may require blockade of two or more inhibitory pathways, or a combination of pathway blockade, vaccination and/or virus suppression via direct-acting antivirals (DAA). Sustained restoration of CD4+ T cell function may be also crucial for a clinical cure, particularly in chronic hepatitis B. How CD4+ T cells are silenced, and the pathways to rescue this silencing, are still unknown.
One factor contributing to loss of function is signaling through the programmed cell death-1 (PD-1) co-inhibitory receptor expressed on exhausted CD8+ T cells. Restoration of CD8+ T cell effector functions and reduced virus replication in mice treated with antibodies to the PD-1 ligand6 spurred research into immune exhaustion in chronic viral hepatitis. Expression of PD-1 by exhausted HCV- and HBV-specific CD8+ T cells in infected humans, and restoration of proliferation and effector functions by in vitro blockade of the pathway, was confirmed in a remarkably short time frame7,8. Notably, a phase 1 clinical trial of PD-1–blocking antibodies was recently completed in subjects with chronic hepatitis C (US ClinicalTrials.gov identifier NCT00703469), indicating how fast a new concept for immune modulation can move into the clinic.
HCV-specific9–11 and HBV-specific12 CD8+ T cells express multiple inhibitory receptors. Two inhibitory receptors, cytotoxic T lymphocyte antigen-4 (CTLA-4) and T cell immunoglobulin domain and mucin domain-3 (TIM-3), act synergistically with PD-1 to enforce exhaustion of HCV-specific CD8+ T cells, at least in vitro10,11. The study involving TIM-3 provided new insight into functional consequences of blocking more than one inhibitory pathway10, which has relevance to immunotherapy. Concurrent expression of PD-1 and TIM-3 was more common on HCV-specific CD8+ T cells from individuals with HCV who followed a chronic versus acute resolving course of infection10. High TIM-3 and PD-1 expression also marked the most profoundly exhausted CD8+ T cells. Notably, HCV antigen–driven proliferation of CD8+ T cells was restored by in vitro blockade of PD-1, TIM-3 or both. Rescue of cytotoxic function, however, depended on suppression of TIM-3 and not PD-1 signaling10. How various CD8+ T cell effector functions will be altered by blockade of other inhibitory receptors, such as CD244 (2B4), CD160 and CD223 (LAG-3) is still being defined9–11.
Selection of the pathways for blockade would be facilitated by better insight into mechanisms important for T cell control of virus replication in the liver. The relative importance of cytotoxic versus noncytotoxic mechanisms in curing liver disease is still debated. The study by McMahan et al.10 hints at the possibility that therapy might someday be tailored to minimize damage of liver tissue when large numbers of hepatocytes are infected, perhaps by sequentially restoring noncytotoxic and then cytotoxic effector functions. Controlling how immunity is reconstituted with therapeutic antibodies is for now highly speculative, but comparing patterns of gene expression in exhausted and rescued virus-specific CD8+ T cells could reveal downstream regulators of survival and effector functions13, which may provide more precise targets for therapy.
Understanding how CD4+ T cells are silenced in chronic viral hepatitis is also crucial, as early loss of helper activity is essential for persistence and its reconstitution may be critical for successful therapy. The study of McMahan et al.10 linked virus persistence with higher expression of TIM-3 on circulating CD4+ T cells during acute hepatitis C. But if mechanisms of CD8+ and CD4+ T cell failure are different, strategies for reconstitution of helper and effector functions may not overlap.
Perhaps the most crucial implication of the studies by McMahan et al.10 and others11,12 is that interruption of a single inhibitory pathway may not be sufficient for immune reconstitution and cure of chronic hepatitis. Treatment with antibodies that block more than one path-way might be feasible, but conditions for their use will probably vary for HCV and HBV. Most persistent HCV infections, especially those where viremia is low or viral and host genotypes are favorable, will be cured by combinations of direct acting antivirals and standard therapy. Cure rates for HCV will continue to increase as new direct-acting compounds are developed, but whether every infection will be cured by this approach is unknown.
Treatment failures might benefit from immune therapy. Reconstitution of T cell immunity is particularly relevant to chronic hepatitis B, where a sustained cure by direct-acting antivirals is uncommon. HBV genomes are not easily eliminated from the liver, and so immune control must be sustained for a lifetime. Pegylated type I interferon is thought to provide a durable cure after discontinuation of therapy by modulating immunity. Blockade of inhibitory pathways might be an important approach to HBV therapy if relatively low response rates to type I interferon are improved.
Finally, most people with chronic viral hepatitis are relatively healthy for years. Safety will therefore be a primary concern when selecting inhibitory pathways for blockade. Although antibodies to most inhibitory receptors or ligands, including TIM-3, have not yet been tested in humans, data from cancer immunotherapy trials of PD-1 and CTLA-4 blockade suggest that the risk might vary by pathway. Autoimmunity occurred after treatment with CTLA-4–specific antibodies14, but PD-1 blockade seems to be well tolerated in the clinic so far. The number of inhibitory pathways that must be blocked to overcome T cell exhaustion may be reduced by combination with other approaches, such as therapeutic vaccination15 and/or direct-acting antivirals or type I interferon. As there is no clinical precedent for combining immunomodulatory antibodies or adding therapeutic antiviral vaccines to the regimen, well-designed preclinical studies for HBV and HCV infection will be crucial to minimize risk.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/.
References
- 1.Dienstag JL. N. Engl. J. Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 2.Brillanti S, Mazzella G, Roda E. Dig. Liver Dis. 2010 Nov 18; doi: 10.1016/j.dld.2010.10.007. published online. [DOI] [PubMed] [Google Scholar]
- 3.Thompson AJ, McHutchison JG. J. Viral Hepat. 2009;16:377–387. doi: 10.1111/j.1365-2893.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 4.Rong L, Dahari H, Ribeiro RM, Perelson AS. Sci. Transl. Med. 2010;2:30ra32. doi: 10.1126/scitranslmed.3000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. J. Exp. Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber DL, et al. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Maier H, Isogawa M, Freeman GJ, Chisari FV. J. Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 8.Walker CM. Adv. Virus Res. 2010;78:43–86. doi: 10.1016/B978-0-12-385032-4.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bengsch B, et al. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahan RH, et al. J. Clin. Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamoto N, et al. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raziorrouh B, et al. Hepatology. 2010;52:1934–1947. doi: 10.1002/hep.23936. [DOI] [PubMed] [Google Scholar]
- 13.Haining WN, Wherry EJ. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Di Giacomo AM, Biagioli M, Maio M. Semin. Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Ha SJ, et al. J. Exp. Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]