Abstract
Three nuclei of the lateral lemniscus are present in the zebra finch, ventral (LLV), intermediate (LLI), and dorsal (LLD). LLV is separate from the superior olive (OS): it lies closer to the spinal lemniscus and extends much further rostrally around the pontine periphery. LLI extends from a caudal position ventrolateral to the principal sensory trigeminal nucleus (LLIc) to a rostral position medial to the ventrolateral parabrachial nucleus (LLIr). LLD consists of posterior (LLDp) and anterior (LLDa) parts, which are largely coextensive rostrocaudally, although LLDa lies medial to LLDp. All nuclei are identifiable on the basis of cytochrome oxidase activity. The cochlear nucleus angularis (NA) and the third-order nucleus laminaris (NL) project on OS predominantly ipsilaterally, on LLV and LLI predominantly contralaterally, and on LLD contralaterally only. The NA projections are heavier than those of NL and differ from them primarily in their terminations within LLD: NA projects to LLDp, whereas NL projects to LLDa. In this the projections are similar to those in the barn owl (Takahashi and Konishi [1988] J Comp Neurol 274:212–238), in which time and intensity pathways remain separate as far as the central nucleus of the inferior colliculus (MLd). In contrast, in the zebra finch, although NA and NL projections remain separate within LLD, the projections of LLDa and LLDp become intermixed within MLd (Wild et al., J Comp Neurol, this issue), consistent with the intermixing of the direct NA and NL projections to MLd (Krützfeldt et al., J Comp Neurol, this issue). J. Comp. Neurol. 518:2135–2148, 2010.
INDEXING TERMS: cochlear nuclei; dorsal; ventral, and intermediate nuclei of the lateral lemniscus; zebra finch; avian
In the first of this series of articles (Krützfeldt et al., 2010) we noted the desirability of a description of the ascending auditory brainstem pathways in a songbird, because although auditory input is known to be crucial for song learning during development and for the stability of song production in adulthood, the only accounts of the ascending auditory projections through the brainstem were in non-songbirds such as pigeons, chickens, and barn owls, in which vocalizations are unlearned. In the companion article, therefore, we charted the projections of the cochlear nucleus magnocellularis (NM) to nucleus laminaris (NL) in the zebra finch, and the projections of NL and the cochlear nucleus angularis (NA) to nucleus mesencephalicus lateralis, pars dorsalis (MLd), the proposed avian homolog of the central nucleus of the mammalian inferior colliculus, and the reptilian torus semicircularis (Karten, 1967). In the present article we chart the projections of NA and NL to the superior olive and the nuclei of the lateral lemniscus, a collection of nuclei within the ascending brainstem auditory pathway that not only also projects on MLd, but has other, more heterogeneous projections, both ascending and descending (Wild et al., 2009, 2010). Before proceeding with a description of the results, however, it should be noted that the terminology for the lateral lemniscal nuclei in birds varies between authors both within the same species and between different species, which can be quite confusing. In the barn owl, Takahashi and Konishi (1988) described a lateral lemniscal complex as consisting of four nuclei: olivaris superior (OS), nucleus lemnisci lateralis, pars ventralis (LLv), nucleus ventralis lemnisci lateralis, pars anterior (VLVa), and nucleus ventralis lemnisci lateralis, pars posterior (VLVp). The inclusion of the superior olive in a lateral lemniscal complex, however, has not been the rule in avian auditory studies (Boord, 1968; Karten and Hodos, 1967; Leibler, 1975; Conlee and Parks, 1986; Arends and Zeigler, 1986; Wild, 1987; Westerberg and Schwartz, 1995), and although there appears to be no equivalent of the avian superior olive in the mammalian superior olivary complex, the latter are generally considered separate from the lemniscal nuclei (e.g., Schwartz, 1992; Webster, 1992; Cant and Benson, 2003; Schofield, 2005). Furthermore, in birds the names of the lemniscal nuclei, and/or their abbreviations, are inconsistent and sometimes refer to different nuclei. For instance, the abbreviation of a ventral nucleus of the lateral lemniscus in pigeons and chickens is either LV for Boord (1968) or LLv for Karten and Hodos (1967), Leibler (1975), and Conlee and Parks (1986). But the LLv of Karten and Hodos (1967) turned out not to be an auditory nucleus at all, but part of the parabrachial complex (Wild et al., 1990), while the LLv of Leibler (1975) and Conlee and Parks (1986) was shown to receive projections from the contralateral NA and NL, but in the barn owl bilaterally only from NA (Takahashi and Konishi, 1988).
A small intermediate nucleus of the lateral lemniscus (LLI) may be present in the barn owl (Takahashi and Konishi, 1988; Wild et al., 2001), but an LLI was not recognized in the pigeon (Boord, 1968; Karten and Hodos, 1967; Leibler, 1975) or chicken (Conlee and Parks, 1986). In the chicken, however, Westerberg and Schwarz (1995) defined an LLIp and an LLIa, which we suggest correspond, respectively, to the VLVp and VLVa of Leibler (1975). Only VLVp, however, do we consider to be an intermediate nucleus (LLI), in agreement with Arends and Zeigler (1986). These authors noted that in pigeon LLI has rostral (LLIr) and caudal (LLIc) parts, although these are in rostrocaudal continuity. Puelles et al. (2007) also identify an intermediate nucleus in the chicken (ILL), but in view of the proximity of this nucleus to the nucleus semilunaris—where the dorsal nucleus is situated in other species—it may be that this is actually the dorsal nucleus, which would therefore correspond to the LLD of pigeons (Arends and Zeigler, 1986), at least in part.
The nomenclature for a dorsal nucleus of the lateral lemniscus is particularly confusing. An LLd in pigeons (Karten and Hodos, 1967) and chickens (Conlee and Parks, 1986) or a DLL in chickens (Puelles et al., 2007) is situated in the dorsolateral mesencephalic tegmentum, dorsal to the nucleus semilunaris. Cytoarchitecturally this nucleus is obviously different from the dorsal nucleus of other avian species; furthermore, neither Leibler (1975) nor Wild (1995) could find an auditory projection to or from this nucleus in pigeons, although Conlee and Parks (1986), using the autoradiographic technique, reported that in chickens it received a small projection from the contralateral NA in three out of eight of their cases receiving injections of tritiated amino acid. In barn owls, Takahashi and Konishi (1988) did not refer to a dorsal nucleus, but Wild et al. (2001) suggested that the VLVa and VLVp in the barn owl should be renamed, in the interests of consistency, LLDa and LLDp, respectively, and this suggestion seems to have been accepted (Konishi, 2003). In the present article in the zebra finch, therefore, the lateral lemniscal nuclei are considered to be, after Arends and Zeigler (1986): LLV, LLI (with rostral (LLIr) and caudal (LLIc) parts in continuity, and LLD (consisting of LLDa and LLDp). LLDa and LLDp are absolutely much smaller in the zebra finch than the comparable nuclei of the barn owl, but appear to be similar to them in terms of their afferent and efferent connections. Whether they are relatively smaller, e.g., with respect to the size of brainstem, awaits careful comparative measurements.
While these attempts to standardize the names of the lateral lemniscal nuclei in birds in accordance with the mammalian situation might help to reduce confusion, they should not be regarded as necessarily homologizing the nuclei across the two classes (see Discussion).
MATERIALS AND METHODS
Because our conclusions are based on material used in the companion article (Krützfeldt et al., 2010), the methods are identical and need not be repeated here. The results are derived from the same cases as described in the companion article, plus an additional 15 cases involving injections of either biotinylated dextran amine (BDA) or cholera toxin B-chain (CTB) in each of the nuclei of the lateral lemniscus and superior olive in order to provide retrograde confirmation of afferent origins. Tissue processing, data collection, and image preparation were the same or similar to those described in the companion article (Krützfeldt et al., 2010) and in that of Wild and Farabaugh (1996). The cross-sectional areas of cells making up OS, LLV, LLI, and LLD were measured in three brains by drawing their outlines in Nissl counterstained transverse sections using a 40× objective and a camera lucida and entering the data into ImageJ (http://rsb.info.nih.-gov/ij). Cells were chosen by overlaying the nucleus with a 1-cm square grid and drawing cells in one of every five randomly chosen fields in the grid.
RESULTS
Cytoarchitectonics
Cochlear nuclei (NA and NM) and NL
A general cytoarchitectural description of these nuclei is given in the companion article (Krützfeldt et al., 2010).
Superior olive (OS)
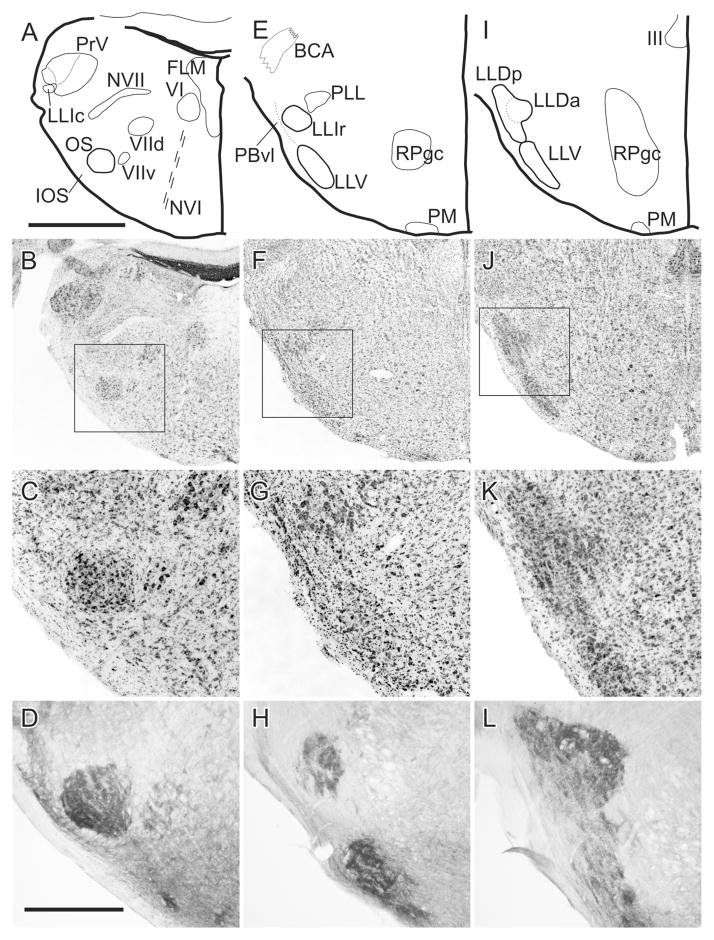
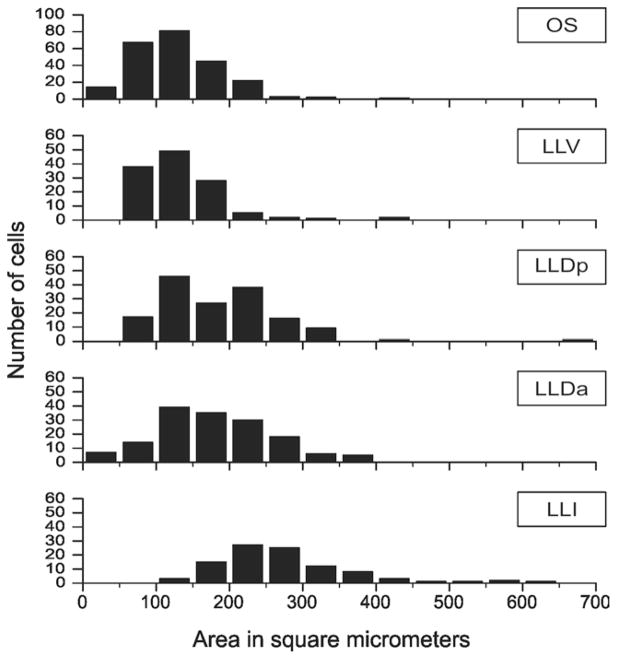
This nucleus is circular or pear-shaped in cross section (Fig. 1A–D). At caudal pontine levels it is located ventral to the spinal trigeminal nucleus but its position gradually descends through more rostral levels to occupy a ventrolateral position, where it is separated from the spinal lemniscus by facial motoneurons and by smaller neurons comprising the nucleus infra-olivaris superior (IOS: Wild, 1993). Other groups of facial motoneurons lie medial and dorsomedial to OS. OS is largely made up of small-to-medium sized neurons having a mean cross-sectional area of ≈128 μm2; n = 235; Fig. 2), considerably smaller than any of those found in OS of the barn owl (Carr et al., 1989).
Figure 1.
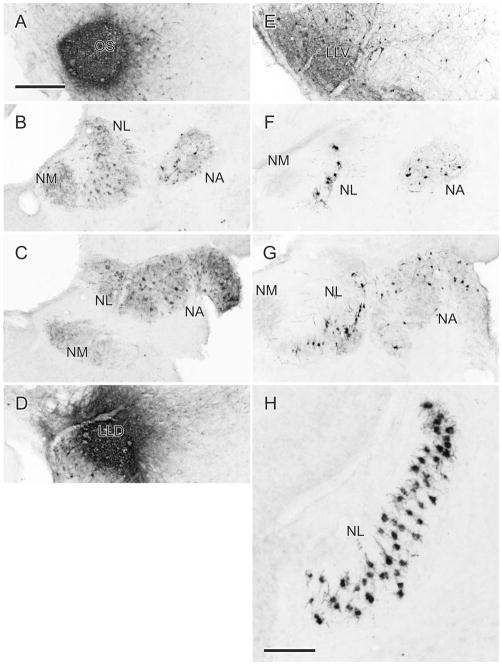
Caudal (A–D), middle (E–H), and rostral (I–L) levels through the superior olive (OS) and lateral lemniscal nuclei (LLV, LLIc, LLIr, LLDa, and LLDp). A,E,I: Schematic outlines of the Nissl-counterstained sections shown in B,F,J. The boxed areas in B,F,J are shown at higher power in C,G,K. D,H,L: Sections at similar levels as C,G,K but have been reacted for cytochrome oxidase. BCA: ascending part of the brachium conjunctivum; FLM: medial longitudinal fasciculus; LLD: dorsal nucleus of the lateral lemniscus; LLIc and LLIr: intermediate nucleus of the lateral lemniscus, caudal and rostral parts; LLV: ventral nucleus of the lateral lemniscus; IOS: nucleus infra-olivaris superior; NVI: abducent nerve; NVII: facial nerve; VIId and VIIv: dorsal and ventral facial motor nuclei; OS: superior olive; PBvl: parabrachial nucleus, ventrolateral part; PLL: para-lateral lemniscal nucleus; PM: medial pontine nucleus; RPgc: pontine reticular nucleus, gigantocellular part; III: oculomotor nucleus; VI: abducens nucleus. Scale bars = 1 mm for the upper two rows and 400 μm for the lower two rows.
Figure 2.
Cell size distributions of neurons in OS and the lateral lemniscal nuclei.
Lateral lemniscal nuclei
The caudal pole of LLV overlaps the rostral pole of OS at the level of the ventral limit of the ventrolateral para-brachial nucleus (PBvl), but LLV lies slightly more peripherally than OS, closer to the spinal lemniscus. It also extends much further rostrally than OS to pass around the ventrolateral periphery of the pons to approximate the ventral border of LLD (Fig. 1E–H). Its neurons are similar in size to those of OS (mean = ≈133 μm2; n = 125; Fig. 2) but without the smallest neurons, and somewhat smaller than LLv neurons in the barn owl (Carr et al., 1989).
LLIc is circular in cross section and lies in the crook of the curved arm of PBvl (Fig. 1E–H). Its cells are distinctly larger than those of either OS or LLV (mean = ≈273 μm2; n = 98; Fig. 2), in fact, as large as the facial motoneurons clearly evident in Fig. 1C. LLIc is a tapered, caudal extension of LLIr, and is situated immediately ventrolateral to, and separate from, the principal sensory trigeminal nucleus (PrV) (Fig. 1A; see also Arends and Zeigler, 1986). Takahashi and Konishi (1988) describe a lateral part of PrV in the barn owl as “nucleus paraprincipalis,” but the relation of this nucleus to LLIc is unclear. Dorsomedial to LLIr is a collection of similar-sized neurons that have been called para-lateral lemniscal (PLL; Fig. 1E) and shown to project to the telencephalic nucleus basorostralis (Bas), as does LLI (Wild and Farabaugh, 1996). This PLL nucleus should not be confused with a different nucleus baring the same name in the chick (Puelles et al., 1997).
LLD is cytoarchitecturally more complex than the other lateral lemniscal nuclei and extends more rostrally and dorsally than both LLV and LLI (Fig. 1I–L). As in the barn owl, LLD in zebra finches has two parts, LLDa and LLDp, although the two parts are for the most part rostrocaudally coextensive, as are the comparable nuclei in the barn owl (Takahshi and Konishi, 1988). The dorsal border of LLDp lies immediately ventromedial to the ventral tip of nucleus semilunaris, while its ventral border merges indistinctly with the dorsal border of LLV. LLDa lies medial to LLDp, such that a part of LLDp lies mostly dorsolateral to LLDa and another part mostly ventrolateral to LLDa (Fig. 1I). The mean areas of cells making up the two subdivisions of LLD are: ≈180 μm2; n = 154 for LLDa and ≈186 μm2; n = 155 for LLDp (Fig. 2). These sizes are somewhat larger than those of the comparable nuclei in the barn owl (Carr et al., 1989). It is possible that LLDa and LLIr have been conflated in various previous studies in other species, their being regarded as rostral and caudal extensions of the same nucleus, usually known as VLV (Karten and Hodos, 1967). But, as we will show, LLDa is definitely a subnuclear component of a dorsal lemniscal nucleus, hodologically distinguishable from LLIr. As can be seen in Fig. 1K, LLDa and LLDp are traversed by horizontal striations (fiber fascicles) of the commissure of Probst, which carries LLD axons across the midline to LLD and MLd on the contralateral side (see below). LLV and LLD are traversed from below by fiber fascicles of the lateral lemniscus as they ascend dorsolaterally, with many fibers also passing both medial and lateral to the nuclei. The lateral lemniscal nuclei and OS are also identifiable on the basis of cytochrome oxidase histochemistry (Fig. 1D,H,L).
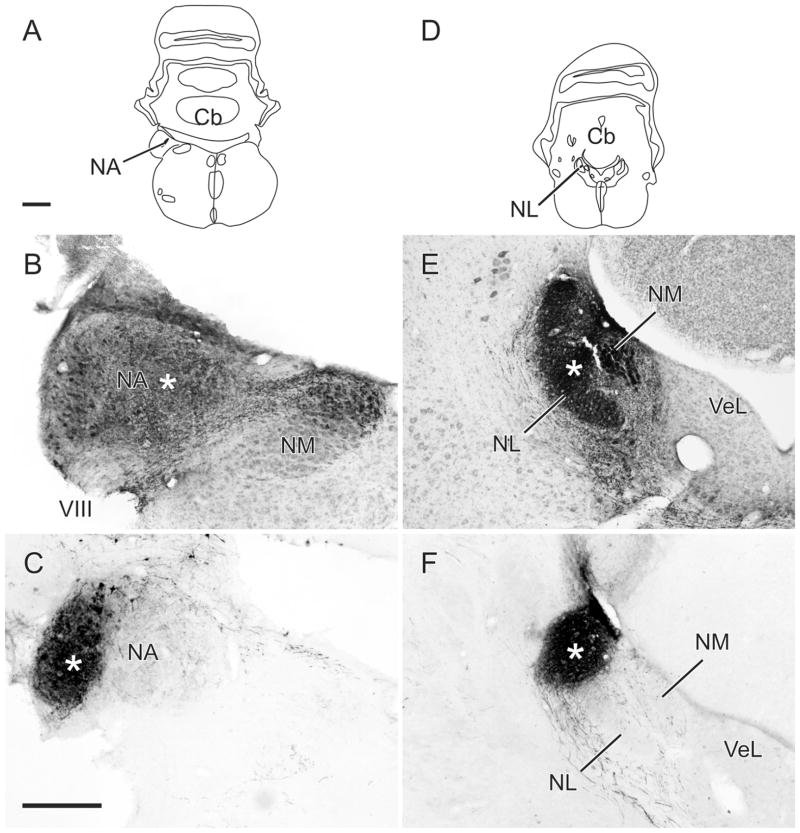
Projections of NA
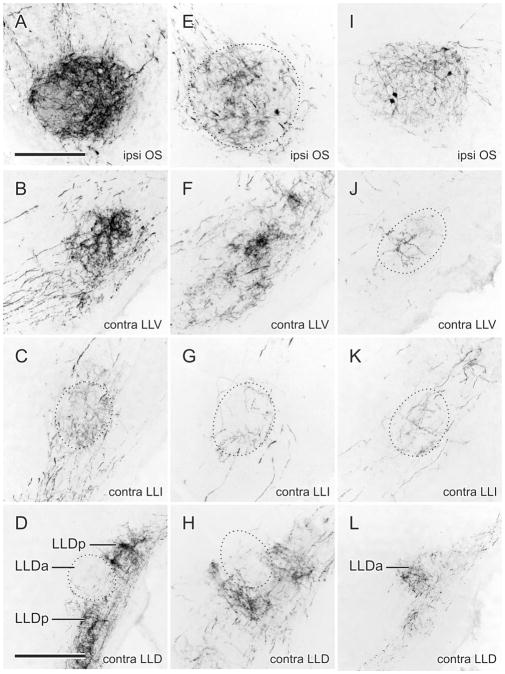
Two injections of BDA in different parts of NA are shown in Fig. 3. One was centered about half-way down the medial part of the nucleus and the other was confined to the lateral part. Via somatopetal and somatofugal transport to and from ganglionic neurons, terminal labeling in the ipsilateral NM was found to result from both these injections. The medial injection produced terminal labeling in the medial part of caudal NM (Fig. 3B), consistent with lower frequency representation (Konishi, 1970), while the lateral injection produced terminal labeling in the most rostral part of NM (not shown), consistent with higher frequency representation (Konishi, 1970). Figures 4 and 5 show the ascending projections resulting from each of these injections. Labeled fibers first descended through the lateral tegmentum in two to three swaths to terminate in the ipsilateral OS. Medial NA injections produced terminal fields predominantly in the medial part of OS, and lateral NA injections produced terminal fields predominantly in lateral parts of OS. Similar but sparser terminal fields were produced in the contralateral OS (Fig. 5) by fibers that crossed in the ventral tegmentum, dorsal to the ipsilateral OS. Thenceforth, the great majority of the NA projections were to the contralateral LLV, LLI, and LLD (Fig. 4B–D,F–H). Terminal labeling in LLV and LLD was heavier than that in LLI. That in LLI was predominantly in LLIr, with only a few fibers in LLIc; that in LLD appeared to avoid LLDa, being confined very largely to LLDp (Fig. 4D,H). There were sparse terminations in the ipsilateral LLV and LLI, but none in the ipsilateral LLD.
Figure 3.
A,D: Schematics showing the location of the centers of injections in NA and NL shown in B,E, respectively. B,C: Injections of BDA in the medial and lateral parts of NA, respectively (B is counterstained). E,F: Injections of BDA in the middle and lateral (dorsal) parts of NL, respectively (E is counterstained). Asterisks mark the approximate center of each injection. Scale bars = 1 mm for the schematics and 250 μm for the photomicrographs.
Figure 4.
A–D: Fiber and terminal labeling in the left (ipsilateral) OS (A) and right (contralateral) LLV (B), LLIr (C), and LLDp (D) following an injection of BDA into medial NA shown in Fig. 3A,B. E–H: Fiber and terminal labeling in the left (ipsilateral) OS (E) and right (contralateral) LLV (F), LLIr (G), and LLDp (H) following an injection of BDA into lateral NA shown in Fig. 3C. I–L: Fiber and terminal labeling in the left (ipsilateral) OS (I) and right (contralateral) LLV (J), LLIr (K), and LLDa (L) following an injection of BDA into mid-NL shown in Fig. 3E. Scale bars = 200 μm for A–K; 400 μm for D,H,L.
Figure 5.
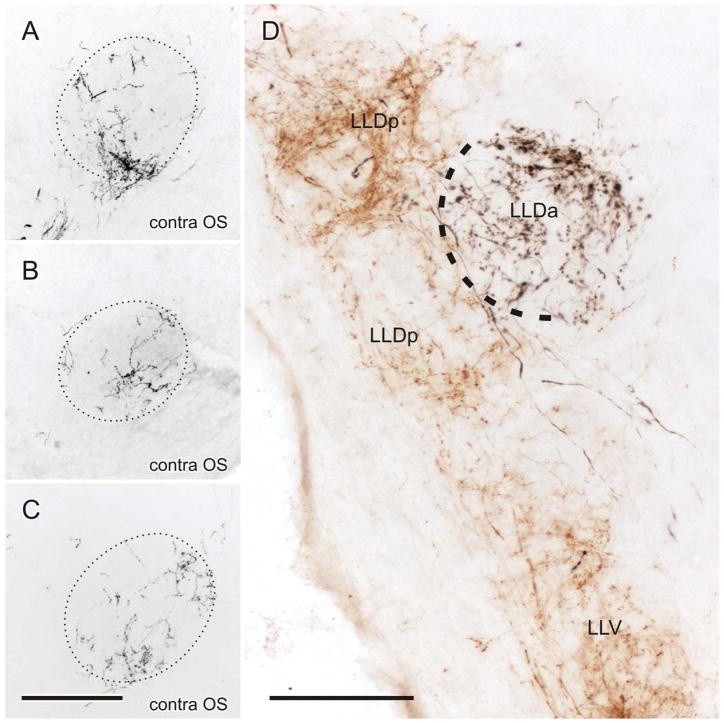
A–C: Fiber and terminal labeling in the contralateral OS following injections of BDA into medial NA, lateral NA, and mid-NL, respectively. D: Brown (CTB) labeling in LLV and LLDp and black (BDA) labeling in LLDa, following injections of CTB and BDA in the contralateral NA and NL of the same case, respectively. Black label was produced with heavy metal (CoCl2) intensification of the DAB reaction product, while brown label was produced without such intensification (see Materials and Methods in Krützfeldt et al., 2010). Scale bars = 200 μm for A–C; 125 μm for D.
Projections of NL
Two typical injections of BDA in NL are shown in Fig. 3, one located in the middle of the nucleus, the other dorsally or laterally. Both produced retrograde labeling of NM neurons on both sides of the brain (not shown). Projections to the ipsilateral OS (Fig. 4I) and to the contralateral LLV, LLI, and LLD (Fig. 4J–L) were sparser than those of NA to these nuclei, and labeling was sparse to very sparse in the contralateral OS (Fig. 5C) and ipsilateral LLV and LLI. Projections were absent from the ipsilateral LLD. Within the ipsilateral OS, a topographic organization of the NL projections resulting from injections in different parts of NL could not be discerned, although, across different cases, the whole of the nucleus appeared to receive NL projections. Within the contralateral LLD, terminations were largely confined to LLDa (Figs. 4L, 5D) and could be distinguished from labeled fibers that traversed this nucleus en route to MLd.
Injections in both NA and NL
Four cases received dual injections, each nucleus receiving either CTB or BDA. Since label resulting from the NL and NA injections was colored either black or brown, the pattern of their distinct projections and terminal fields could be observed in the same sections. As shown for a typical case in Fig. 5D, LLDp was anterogradely labeled from the NA injections, while LLDa was separately labeled from the NL injection. Variable degrees of overlap were present in OS and LLV and in LLI the NL terminations tended to lie medial to those of NA.
Retrograde confirmation of afferent origins
Injections of either BDA or CTB were made into OS (n = 5), LLV (n = 5), and LLD (n = 2) (Fig. 6). The OS and LLV injections retrogradely labeled cells throughout the ipsilateral NL and NA, with smaller numbers in the contra-lateral NL and NA. No labeled cells were present in NM. The two injections in LLD were centered on LLDa, with a variable amount of involvement of LLDp. The great majority of labeled cells from these injections were found in NL contralateral to the injection (Fig. 6H), with some scattered cells in NA. No labeled cells were present in NL ipsilateral to the injection and no labeled cells were present in NM.
Figure 6.
A–C: An injection of CTB in the left OS (A) produces retrogradely labeled cells predominantly in the right NA and NL at rostral (B) and more caudal (C) levels. E–G: An injection of BDA in the left LLV (E) produces retrogradely labeled cells predominantly in the right NA and NL at rostral (F) and more caudal (G) levels. D,H: An injection of CTB centered on the left LLDa, with some involvement of LLDp (D), produces retrogradely labeled cells predominantly in the right NL (H), with a few in the right NA (not shown). Scale bars = 100 μm; 250 μm for all others.
DISCUSSION
In the companion article (Krützfeldt et al., 2010) we drew attention to a technical problem that, despite our best efforts to confine the injections to either NA or NL, could have led to a small contamination of NA efferents by NL injections. This was because some axons of NA neurons were observed to travel medially as far as the wall of glia that flanks NL on its lateral side, before turning ventrally through the tegmentum. This meant that such axons could have taken up the tracer injected into NL, perhaps as a result of damage to those axons, thereby resulting in transport by NA neurons. That this actually occurred, albeit on a small scale, was deduced from the fact that a few NA cell bodies were retrogradely labeled by some of the NL injections. We suggested that, although it was impossible to quantify the proportion of the projections resulting from this inadvertent uptake, it was likely to be small and therefore unlikely to account for the very substantial overlap of the NA and NL projections to MLd that we observed. Since in the present study the same injections were used to define the NA and NL projections to the OS and the lateral lemniscal nuclei, the same proviso applies. Again, however, we suggest that the interpretation of the results is unlikely to be significantly compromised by the small amount of uptake by NA fibers of passage. Information relevant to this suggestion is presented below.
Superior olive
In the zebra finch we found that, following injections in either NA or NL, anterograde labeling was found in both the ipsilateral and the contralateral OS, although in each case the contralateral labeling was less than the ipsilateral labeling. These results differ from those reported in other species such as pigeons (Leibler, 1975), chickens (Conlee and Parks, 1986), mallards (Arends, 1981), and barn owls (Takahashi and Konishi, 1988), in that, unlike NA, NL in these species has not been found to project to the contralateral OS. The question here, however, is whether the contralateral labeling we observed in the zebra finch is real, i.e., derives from NL or is a result of uptake by NA fibers of passage (see above), NA having been shown to project to the contralateral OS (see Results). We suspect the NL labeling is real 1) because of its consistency across cases having either none or a variable number of retrogradely labeled cells in NA; 2) because in some sections from dual injection cases black BDA (NL) labeling was present in the contralateral OS in the absence of brown CTB (NA) labeling, even though the black retrogradely labeled cells in NA were located in the center of the CTB injection in NA; and 3) retrogradely labeled cells were present in NL bilaterally following unilateral injections in OS.
The projections of NA and NL to the ipsilateral OS have been described in the chicken (Conlee and Parks, 1986; see also Burger et al., 2005) and barn owl (Takahashi and Konishi, 1988), such that there is at least partial separation of the terminations from the two nuclei. In the chicken the NL projections appear to be restricted to dorsal regions of the nucleus, whereas the NA projections occupy a more ventrolateral position at caudal levels and most of the nucleus at more rostral levels (Conlee and Parks, 1986). In the barn owl the NA projections occupy largely dorsolateral and ventromedial regions of the ipsilateral OS, whereas the NL projections occupy medial to dorsomedial regions. Contralaterally, the NA projections are much more sparse and diffuse, and therefore could overlap with the ipsilateral NL projection to some extent (Takahashi and Konishi, 1988). In the zebra finch we found good evidence of a topographic organization of the NA projections to OS, but not for the NL projections. Injections in medial NA produced predominantly medial terminal fields in the ipsilateral OS, while lateral NA injections produced lateral terminal fields. Over a series of cases the NL projections to OS appeared to cover the whole nucleus, implying substantial overlap of the NL and NA projections. In the barn owl OS units excited by stimulation of the ipsilateral ear are tonotopically organized in lateral and posterior parts of the nucleus, with best frequencies increasing from dorsal to ventral (Moiseff and Konishi, 1983). Binaural units excited by input from either ear are found more medially and centrally in the nucleus.
Lateral lemniscal nuclei: NA projections
In the zebra finch NA to projects bilaterally to LLV and LLI, with a strong contralateral predominance, and to LLDp contralaterally only. Similarly in barn owls, NA projects to LLV bilaterally and to LLDp contralaterally (Takahashi and Konishi, 1988), although, as the authors point out, the first finding is curiously at variance with that of Moiseff and Konishi (1983), who found strictly monaural units in LLV. LLDp in the barn owl is said to be the first binaural site in the pathway that processes differences in intensity of sounds arriving at the two ears. The excitatory input originates from the contralateral NA and the inhibitory input from the contralateral LLDp (Manley et al., 1988; Takahashi and Keller, 1992). Comparable physiological information is not available for the zebra finch, although the pattern of inputs from NA and the contralateral LLDp seems similar (present results; Wild et al., 2010). In pigeons and chickens NA projects to LLV contralaterally only, but bilaterally to a nucleus Leibler (1975) called VLVp and Conlee and Parks (1986) called VLV. As we point out in the introduction, we consider the nucleus designated by these last two abbreviations to be equivalent to LLIr in the zebra finch (see also Arends and Zeigler, 1986), so the results in the zebra finch are probably congruent with those in pigeons and chickens, at least with respect to LLI. An LLI was not initially recognized in the barn owl (Takahashi and Konishi, 1988).
NL projections
In the zebra finch NL projects to the contralateral LLV, LLIr (with only a very sparse projection to LLIc in one case), and LLDa. In one other case we found a sparse projection to the ipsilateral LLV as well. Similarly in pigeons (Leibler, 1975) and chickens (Conlee and Parks, 1986), NL projects to the equivalent of the zebra finch LLV (LLv) contralaterally and to the equivalent of LLI (Leibler’s VLVp and Conlee and Parks’ VLV). In the barn owl, however, NL does not project to LLV, a finding which is congruent with the apparent lack of binaural units in the nucleus (Moiseff and Konishi, 1983; see above). As in zebra finches, however, NL in barn owls projects to LLDa, which is large enough in that species to permit a mapping of different frequencies (Takahashi and Konishi, 1988). Whether LLDa in the zebra finch is part of a pathway that registers interaural time differences, as LLDa does in the barn owl (Konishi, 2003), is presently unknown. Like LLDp, it receives a projection from its contralateral counterpart and it projects to the contralateral MLd (Wild et al., 2010).
Dual NA and NL injections
The main purpose of these combined injection cases was 1) to assess the amount of overlap of the projections to MLd in the same sections (see companion article, Krützfeldt et al., 2010), and 2) to distinguish between the NA and NL projections to LLD. In this they were successful in showing that NA projects to LLDp, while NL projects to LLDa, an important distinction that supports the comparison of LLD in zebra finches and barn owls, despite the obvious difference in nuclear size between the two species. A similar pattern of NA and NL projections to LLD may have been shown by Leibler (1975) in pigeons. He found NL to project specifically on a ventral part of his VLVa, which possibly corresponds to LLDa in the zebra finch, and NA to project predominantly on a dorsal part of his VLVa, which may correspond to the dorsal part of LLDp in the zebra finch. Therefore, Leibler may have been the first to define NL and NA inputs to separate LLD sub-nuclei. If correct, the findings of separate NL and NA inputs to LLD are congruent across three species: barn owls, pigeons, and zebra finches. Whether these inputs process time and intensity parameters in the last two species, as they have been shown to do in barn owls, remains to be investigated.
Comparison with mammals
Takahashi and Konishi (1988) compared their findings with respect to the lateral lemniscal nuclei in barn owls with those in cats, the species for which there was at that time the most information. Since then there has been a host of studies in a variety of species dealing with the projections of the cochlear nuclei to one or more of the superior olivary nuclei and/or the three lateral lemniscal nuclei (e.g., Friauf and Ostwald, 1988; Kuwabara et al., 1991; Smith et al., 1991, 1993; Huffman and Covey, 1995; Schofield, 1995; Schofield and Cant, 1997; Adams, 1997; Glendenning and Hutson, 1998; Thompson and Schofield, 2000; Doucet and Ryugo, 2003), findings from these and other studies having been extensively reviewed (Schwartz 1992; Helfert and Aschoff, 1997; Oertel and Wickesberg, 2002; Cant and Benson, 2003; Schofield, 2005; Benson and Cant, 2008).
The avian superior olive has no known equivalent in the superior olivary complex of mammals, Boord’s (1968) suggestion that it corresponds with the LSO of mammals notwithstanding (Takahashi and Konishi, 1988). OS receives bilateral inputs from NA and, in the zebra finch, bilateral inputs from NL, but information as to whether OS cells are monaural or binaural is available only for the barn owl: 72% of cells were monaural (“OE,” indicating a response only to ipsilateral stimulation) and 23% binaural (“EE,” excited by stimulation of either ear) (Moiseff and Konishi, 1983).
In mammals the ventral nucleus of the lateral lemniscus (VNLL) receives its ascending input predominantly from the contralateral ventral cochlear nucleus, with additional inputs from the OS complex (reviewed in Benson and Cant, 2008). Dorsal and ventral parts of VNLL have been recognized in some species, with some cell-type specific projections from the VCN to VNLLv. VNLL provides a major brainstem input to the ICc (Helfert and Aschoff, 1997). This projection is ipsilateral and probably inhibitory, VNLL cells being either GABAergic and/or glycinergic (reviewed in Schofield, 2005). VNLL has generally been considered a monaural nucleus, especially in bats (Casseday and Covey, 1995), but this is clearly not the case in all mammals (Batra and Fitzpatrick, 2002). Functionally, VNLL is concerned with the analysis of temporal aspects of auditory stimuli, and is possibly involved in auditory pattern recognition (see Schofield, 2005).
In the zebra finch LLV receives its cochlear nuclear projection predominantly from the contralateral NA, with additional inputs from NL and OS, and hence is likely to be a binaural nucleus, the finding of only “EO” cells in LLv by Moiseff and Konishi (1983) notwithstanding (see Takahashi and Konishi, 1988). Different parts of LLV have not been recognized in birds. Some evidence exists for glycinergic cells in the LLV of chickens, but their projections are unknown (Westerberg and Schwarz, 1995). Most cells in LLV of barn owl are GABAergic (Carr et al., 1989) and possibly in pigeons as well (Domenici et al., 1988; Veenman and Reiner, 1994). Like VNLL in mammals, LLV in birds is also a major source of brainstem projections to the ICc (MLd) (Wild et al., 2010). In pigeons LLV also provides a projection that bypasses the midbrain to terminate directly in the nucleus ovoidalis (Ov) of the auditory thalamus, from where information is probably relayed to a broad band or low-frequency region of the auditory “cortex” (Wild, 1987; Wild et al., 1993). A direct LLV projection to Ov is also present in the zebra finch (Wild et al., 2009). Because auditory pattern recognition is a requirement for song perception, it is possible that LLV is also involved in this function in songbirds.
An intermediate nucleus of the lateral lemniscus (INLL) is not recognized in all mammalian species, bats being an exception. It is considered a monaural nucleus, with inputs from the contralateral VCN and ipsilateral MNTB (Oertel and Wickesberg, 2002). As for the other two lateral lemniscal nuclei, its outputs are to the inferior colliculus. In contrast, in birds LLI not only receives inputs from the contralateral NL and NA, but also bilateral inputs from OS and is, therefore, likely to be part of the binaural system. Moreover, LLI does not project on the inferior colliculus, but in all avian species studied projects on a telencephalic nucleus known as basorostralis (Bas), located laterally adjacent to the lateral striatum (Arends and Zeigler, 1986; Hall et al., 1993; Wild and Farabaugh, 1996; Wild et al., 1997, 2001). LLI provides a short latency auditory projection to Bas (Delius et al., 1979) and in the barn owl it provides for a tonotopic organization of Bas (Wild et al., 2001).
The dorsal nucleus of the lateral lemniscus (DNLL) in mammals is a single nucleus that is considered part of the binaural system (Schofield, 2005). It is reciprocally connected across the midline, via GABAergic neurons, with its contralateral counterpart and it projects bilaterally to the ICc (Pollak et al., 2003; Schofield, 2005). In birds two parts of LLD are present, an anterior part receiving input mainly from the contralateral NL and a posterior part mainly from the contralateral NA (Wild, 1987; Müller, 1987; Takahashi and Konishi, 1988; present results). Consequently, in barn owls “EE” cells sensitive to interaural phase differences were found in LLDa, whereas “EI” cells sensitive to interaural level differences were found in LLDp (Moiseff and Konishi, 1983; Manley et al., 1988; Mogdans and Knudsen, 1994). In barn owls, chickens, and pigeons, LLD neurons are GABAergic and inhibitory (Müller, 1987; Domenici et al., 1988; Carr et al., 1989; Takahashi and Keller, 1992; Veenman and Reiner, 1994) and in the zebra finch both LLDa and LLDp are connected with their contralateral counterparts via the commissure of Probst (Wild et al., 2010), as is LLDp in the barn owl (Takahashi and Keller, 1992; Takahashi et al., 1995). Furthermore, both parts also project to MLd, but in the zebra finch the projection of LLDa is entirely contralateral and that of LLDp almost entirely contralateral (Wild et al., 2010). In zebra finches both LLD nuclei also project directly on the auditory thalamus, although less densely than LLV (Wild et al., 2010).
One of the reasons for conducting the present studies was to define the ascending pathways that could mediate the auditory feedback that is important in the control of song learning and production. Similar concerns have been the focus of studies in bats that have attempted to determine the location of the audio-vocal interface important in echolocation (Metzner, 1993, 1996). In birds, however, there is as yet no evidence that an audio-vocal interface that might be involved in vocal control is present at brainstem levels. Rather, such an interface in songbirds is generally thought to occur at telencephalic levels (Janata and Margolish, 1999; Coleman and Mooney, 2004; Coleman et al., 2007).
Acknowledgments
Grant sponsor: Royal Society of New Zealand Marsden Fund (to J.M.W); Grant sponsor: National Institutes of Health (NIH); Grant number: R01 NS029467 (to R.A. Suthers and J.M.W.).
We thank Silke Fuchs for technical assistance.
LITERATURE CITED
- Adams JC. Projections from octopus cells of the posteroventral cochlear nucleus to the ventral nucleus of the lateral lemniscus in cat and human. Aud Neurosci. 1997;3:335–350. [Google Scholar]
- Arends JJ. PhD Thesis. Leiden, Netherlands: University of Leiden; 1981. Sensory and motor aspects of the trigeminal system in the mallard (Anas Platyrhynchos L.) [Google Scholar]
- Arends JJ, Zeigler HP. Anatomical identification of an auditory pathway from a nucleus of the lateral lemniscal system to the frontal telencephalon (nucleus basalis) of the pigeon. Brain Res. 1986;398:375–381. doi: 10.1016/0006-8993(86)91499-x. [DOI] [PubMed] [Google Scholar]
- Batra R, Fitzpatrick DC. Monaural and binaural processing in the ventral nucleus of the lateral lemniscus: a major source of inhibition to the inferior colliculus. Hear Res. 2002;168:90–97. doi: 10.1016/s0378-5955(02)00368-4. [DOI] [PubMed] [Google Scholar]
- Benson CG, Cant NB. The ventral nucleus of the lateral lemniscus of the gerbil (Meriones unguiculatus): organization of connections with the cochlear nucleus and the inferior colliculus. J Comp Neurol. 2008;510:673–690. doi: 10.1002/cne.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boord RL. Ascending projections of the primary cochlear nuclei and nucleus laminaris in the pigeon. J Comp Neurol. 1968;133:523–541. doi: 10.1002/cne.901330410. [DOI] [PubMed] [Google Scholar]
- Burger RM, Cramer KS, Pfeiffer JD, Rubel EW. Avian superior olivary nucleus provides divergent inhibitory input to parallel auditory pathways. J Comp Neurol. 2005;481:6–18. doi: 10.1002/cne.20334. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Covey E. Mechanisms for analysis of auditory temporal patterns in the brainstem of echolocating bats. In: Covey E, Hawkins HL, Port RF, editors. Neural representation of temporal patterns. New York: Plenum Press; 1995. pp. 25–51. [Google Scholar]
- Coleman MJ, Mooney R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J Neurosci. 2004;24:7251–7265. doi: 10.1523/JNEUROSCI.0947-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlee JW, Parks TN. Origin of ascending auditory projections to the nucleus mesencephalicus lateralis pars dorsalis in the chicken. Brain Res. 1986;367:96–113. doi: 10.1016/0006-8993(86)91583-0. [DOI] [PubMed] [Google Scholar]
- Delius JD, Runge TE, Oeckinghaus H. Short-latency auditory projection to the frontal telencephalon of the pigeon. Exp Neurol. 1979;63:594–609. doi: 10.1016/0014-4886(79)90174-2. [DOI] [PubMed] [Google Scholar]
- Domenici L, Waldvogel HJ, Streit P. Distribution of GABA-like immunoreactivity in the pigeon brain. Neuroscience. 1988;25:931–950. doi: 10.1016/0306-4522(88)90047-4. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Axonal pathways to the lateral superior olive labeled with biotinylated dextran amine injections in the dorsal cochlear nucleus of rats. J Comp Neurol. 2003;461:452–465. doi: 10.1002/cne.10722. [DOI] [PubMed] [Google Scholar]
- Friauf E, Ostwald J. Divergent projections of physiologically characterized rat ventral cochlear nucleus neurons as shown by intra-axonal injection of horseradish peroxidase. Exp Brain Res. 1988;73:263–284. doi: 10.1007/BF00248219. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Hutson KA. Lack of topography in the ventral nucleus of the lateral lemniscus. Microsc Res Tech. 1998;41:298–312. doi: 10.1002/(SICI)1097-0029(19980515)41:4<298::AID-JEMT3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hall WS, Cohen PL, Brauth SE. Auditory projections to the anterior telencephalon in the budgerigar (Melopsittacus undulatus) Brain Behav Evol. 1993;41:97–116. doi: 10.1159/000113827. [DOI] [PubMed] [Google Scholar]
- Helfert RH, Aschoff A. Superior olivary complex and nuclei of the lateral lemniscus. In: Ehret G, Romand R, editors. The central auditory system. New York: Oxford University Press; 1997. pp. 193–258. [Google Scholar]
- Huffman RF, Covey E. Origin of ascending projections to the nuclei of the lateral lemniscus in the big brown bat, Eptesicus fuscus. J Comp Neurol. 1995;357:532–545. doi: 10.1002/cne.903570405. [DOI] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. J Neurosci. 1999;19:5108–5118. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ. The organization of the ascending auditory pathway in the pigeon (Columba livia). I. Diencephalic projections of the inferior colliculus (nucleus mesencephali lateralis, pars dorsalis) Brain Res. 1967;6:409–427. doi: 10.1016/0006-8993(67)90055-8. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Hodos W. A stereotaxic atlas of the brain of the pigeon (Columba livia) Baltimore, MD: Johns Hopkins Press; 1967. [Google Scholar]
- Konishi M. Evolution of design features in the coding of species-specificity. Am Zool. 1970;10:67–72. doi: 10.1093/icb/10.1.67. [DOI] [PubMed] [Google Scholar]
- Konishi M. Coding of auditory space. Annu Rev Neurosci. 2003;26:31–55. doi: 10.1146/annurev.neuro.26.041002.131123. [DOI] [PubMed] [Google Scholar]
- Krützfeldt NOE, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. I. Projections of nucleus angularis and nucleus laminaris to the auditory torus. J Comp Neurol. 2010;518:2109–2134. doi: 10.1002/cne.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, DiCaprio RA, Zook JM. Afferents to the medial nucleus of the trapezoid body and their collateral projections. J Comp Neurol. 1991;314:684–706. doi: 10.1002/cne.903140405. [DOI] [PubMed] [Google Scholar]
- Leibler LM. PhD Thesis. Cambridge, MA: Massachusetts Institute of Technology; 1975. Monaural and binaural pathways in the ascending auditory system of the pigeon. [Google Scholar]
- Manley GA, Köppl C, Konishi M. A neural map of interaural intensity differences in the brain stem of the barn owl. J Neurosci. 1988;8:2665–2676. doi: 10.1523/JNEUROSCI.08-08-02665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner W. An audio-vocal interface in echolocating horseshoe bats. J Neurosci. 1993;13:1899–1915. doi: 10.1523/JNEUROSCI.13-05-01899.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner W. Anatomical basis for audio-vocal integration in echolocating horseshoe bats. J Comp Neurol. 1996;368:252–269. doi: 10.1002/(SICI)1096-9861(19960429)368:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Mogdans J, Knudsen EI. Representation of interaural level difference in the VLVp, the first site of binaural comparison in the barn owl’s auditory system. Hear Res. 1994;74:148–164. doi: 10.1016/0378-5955(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. Binaural characteristics of units in the owl’s brainstem auditory pathway: precursors of restricted spatial receptive fields. J Neurosci. 1983;3:2553–2562. doi: 10.1523/JNEUROSCI.03-12-02553.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CM. gamma-Aminobutyric acid immunoreactivity in brainstem auditory nuclei of the chicken. Neurosci Lett. 1987;77:272–276. doi: 10.1016/0304-3940(87)90511-8. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wickesberg RE. Ascending pathways through ventral nuclei of the lateral lemniscus and their possible role in pattern recognition in natural sounds. In: Oertel D, Fay RR, Popper AN, editors. Springer handbook of auditory research. New York: Springer; 2002. pp. 207–237. [Google Scholar]
- Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends Neurosci. 2003;26:33–39. doi: 10.1016/s0166-2236(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martínez-de-la-Torre M, Paxinos G, Watson C, Martínez S. An atlas featuring neuromeric subdivisions and mammalian homologies. Amsterdam: Elsevier Academic Press; 2007. The chick brain in stereotaxic coordinates. [Google Scholar]
- Schofield BR. Projections from the cochlear nucleus to the superior paraolivary nucleus in guinea pigs. J Comp Neurol. 1995;360:135–149. doi: 10.1002/cne.903600110. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Superior olivary complex and lateral lemniscal connections of the auditory midbrain. In: Winer JA, Schreiner CE, editors. The inferior colliculus. New York: Springer; 2005. pp. 132–154. [Google Scholar]
- Schofield BR, Cant NB. Ventral nucleus of the lateral lemniscus in guinea pigs: cytoarchitecture and inputs from the cochlear nucleus. J Comp Neurol. 1997;379:363–385. doi: 10.1002/(sici)1096-9861(19970317)379:3<363::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Schwartz IR. The superior olivary complex and lateral lemniscal nuclei. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: neuroanatomy. New York: Springer; 1992. pp. 117–167. [Google Scholar]
- Smith PH, Joris PX, Carney LH, Yin TC. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol. 1991;304:387–407. doi: 10.1002/cne.903040305. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Banks MI, Yin TCT. Responses of cochlear nuclear cells and projections of their axons. In: Merchan MA, Juiz J, Godfrey D, Mugnaini E, editors. The mammalian cochlear nuclei: organization and function. New York: Plenum Press; 1993. pp. 349–360. [Google Scholar]
- Takahashi TT, Keller CH. Commissural connections mediate inhibition for the computation of interaural level difference in the barn owl. J Comp Physiol [A] Neuroethol Sens Neural Behav Physiol. 1992;170:161–169. doi: 10.1007/BF00196898. [DOI] [PubMed] [Google Scholar]
- Takahashi TT, Konishi M. Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. J Comp Neurol. 1988;274:212–238. doi: 10.1002/cne.902740207. [DOI] [PubMed] [Google Scholar]
- Takahashi TT, Barberini CL, Keller CH. An anatomical substrate for the inhibitory gradient in the VLVp of the owl. J Comp Neurol. 1995;358:294–304. doi: 10.1002/cne.903580210. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Schofield BR. Afferent projections of the superior olivary complex. Microsc Res Tech. 2000;51:330–354. doi: 10.1002/1097-0029(20001115)51:4<330::AID-JEMT4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A. The distribution of GABA-containing perikarya, fibers, and terminals in the forebrain and midbrain of pigeons, with particular reference to the basal ganglia and its projection targets. J Comp Neurol. 1994;339:209–250. doi: 10.1002/cne.903390205. [DOI] [PubMed] [Google Scholar]
- Webster DB. An overview of mammalian auditory pathways with an emphasis on humans. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: neuroanatomy. New York: Springer; 1992. pp. 1–22. [Google Scholar]
- Westerberg BD, Schwarz DW. Connections of the superior olive in the chicken. J Otolaryngol. 1995;24:20–30. [PubMed] [Google Scholar]
- Wild JM. Nuclei of the lateral lemniscus project directly to the thalamic auditory nuclei in the pigeon. Brain Res. 1987;408:303–307. doi: 10.1016/0006-8993(87)90393-3. [DOI] [PubMed] [Google Scholar]
- Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol. 1993;338:225–241. doi: 10.1002/cne.903380207. [DOI] [PubMed] [Google Scholar]
- Wild JM. Convergence of somatosensory and auditory projections in the avian torus semicircularis, including the central auditory nucleus. J Comp Neurol. 1995;358:465–486. doi: 10.1002/cne.903580402. [DOI] [PubMed] [Google Scholar]
- Wild JM, Farabaugh SM. Organization of afferent and efferent projections of the nucleus basalis prosencephali in a passerine, Taeniopygia guttata. J Comp Neurol. 1996;365:306–328. doi: 10.1002/(SICI)1096-9861(19960205)365:2<306::AID-CNE8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Wild JM, Arends JJ, Zeigler HP. Projections of the para-brachial nucleus in the pigeon (Columba livia) J Comp Neurol. 1990;293:499–523. doi: 10.1002/cne.902930402. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory fore-brain in the pigeon (Columba livia) J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- Wild JM, Reinke H, Farabaugh SM. A non-thalamic pathway contributes to a whole body map in the brain of the budgerigar. Brain Res. 1997;755:137–141. doi: 10.1016/s0006-8993(97)00026-7. [DOI] [PubMed] [Google Scholar]
- Wild JM, Kubke MF, Carr CE. Tonotopic and somatotopic representation in the nucleus basalis of the barn owl, Tyto alba. Brain Behav Evol. 2001;57:39–62. doi: 10.1159/000047225. [DOI] [PubMed] [Google Scholar]
- Wild JM, Krützfeldt NOE, Kubke MF. Affferents to the cochlear nuclei and nucleus laminaris for the ventral nucleus of the lateral lemniscus in the zebra finch (Taeniopygia guttata) Hear Res. 2009;212:3119–3124. doi: 10.1016/j.heares.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Wild JM, Krützfeldt NOE, Kubke MF. Connections of the auditory brainstem in a songbird (Taeniopygia guttata). III. Projections of the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010;518:2149–2167. doi: 10.1002/cne.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]