Abstract
Purpose of review
The continued emergence of multiresistant pathogens and widespread antimicrobial use has led to a greater emphasis on antimicrobial stewardship programs. Concurrently, an increased awareness of the rising number of antibiotic allergy labels and impact on antimicrobial use has surfaced. The integration of antibiotic allergy de-labeling and antimicrobial stewardship programs may be a pathway worthy of further focus and investigation.
Recent findings
Recent literature has evaluated the efficacy of antibiotic allergy management (historical de-labeling, in-vitro testing, skin prick testing, intradermal testing, and oral challenges) and impact of antibiotic allergy labels on patient outcome. The importance of true and perceived antibiotic allergy cross-reactivity in the setting of β-lactam allergies has been highlighted. The impact of dedicated antibiotic allergy de-labeling clinics, inpatient antibiotic allergy testing, and integrated antimicrobial stewardship programs has been recently appraised.
Summary
More recent literature supports that appropriate antibiotic allergy in-vitro and in-vivo testing and subsequent antibiotic allergy de-labeling, particularly in regard to β-lactams, can decrease broad-spectrum antibiotic use, costs, patient length of stay, and mortality. Integration of antibiotic allergy management into the decision support systems of inpatient and outpatient antimicrobial stewardship programs represents an important opportunity to further improve measured outcomes from antibiotic utilization.
Keywords: antibiotic allergy, antimicrobial stewardship, β-lactam, de-labeling
INTRODUCTION
Antibiotic allergy labels are commonly reported but misleading, and negatively impact on antibiotic utilization and patient outcome. Antibiotic allergies have primarily been reported for β-lactams, with a wide range of clinical manifestations and a diverse immunopathogenetic basis [1▪▪,2].
Approximately, 10–15% of hospitalized patients are labeled as penicillin allergic, whereas 80–90% of penicillin allergic labeled patients are negative on penicillin skin testing [3]. The overestimation of cross-reactivity between and among β-lactams leads to further errors in antibiotic allergy labeling. Imprecise antibiotic allergy labeling is associated with increased antibiotic costs, antimicrobial use, risk of acute care admission, and mortality [4,5▪]. Preliminary studies highlight an improvement in antibiotic utilization and patient outcomes with integrated in-vitro and in-vivo antibiotic allergy testing, re-challenging, de-labeling, and antimicrobial stewardship programs [6▪▪,7▪▪]. We review the current understanding of antibiotic allergy classification, pathogenesis, testing, and the impact of the antibiotic allergy label, and highlight the opportunity for integrated and proactive antimicrobial allergy, stewardship and decision support programs aimed at improving antibiotic prescribing and patient outcome.
ANTIBIOTIC ALLERGY CLASSIFICATION
Type A adverse drug reactions (ADRs) comprise more than 80% of all ADRs and relate to the drug’s predictable pharmacological properties. Type B ADRs are largely immunologically mediated with less predictable relation to dose and pharmacological action (Table 1) [1▪▪,8,9,10▪▪,11▪▪]. Immunologically mediated (allergic or hypersensitivity) reactions have been historically classified as immediate (<1 h) or nonimmediate (>1 h) [12]. Accelerated antibiotic reactions are defined as occurring more than 1 hour and less than 72 hours after exposure and although these can be immunoglobin E (IgE)-mediated, alternative mechanisms (e.g., T-cell-mediated) are likely. Drug hypersensitivity is traditionally classified based on immunological mechanism [8,9,12,13]. The diagnostic sensitivity and negative predictive value (NPV) for antibiotic allergy testing is greatest with immediate/IgE-mediated (type I) reactions and in particular for penicillin skin testing using validated testing determinants [3].
Table 1.
Classification of antibiotic allergy and recommended testing
| Gell and Coombs classificationa/ ADR type | Immunological mechanism | Timing | Clinical | Commonly involved antibiotics | Testingb |
|---|---|---|---|---|---|
| Class I: type B ADR | IgE-mediated; <30 min to 1 h | Immediate or accelerated (30 min to 1 h and less commonly 6–48 h) | Pruritus/urticaria; angioedema; bronchospasm; laryngeal edema; anaphylaxis | β-lactams; sulfa antimicrobials; macrolides; fluoroquinolones | IgE/RASTc; SPT; IDT; oral challenged |
| Class II: type B ADR | Cytotoxic; IgG-mediated; 5 to >72 h | Accelerated or delayed (5 to >72 h) | Hemolytic anemia; thrombocytopenia | Sulfa antimicrobials; rifampin; dapsone; β-lactams; vancomycin | Drug-specific antiplatelet antibodies |
| Class III:type B ADR | Immune complex; IgG-mediated; 3 to >72 h | Accelerated or delayed; (3 to >72 h) | Serum sickness | β-lactams; sulfa antimicrobials; minocycline | No |
| Hypersensitivity, small vessel vasculitis | |||||
| Class IV: type B ADR (subclass a–d) | T-cell-mediated; IVa: macrophages; IVb: eosinophils; IVc: T cells; IVd: neutrophils | Delayed (24 to >72 h)e | Contact dermatitis, SCAR (DRESS/DIHS/ HSS/SJS/TEN, AGEP), DILI, AIN, FDE; nonspecific (maculopapular) exanthem | β-lactams; sulfa antimicrobials; fluoroquinolones; tetracyclines; macrolides; antiretrovirals (abacavir, nevirapine, and other NNRTIs); dapsone; vancomycin; antituberculous drugs; telaprevir (hepatitis C) | LTT/ELISpot/ICSc; Patch testing; delayed IDT; oral challenge; HLA screene |
AGEP, acute generalized exanthematous pustulosis; AIN, acute interstitial nephritis; BAT, basophil activation testing; DIHS, drug-induced hypersensitivity syndrome; DILI, drug-induced liver injury; DRESS, drug reaction with eosinophilia and systemic symptoms; ELISpot, enzyme-linked immunospot; FDE, fixed drug eruption; HLA, human leukocyte antigen; HSS, hypersensitivity syndrome; ICS, intracellular cytokine staining; IDT, intradermal testing; Ig, immunoglobulin; LTT, lymphocyte transformation test; SJS, Stevens–Johnson syndrome; SPT, skin prick testing; TEN, toxic epidermal necrolysis.
Type B adverse drug reactions (ADRs) – immunologically mediated allergies, further subclassified into classes I–IV based on Gell and Coombs classification [8], recently updated by Pichler [9]. Type A ADRs are common and predictable drug reactions that are dose-dependent and based on pharmacological properties.
Combination in-vivo testing recommended; SPT/IDT and oral challenge are considered gold standard.
RAST screening only (specific not sensitive) and not available in all countries. LTT and ELISpot not recommended for IgE-mediated drug allergy testing, but can be used for type IV-mediated reactions.
Can occur from 1–2 days to 8 weeks following drug. No specific test for delayed drug hypersensitivity has adequate sensitivity or 100% negative predictive value to be used as the sole basis for rechallenge in the case of severe drug reaction. May be more rapid/severe on second exposure [10▪▪].
ANTIBIOTIC ALLERGY MECHANISMS: THE IMPORTANCE OF SIDE CHAINS AND CROSS-REACTIVITY
In penicillin allergic patients, 5% or less (first generation cephalosporin) and 2% or less (second, third or fourth generation cephalosporin) will be expected to have in-vivo cross-reactivity [14]. Reports of much higherpenicillin cross-reactivity with first generation cephalosporins prior to 1980 were probably due to contamination with penicillin during manufacturing, shared side chains of earlycephalosporins such as cephaloridine and cephalothin with penicillin G, and reporting methodology [15–17]. Identifying true cross-reactive allergies is paramount to safe and effective future antibiotic use [17].
Allergic responses to β-lactam antibiotics can involve sensitization to the β-lactam ring, subclass-specific side ring, and/or side chains [‘R group(s)’], as demonstrated in Fig. 1 [1▪▪,3,17,18]. The side chains of β-lactams play a dominant role in both immediate and delayed allergic reactions [1▪▪]. There is a wide range of shared side chains between different classes of β-lactam drugs as well as within subclasses (Tables 2 and 3) [1▪▪,18,19▪▪].
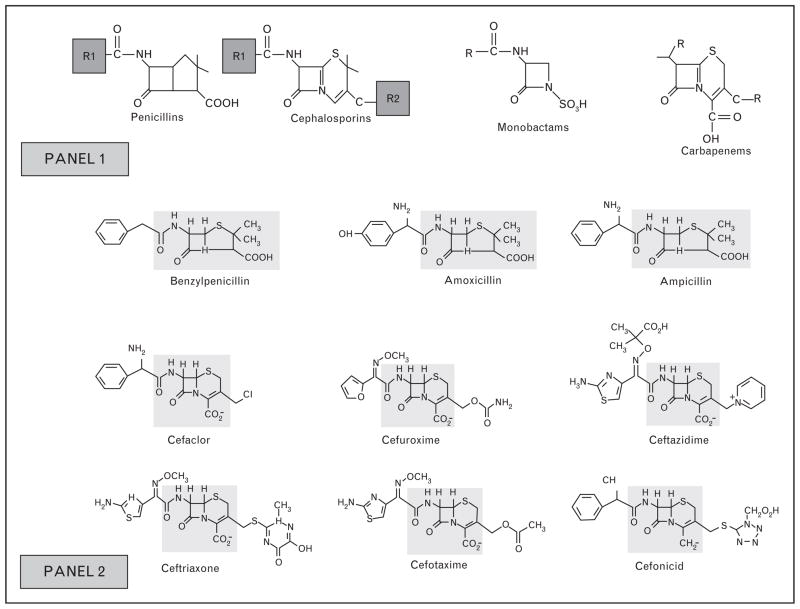
FIGURE 1.
Structure of conserved regions and side chains of common β-lactam antibiotics. Adapted from Antunez et al. [18]. Panel 1: Demonstrates structures of β-lactams. β-Lactams consist of a four-member β-lactam ring, the penicillins connnected to a five-member thiazolidine ring and cephalosporins to a six-member dihydrothiazine ring. Penicillins have one side chain (R1) and cephalosporins have two (R1 & R2). Whereas R2 provides useful pharmacological properties, R1 has the greatest immunogenic properties [1▪▪]. Monobactams and carbapenems also have side chains (R) that commonly do not cross-react. Panel 2: Demonstrates the common penicillin, aminopenicillin, and cephalosporin core structures (shaded regions) and R1/R2 side chains. Cross-reactivity between cephalosporins and penicillins with different side chains due to IgE against the β-lactam core is uncommon [3,17], as rapid degradation of cephalosporins forms molecules with no clear structural similarities to the major and minor determinants of penicillin [1▪▪].
Table 2.
β-Lactam antibiotics that share identical R1-group side chain
| Penicillin G | Amoxicillin | Ampicillin | Ceftriaxone | Cefoxitin | Cefamandole | Ceftazadime |
| Cephaloridine | Cefadroxil | Cefaclorb | Cefotaxime | Cephaloridine | Cefonicid | Aztreonam |
| Cephalothin | Cefprozil | Cephalexin | Cefpodoxime | Cephalothin | ||
| Cefoxitina | Cefatrizine | Cephadrine | Cefditoren | |||
| Cephalexina | Cephaloglycin | Ceftizoxime | ||||
| Loracarbef | Cefmenoxime | |||||
| Cefepimea |
Table 3.
β-Lactam antibiotics that share identical R2-group side chains
| Cephalexin | Cefotaxime | Cefuroxime | Cefotetan | Cefaclor | Ceftibuten |
| Cefadroxil | Cephalothin | Cefoxitin | Cefamandole | Loracarbef | Ceftizoxime |
| Cepharadine | Cepahloglycin | Cefmetazole | |||
| Cephapirin | Cefpiramide |
Rates of β-lactam cross-reactivity in cephalosporin allergic patients vary (8.3–50%) [20], and when oral challenge is utilized (the gold standard for tolerance) this is much lower [21▪▪]. In addition, commonly used cephalosporins such as cefazolin that have distinct side chains have been shown to primarily cause selective IgE-mediated reactions with low cross-reactivity with other β-lactams [22]. Cross-reactivity between cephalosporins is low due to the heterogeneity between side chains (Fig. 1) [22,23]. The in-vivo cross-reactivity between penicillins and carbapenems is less than 1% [23]. Aztreonam, a monobactam, can be safely administered to those with confirmed IgE or T-cell-mediated β-lactam allergy despite earlier concerns over a shared β-lactam ring (Fig. 1) [23,24]. For ceftazidime and aztreonam, which share an identical side chain, in-vivo cross-reactivity has been reported [20].
Cross-reactivity also occurs between fluoroquinolones via IgE and T-cell-mediated mechanisms [25–27], although ciprofloxacin has been tolerated in patients with immediate hypersensitivity to moxifloxacin [28]. Like vancomycin, ciprofloxacin, particularly in higher intravenous dosing, can induce nonspecific release of histamine from mast cells inducing a pseudoallergic (‘red-man, anaphy-lactoid’) reaction, often confused clinically with true IgE-mediated reactions and more common in patients with uncontrolled HIV [29].
IgE and non-IgE-mediated (primarily T-cell-mediated) reactions to vancomycin [30▪], amino-glycosides, [31], sulfa antimicrobials, bacitracin, dapsone, and antimycobacterial therapies have been described [16,32–35]. Nonirritating concentrations of these drugs can be employed for in-vivo testing; however, most macrolides and vancomycin are generally too irritating to perform skin prick testing/intradermal testing (SPT/IDT) and the NPV is low. Therefore, for drugs such as clindamycin [36] the very low NPV of SPT/IDT usually warrants going straight to observed oral challenge when possible as a way of determining drug tolerance [16].
TESTING FOR TYPE I-MEDIATED REACTIONS TO ANTIBIOTICS
Testing modalities for antibiotic allergy are dependent on type of clinical presentation and suspected immunological mechanism, as outlined below.
In-vitro testing
Radioallergosorbent testing (RAST) involves the detection of specific IgE to antibiotics in vitro, conjugated to a carrier. RAST testing for antibiotics is less sensitive than in-vivo (SPT/IDT/oral challenge) methods [37] and is invalid for penicillin minor determinants. A recent publication highlighted that in-vitro testing (ImmunoCAP) measuring IgE to penicillin V and G major determinants produced a large number of false positives (26%), raising concerns over its validity [38▪▪]. Furthermore, Bourke et al. [21▪▪] found no correlation between RAST and oral challenge positivity. The basophil activation test (BAT) measures drug-induced activation of basophils and has a sensitivity of 22–55% and specificity of 79–100% for IgE-mediated β-lactam allergies [39]. RAST and other available in-vitro tests for IgE-mediated reactions do not exclude or confirm allergy and should not be used as a substitute for in-vivo testing or as the basis for which oral challenge is performed [16].
In-vivo cutaneous testing: skin prick testing and intradermal testing
SPT followed by IDT is safe and well validated, particularly for immediate antibiotic allergy, particularly penicillin when using benzyl penicillin and its validated major [benzylpenicilloyl-poly-L-lysine (PPL)] and minor (benzylpenicillin, sodium benzylpenilloic acid, benzylpenicilloic acid) determinants [10▪▪]. PPL is a stable group formed from the spontaneous opening of the β-lactam ring [1▪▪]. PPL accounts for 95% of metabolites and the minor determinants the remainder [40]. Amoxicillin minor determinants amoxicilloic acid and diketo-piperazine have also been studied, but they are not more sensitive than amoxicillin alone in SPT/IDT [41]. In contemporary practice, when PPL and minor determinant mixture (MDM) are used in combination with other reagents such as benzylpenicillin and an aminiopenicillin (amoxicillin/ampicillin), the sensitivity of SPT/IDT is 70%, specificity 97–100%, NPV 97–99%, and positive predictive value (PPV) 40–100% [16,42]. The relevant haptens have not been determined for most other β-lactams and are, therefore, applied in their parent form [3]. The sudden lack of commercial availability of PPL/MDM in 2005 left a void; more recently Diater (DAP; Madrid, Spain) and Allerquest (PrePen; Plainville, CT, USA; www.allerquest.com) now supply PPL/ MDM (Diater) and PPL (PrePen), respectively, under restricted prescriber use to many countries, and centers also manufacture their own MDMs [40]. The reagents used for β-lactam SPT/IDT are available in current practice guidelines [16] and a clinical example of positive SPT/IDT is demonstrated in Fig. 2.
FIGURE 2.
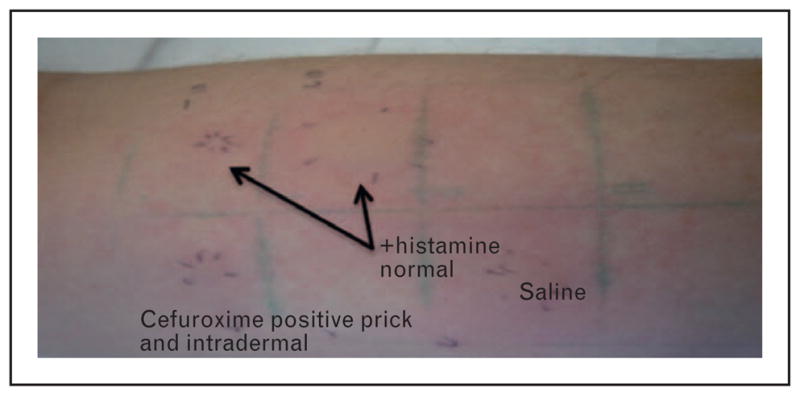
Clinical image of positive skin prick testing/ intradermal testing (SPT/IDT) to cefuroxime. Positive selective immediate cefuroxime SPT (9 mm wheal/36 mm flare)/ IDT (15 mm wheal/45 mm flare) with flare spreading to adjacent sites: patient had a history of an immediate/IgE type reaction to oral cefuroxime 4 months prior. Negative IDT/patch testing to all other penicillin and cephalosporin determinants including cefoxitin (shared side chain; not shown). Tolerated 5-day challenge with penicillin VK 500 mg four times daily (q.i.d.).
Selective skin test positive and IgE-mediated allergy to aminopenicillins (amoxicillin, ampicillin) was originally reported to comprise less than 1% of penicillin allergic patients in earlier large US studies [43], and higher in Europe [43]; however, this has increased over the past 10–15 years, presumably secondary to increased community use, and now comprises more than 30% of SPT/IDT positive results in recent studies [21▪▪,44,45]. It has been estimated in patients who develop immediate reactions on amoxicillin–clavulanate that up to 16% may be selective reactors to the clavulanate determinant [46]. Patients who are selectively positive to amoxicillin or ampicillin on SPT/IDT commonly have selective reactions and are tolerant of benzyl penicillin and other penicillins [10▪▪,21▪▪,47▪]. Ampicillin shares side chains with cephalosporins (Tables 2 and 3); hence, patients with IgE-mediated reactions to ampicillin and amoxicillin containing antibiotic evidenced by history and positive SPT/IDT should undergo observed and monitored challenge to cephalosporins such as cephalexin prior to prescription.
Previous studies have shown a significant number of previously skin test positive patients will revert to negative [48,49]. Resensitization is rare in patients not exposed to multiple courses of parenteral antibiotics and re-testing is not recommended in the absence of a recurrent clinical reaction [50,51]. In addition, for patients who have selective IgE responses presumably as side chain reactions to amoxicillin, these become negative over time and their natural history does not appear to be influenced by repeated and periodic use of penicillin VK or penicillin G [48].
TESTING FOR TYPE IV REACTIONS TO ANTIBIOTICS
Delayed antibiotic reactions (type IV) have a varied spectrum of presentation from mild skin drug eruptions that may resolve with continued treatment, to life-threatening systemic reactions (Table 1). Unlike SPT/IDT for type I reactions, no in-vivo or ex-vivo diagnostic testing for type IV reactions has a NPV of close to 100%. Therefore, any decision to rechallenge a patient with a drug or structurally similar drug following negative testing must be based on a combination of clinical and laboratory criteria. Patients who have experienced a severe drug reaction, such as drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP), acute interstitial nephritis (AIN), or drug-induced liver disease (DILI), should be given advice for permanent avoidance of the causative and structurally related drugs.
Delayed intradermal testing
Whereas immediate IDT readings are read at 15 min for IgE-mediated type reactions, delayed IDT readings are read at 20–30 min, 6 h, and 24 h. Delayed IDT has the advantage of the shorter read time and the lack of need to keep patch tape attached and undisturbed for 48 h. In addition, some studies have demonstrated a higher sensitivity for type IV reactions via delayed IDT than patch testing, despite the same immunological mechanisms [10▪▪]. A positive delayed IDT for amoxicillin is shown (Fig. 3).
FIGURE 3.
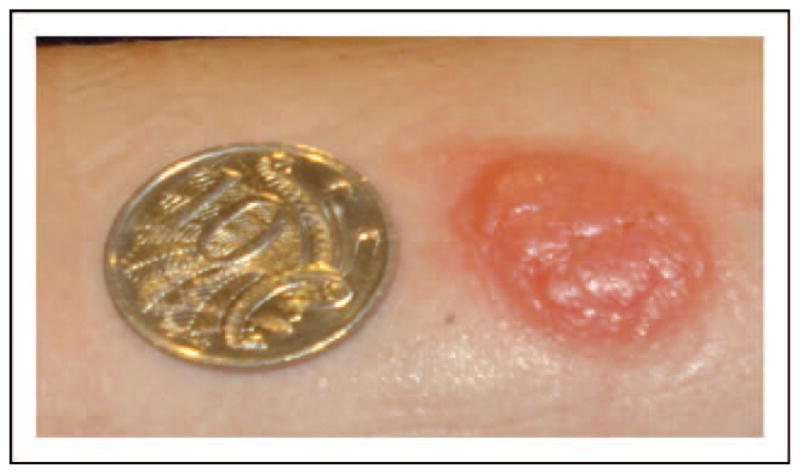
Positive delayed intradermal testing (IDT) for amoxicillin. Positive selective delayed IDT reaction to amoxicillin. Positive IDT with blistering reaction at site of amoxicillin IDT and negative skin prick testing (SPT)/IDT to all other penicillin and cephalosporin reagents. Reaction started at 6 h post-IDT and pictured here at 48 h in patient with previous delayed rash associated with amoxicillin. Patient tolerated 5-day challenge with penicillin VK 500 mg four times daily (q.i.d.). Note: Coin size diameter 23.60 mm.
Patch testing
This involves applying a mixture of the highest nonirritating concentration of antibiotic in the appropriate vehicle, which is usually petrolatum, but can vary dependent on drug solubility characteristics. The antibiotic in the vehicle and vehicle control are placed in patch wells and attached to the skin, removed at 48 h, with additional readings at 96 h and 7 days [52]. The sensitivity of patch testing is variable and most reactions are limited to the site of application with systemic reactions rarely noted [52]. The sensitivity of patch testing is highest for AGEP and DRESS, greater than fixed drug eruption (FDE; at the site of the reaction), and lowest for SJS/ TEN. Patch testing for the antiretroviral drug abacavir showed 100% specificity, used to identify true immunologically mediated abacavir hypersensitivity. Although abacavir patch testing had one of the highest diagnostic sensitivities of any patch testing at 87%, given the severity of abacavir hypersensitivity, a negative patch test should never be used as the basis for abacavir re-exposure [10▪▪,53]. Positive patch testing has also been reported with delayed reactions to penicillins/aminopenicillins [54,55], cephalosporins [55], clindamycin [56], glycopeptides [57], imipenem–cilastatin [54], and aztreonam [24]. Patch testing is an alternative to delayed IDT with antibiotics that do not have an intravenous formulation. The low sensitivity of patch testing means that a negative result cannot be used as the basis for oral rechallenge or graded re-introduction, except with mild-to-moderate skin rashes without systemic features in which the antimicrobials are required [58].
Ex-vivo testing
Additional research assays have investigated T-cell-mediated reactions for penicillins, aminopenicillins, cephalosporins, and quinolones [27]. Lymphocyte transformation testing (LTT) stimulates peripheral blood mononuclear cells (PBMCs) with the offending antibiotic and records the stimulation response after 5–7 days [59]. LTT does not provide a diagnostic advantage over patch testing or delayed IDT due to the variable sensitivity/specificity and described false positives [58]. ELISpot and flow cytometry assays (intracellular cytokine staining, ICS) are emerging tests that may be useful for T-cell-mediated reactions [60].
Human leukocyte antigen typing
A number of drug hypersensitivity reactions have been recently found to be human leukocyte antigen (HLA) class I and/or II related. The association between abacavir and HLA-B*57:01 provided the opportunity to use the HLA-B*57:01 screening test, which has a 100% NPV, making abacavir treatment safer [11▪▪,61]. Other HLA associations have been described for nevirapine, flucloxacillin, and amoxicillin–clavulanate (Table 4) [11▪▪,61–64,65▪,66▪,67–72]. Less than 100% NPV and very low PPVs of other antimicrobial drug hypersensitivity HLA associations will limit the feasibility and translation of these tests into routine clinical practice as screening tests [11▪▪]. However, HLA associations have significantly advanced our understanding of drug hypersensitivity syndromes (Table 4).
Table 4.
Antimicrobial hypersensitivity syndromes and human leukocyte antigen
| Antimicrobial | Clinical presentation | HLA |
|---|---|---|
| Abacavir | Hypersensitivity reaction (fever, malaise, GI, rash later and incomplete (70%) | HLA-B*57:01a |
| Nevirapine | Rash | HLA-B*35:05, HLA-Cw4 |
| Hypersensitivity | HLA-B*14/Cw8 | |
| Hepatitis | HLA-DRB1*01:01 + CD4+ >25%/DRB1*01:02; | |
| HLA-Cw4 | ||
| Hepatitis (DILI) | HLA-C*04:01 | |
| Flucloxacillin | SJS/TEN | HLA-B*57:01 |
| Amoxicillin–clavulanate | Hepatitis (cholestatic) | HLA-A*02:01/HLA-DRB1*15:01 |
Re-challenge
Combination of in-vivo testing, SPT/IDT and oral challenge remains the gold standard for IgE-mediated reactions [10▪▪,73▪,74▪]. Acute anaphylaxis with oral penicillin challenge is extremely rare [75]. For most antibiotic classes other than β-lactams, the NPV value of SPT/IDT falls significantly short of 100% and oral challenge provides more definitive information regarding drug safety [10▪▪]. In appropriately selected patients, oral re-challenge has a high NPV (94–100%) for immediate/IgE-mediated β-lactam allergies [75,76]. However, a single dose oral challenge will not rule out a delayed T-cell-mediated reaction, which requires repeat or extended dosing challenges. Due to the lower sensitivity of SPT/IDT/patch testing for nonimmediate type IV reactions, oral challenge or graded reintroduction is used successfully in mild-to-moderate skin reactions [77]. Oral challenge to the suspected or structurally related drug is contraindicated in patients with severe systemic syndromes (SJS/TEN, DRESS, DILI, AGEP, or AIN). Recent literature supports an extended oral challenge of 3–7 days in patients with delayed mild-to-moderate rash without internal organ involvement, mucosal or systemic features [74▪,78]. Hjortlund et al. [79▪▪] demonstrated in a population with primarily cutaneous hypersensitivity to β-lactams that an additional 20% (23/111) were found to be positive during a 7-day oral challenge.
THE EFFICACY, SAFETY, AND IMPACT OF SPECIALIST ANTIBIOTIC ALLERGY DE-LABELING
Recent studies have demonstrated the high NPV of penicillin allergy testing in modern specialized allergy clinics [74▪,80]. Combination testing (SPT/IDT/oral challenge) has been the most validated approach to antibiotic allergy assessment [10▪▪,75]. Bourke et al. [21▪▪] highlighted the efficacy, safety, and near 100% NPV in a retrospective review of more than 400 patients in a comprehensive penicillin de-labeling program in a tertiary care antibiotic allergy clinic. However, concerns regarding the efficacy–effectiveness gap were raised in a patient questionnaire, which suggested a lack of complete adherence to de-labeling recommendations in the SPT-negative cohort [21▪▪]. This failure to actively follow de-labeling recommendations remains a major barrier to program uptake [81▪,82]. A different safety concern is highlighted by an NHS National Patient Safety Agency report documenting incidents in which penicillin allergic patients had been prescribed full-dose penicillin antimicrobials despite their allergic label. In the majority, this was due to prescription of branded β-lactam combination products such as piperacillin–tazobactam (Tazocin) or amoxicillin–clavulanate (Augmentin), in which the trade name concealed the presence of penicillin [83]. A comprehensive allergy assessment reduces reported hypersensitivity labels and produces durable negative re-challenging responses [6▪▪]. Increased β-lactam use and decreased vancomycin and fluoroquinolone prescription have been demonstrated in inpatient and outpatient antibiotic SPT/IDT testing studies [14,84,85].
Managing penicillin allergy is complex [86], with a clear tendency to use broader spectrum therapy in inappropriately labeled patients [16]. The consequences of an inappropriate antibiotic allergy label include limiting therapeutic options, increased broad-spectrum antibiotic use, increased risk of toxicity, and hospital costs [6▪▪,84–87]. A recent retrospective study by Charneski et al. [4] of 11 872 inpatients showed that 11.2% of patients had an antimicrobial allergy label and they had an increased length of stay, higher ICU admission rate (17 versus 12%, P <0.0001), greater antimicrobial use (62 versus 51%, P <0.0001), higher readmission rate (60 versus 50%, P <0.0001), and higher mortality (5 versus 3%, P =0.009).
AN APPROACH TO ANTIBIOTIC ALLERGY TESTING AND DE-LABELING
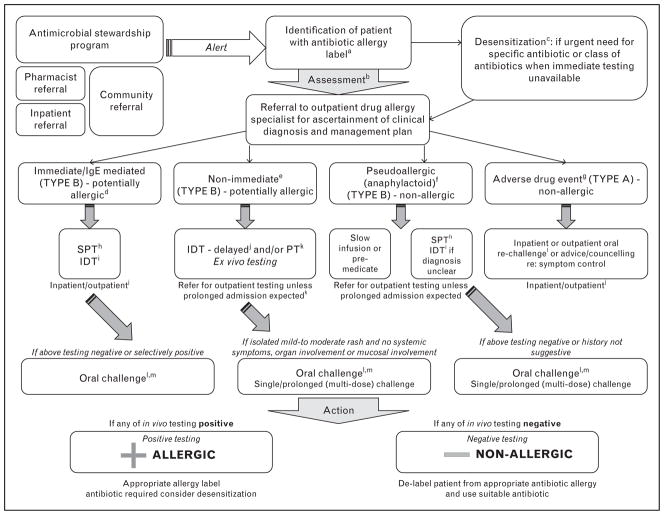
Clinical evaluation is essential in allergy classification (Table 1). A strong suspicion of antibiotic allergy from history and particularly more recent reactions and immediate reactions historically consistent with IgE mediated reactions predict the likelihood of in-vivo SPT/IDT positivity [10▪▪,21▪▪]. The history may support a virus–antibiotic interaction, for example, delayed rash to aminopenicillins in acute Epstein–Barr virus (EBV) infection [10▪▪]. The appropriate labeling or ‘de-labeling’ of antibiotic allergies requires a complete approach from point of label identification to re-challenge (Fig. 4).
FIGURE 4.
Comprehensive approach to antibiotic allergy label identification, clinical assessment, in-vitro/in-vivo investigations and de-labeling. aAntimicrobial Stewardship system: Inpatient identification via antimicrobial stewardship round, antimicrobial stewardship decision support software alerts, infectious disease physicians, and pharmacists. bHistory: Exclude concurrent viral infection, other diagnosis, or other offending drugs. Obtain information regarding age of onset, type of reaction, time after drug exposure, route of antibiotic administration, concurrent medications at time of reaction, tolerability of other antibiotics and previous re-challenge. cDesensitization: Slow reintroduction or desensitization is occasionally used for delayed mild–moderate skin reactions without systemic features, internal organ or mucosal involvement (e.g., trimethoprim sulfamethoxazole). dImmediate/IgE mediated: Withhold β-blocker therapy and strict hospital supervision for 1 h after skin test. eDelayed/T-cell-mediated: Perform in-vivo skin testing, although diagnosis requires delayed intradermal testing (IDT) or patch testing. Patients with histories of non-immediate but accelerated reactions occurring > 1 hour and less than 72 hours after drug exposure which could be IgE mediated may warrant SPT/IDT with immediate readings +/− oral challenge(s) for delabeling fPseudoallergic/anaphylactoid: for example, red-man/histamine syndrome with vancomycin or ciprofloxacin. Not a contraindication to re-prescription, but may require specific care (e.g., slow infusion or antihistamine premedication). In some cases, testing should be performed to rule out IgE-mediated delayed urticarial reaction. gAdverse drug event (ADR): Side-effect or a nonspecific reaction not consistent with immunological mechanism. hSkin prick testing (SPT): Performed for reagents described in Tables 2 and 3. iIDT: Performed for reagents described in Tables 2 and 3. jDelayed reading of IDT: For IDT with delayed readings, read at 20–30 min, 6, and 24 h. kPatch testing (PT): Should only be performed 4–6 weeks after drug reaction. Preferred to delayed IDT, if no intravenous formulation available. Can be performed concurrently with delayed IDT. Read at 48, 96 h, and 7 days. lInpatient (higher risk) and outpatient (lower risk) re-challenge: If low index of suspicion for IgE-mediated or T-cell mediated mechanism, then perform inpatient re-challenge at lowest possible dose/graded doses. Prolonged (multidose challenge for T-cell mediated). Avoid if severe T-cell mediated reaction (DRESS/DIHS, SJS/TEN, AGEP, DILI/AIN). mCombination in-vivo testing recommended; SPT/IDT and oral challenge is considered gold standard. If negative SPT/IDT, then oral challenge with penicillin VK 250 mg. If positive penicillin SPT/IDT and negative cephalosporin SPT, re-challenge with cephalexin.
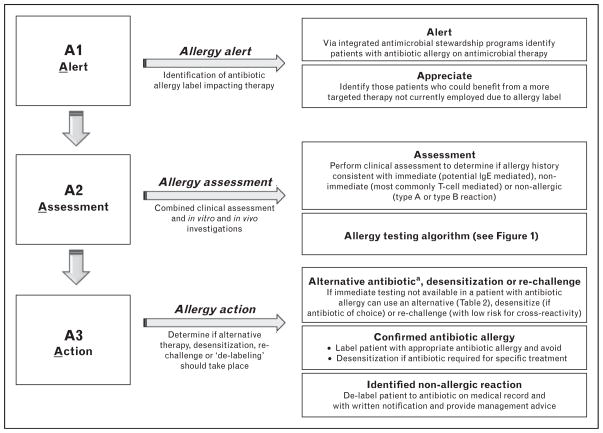
AN ANTIBIOTIC ALLERGY AND ANTIMICROBIAL STEWARDSHIP PARTNERSHIP MODEL
An emphasis on addressing antibiotic allergy labels can reduce restricted antibiotic use and provide another ‘strong arm’ to antimicrobial stewardship [88]. Park et al. [89] demonstrated that pharmacist-facilitated referral to allergists can increase β-lactam antibiotic prescriptions in patients with a reported penicillin allergy, as 94% of patients referred to an allergist were SPT/IDT-negative. Furthermore, 66% of those referred to an allergist were subsequently prescribed a β-lactam compared with 26% who were not referred (P <0.0001). The economic and clinical feasibility of implementing antibiotic combined SPT/IDT antimicrobial stewardship programs was recently evaluated [19▪▪], demonstrating penicillin SPT/IDT in the acute care/ICU, anesthetic preoperative unit, and emergency department reduced nonstandard antibiotic therapy and increased β-lactam use [19▪▪].
Rimawi et al. [7▪▪] highlighted the successful impact of penicillin SPT/IDT on antimicrobial stewardship. A NPV of 100% for penicillin SPT/IDT and oral challenge testing allowed confident prescribing of β-lactam therapy (in a low-risk population with more remote histories of β-lactam allergy) within 3 h of ‘de-labeling’. This approach provided a substantial cost saving ($82 000/5-month period). The exclusion of delayed reactions and nonpenicillin allergy limits its impact. In addition these authors included only PPL and benzylpenicillin in their testing, which would not be considered safe in higher-risk populations with immediate and recent (<1 year) reaction where use of multiple reagents would be considered the minimum standard. Ideally such programs would integrate with the same type of decision support programs fundamental to the success of any antibiotic stewardship program and would entail incorporation of patient history and risk stratification, in-vivo testing, β-lactam cross-reactivity profiles, oral rechallenge as well as desensitization protocols, if necessary (Tables 2 and 3) [16]. A proposed antibiotic allergy model is outlined in Fig. 5.
FIGURE 5.
A proposed model for an integrated antimicrobial stewardship and antibiotic allergy de-labeling program. aAlternative: In patients with low clinical suspicion of antibiotic allergy or previous negative in-vivo testing, immediate re-challenge should be considered. In patients with non-IgE-mediated allergy (confirmed or nonconfirmed) to a β-lactam antibiotic, avoid antibiotics that share an identical R1 or R2 group side chain (Tables 2 and 3). In patients with proven isolated IgE-mediated β-lactam antibiotic allergy or high clinical suspicion prior to in-vivo testing, preferred antibiotic therapies include carbapenems, aztreonam, fluoroquinolone, glycopeptides, or lincosamide.
CONCLUSION
The process through which an antibiotic allergy label gets assigned, acted on, and maintained is currently imprecise and results in adverse effects on the safety, efficacy, and effectiveness of a patient’s medical care over the course of their lifetime. From preliminary studies, antibiotic allergy programs reduce antimicrobial use, patient mortality, and hospitalization costs. Further prospective and randomized studies modeled on our schematics are required to evaluate the impact of antibiotic allergy de-labeling pathways.
KEY POINTS.
Antibiotic allergy labels are often misrepresented and this puts patients at risk for negative outcomes.
Combined antibiotic allergy testing (SPT/IDT/oral challenge) has a very high negative predictive value.
Dedicated antibiotic allergy inpatient and outpatient programs can effectively ‘de-label’ patients and increase standard β-lactam use.
Barriers to ‘de-labeling’ primarily stem from a lack of community and physician understanding of antibiotic cross-reactivity and safety of appropriate re-challenging.
A model of antibiotic allergy testing and antimicrobial stewardship program integration may decrease broad-spectrum antibiotic use and associated costs.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1▪▪.Lagace-Wiens P, Rubinstein E. Adverse reactions to beta-lactam antimicrobials. Expert Opin Drug Saf. 2012;11:381–399. doi: 10.1517/14740338.2012.643866. A recent review of the current causes of and approach to patients with β-lactam antibiotic allergies, and of the mechanisms and impact of β-lactam cross-reactivity. [DOI] [PubMed] [Google Scholar]
- 2.Torres MJ, Blanca M. The complex clinical picture of beta-lactam hypersensitivity: penicillins, cephalosporins, monobactams, carbapenems, and clavams. Med Clin North Am. 2010;94:805–820. doi: 10.1016/j.mcna.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Blanca M, Romano A, Torres MJ, et al. Update on the evaluation of hypersensitivity reactions to beta lactams. Allergy. 2009;64:183–193. doi: 10.1111/j.1398-9995.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 4.Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31:742–747. doi: 10.1592/phco.31.8.742. [DOI] [PubMed] [Google Scholar]
- 5▪.Sastre J, Manso L, Sanchez-García S, Fernández-Nieto M. Medical and economic impact of misdiagnosis of drug hypersensitivity in hospitalized patients. J Allergy Clin Immunol. 2012;129:566–567. doi: 10.1016/j.jaci.2011.09.028. A point prevalence study detailing the impact of drug hypersensitivity syndromes on hospitalization. A four-fold increase in cost was noted with a labeled drug hypersensitivity, with 38% of drug hypersensitivities noted involving antibiotics. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Caimmi S, Sanfiorenzo C, Caimmi D, et al. Comprehensive allergy work-up is mandatory in cystic fibrosis patients who report a history suggestive of drug allergy to beta-lactam antibiotics. Clin Transl Allergy. 2012;2:10. doi: 10.1186/2045-7022-2-10. This study highlights the impact of β-lactam antibiotic allergy labels on cohorts of patients who require directed and frequent antimicrobial therapy. With a targeted allergy work-up in cystic fibrosis patients with a suggestive history of β-lactam allergy, the rate of true drug allergy was 2.3% from 171 labeled patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Rimawi RH, Cook PP, Gooch M, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8:341–345. doi: 10.1002/jhm.2036. An important study that examined an antimicrobial stewardship program that led an inpatient in-vivo allergy testing model for patients with a history of presumed immediate penicillin allergy. De-labeling was achieved in 145 patients over a 12-month period, increasing targeted β-lactam use and hospital cost savings. [DOI] [PubMed] [Google Scholar]
- 8.Gell PGH, Coombs RRA. The classification of allergic reactions underlying disease. In: Gell PGH, Coombs RRA, editors. Clinical aspects of immunology. 2. Oxford: Blackwell Scientific; 1963. [Google Scholar]
- 9.Pichler WJ. Drug hypersensitivity reactions: classification and relationship to T-cell activation. In: Pichler WJ, editor. Drug Hypersensitivity. Basel: S Karger Pub; 2007. pp. 168–189. [Google Scholar]
- 10▪▪.Rive CM, Bourke J, Phillips EJ. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. 2013;34:15–38. A complete review of the current understanding of mechanisms and classifications of drug hypersensitivity syndromes. The management of these syndromes is also detailed, including the role of HLA typing in diagnosis and management. [PMC free article] [PubMed] [Google Scholar]
- 11▪▪.Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13:1285–1306. doi: 10.2217/pgs.12.108. A recent review of the area of HLA-associated hypersensitivity highlighting recent research of the altered peptide repertoire model of abacavir hypersensitivity. [DOI] [PubMed] [Google Scholar]
- 12.Uzzaman A, Cho SH. Chapter 28: classification of hypersensitivity reactions. Allergy Asthma Proc. 2012;33 (Suppl 1):S96–S99. doi: 10.2500/aap.2012.33.3561. [DOI] [PubMed] [Google Scholar]
- 13.Bircher AJ, Scherer Hofmeier FK. Drug hypersensitivity reactions: inconsistency in the use of the classification of immediate and nonimmediate reactions. J Allergy Clin Immunol. 2012;129:263–264. doi: 10.1016/j.jaci.2011.08.042. author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 14.Park MA, Li JT. Diagnosis and management of penicillin allergy. Mayo Clin Proc. 2005;80:405–410. doi: 10.4065/80.3.405. [DOI] [PubMed] [Google Scholar]
- 15.Anne S, Reisman RE. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Ann Allergy Asthma Immunol. 1995;74:167–170. [PubMed] [Google Scholar]
- 16.Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis. 1978;137 (Suppl):S74–S79. doi: 10.1093/infdis/137.supplement.s74. [DOI] [PubMed] [Google Scholar]
- 18.Antunez C, Blanca-Lopez N, Torres MJ, et al. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J Allergy Clin Immunol. 2006;117:404–410. doi: 10.1016/j.jaci.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 19▪▪.Unger NR, Gauthier TP, Cheung LW. Penicillin skin testing: potential implications for antimicrobial stewardship. Pharmacotherapy. 2013;33:856–867. doi: 10.1002/phar.1288. A recent review suggesting that penicillin skin testing has a vital role in antibiotic stewardship programs. It provides insight into the current evidence for and barriers to allergy testing and its integration into antimicrobial stewardship. [DOI] [PubMed] [Google Scholar]
- 20.Romano A, Gaeta F, Valluzzi RL, et al. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010;126:994–999. doi: 10.1016/j.jaci.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Bourke J, Hollingsworth PR, McLean-Tooke P, et al. Penicillin de-labelling in tertiary care clinics: safe and efficacious but incomplete effectiveness. Int Med J. 2012;42 (Supplement 4):21–22. A modern retrospective study of more than 400 patients examining the effectiveness of specialized outpatient antibiotic allergy clinics and high NPV of in-vivo testing in de-labeling patients, in particular in regard to β-lactam antibiotics. [Google Scholar]
- 22.Pipet A, Veyrac G, Wessel F, et al. A statement on cefazolin immediate hypersensitivity: data from a large database, and focus on the cross-reactivities. Clin Exp Allergy. 2011;41:1602–1608. doi: 10.1111/j.1365-2222.2011.03846.x. [DOI] [PubMed] [Google Scholar]
- 23.Patriarca G, Schiavino D, Lombardo C, et al. Tolerability of aztreonam in patients with IgE-mediated hypersensitivity to beta-lactams. Int J Immunopathol Pharmacol. 2008;21:375–379. doi: 10.1177/039463200802100215. [DOI] [PubMed] [Google Scholar]
- 24.Buonomo A, Nucera E, De Pasquale T, et al. Tolerability of aztreonam in patients with cell-mediated allergy to beta-lactams. Int Arch Allergy Immunol. 2011;155:155–159. doi: 10.1159/000318844. [DOI] [PubMed] [Google Scholar]
- 25.Burke P, Burne SR. Allergy associated with ciprofloxacin. BMJ. 2000;320:679. doi: 10.1136/bmj.320.7236.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez I, Lobera T, Blasco A, del Pozo MD. Immediate hypersensitivity to quinolones: moxifloxacin cross-reactivity. J Investig Allergol Clin Immunol. 2005;15:146–149. [PubMed] [Google Scholar]
- 27.Schmid DA, Depta JP, Pichler WJ. T cell-mediated hypersensitivity to quinolones: mechanisms and cross-reactivity. Clin Exp Allergy. 2006;36:59–69. doi: 10.1111/j.1365-2222.2006.02402.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang B, Knowles SR, Weber E. Immediate hypersensitivity to moxifloxacin with tolerance to ciprofloxacin: report of three cases and review of the literature. Ann Pharmacother. 2010;44:740–745. doi: 10.1345/aph.1M579. [DOI] [PubMed] [Google Scholar]
- 29.Mori K, Maru C, Takasuna K. Characterization of histamine release induced by fluoroquinolone antibacterial agents in-vivo and in-vitro. J Pharm Pharmacol. 2000;52:577–584. doi: 10.1211/0022357001774228. [DOI] [PubMed] [Google Scholar]
- 30▪.Bosse D, Lemire C, Ruel J, et al. Severe anaphylaxis caused by orally administered vancomycin to a patient with Clostridium difficile infection. Infection. 2013;41:579–582. doi: 10.1007/s15010-012-0328-4. An example of true vancomycin-mediated IgE allergic response, questioning the belief that vancomycin only induces anaphylactoid reactions. [DOI] [PubMed] [Google Scholar]
- 31.Schulze S, Wollina U. Gentamicin-induced anaphylaxis. Allergy. 2003;58:88–89. doi: 10.1034/j.1398-9995.2003.23710_5.x. [DOI] [PubMed] [Google Scholar]
- 32.Cribb AE, Lee BL, Trepanier LA, Spielberg SP. Adverse reactions to sulphonamide and sulphonamide-trimethoprim antimicrobials: clinical syndromes and pathogenesis. Adverse Drug React Toxicol Rev. 1996;15:9–50. [PubMed] [Google Scholar]
- 33.Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–1477. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 34.Sood A, Taylor JS. Bacitracin: allergen of the year. Am J Contact Dermat. 2003;14:3–4. doi: 10.2310/6620.2003.38621. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal S, Agarwalla A. Dapsone hypersensitivity syndrome: a clinico-epidemiological review. J Dermatol. 2005;32:883–889. doi: 10.1111/j.1346-8138.2005.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 36.Notman MJ, Phillips EJ, Knowles SR, et al. Clindamycin skin testing has limited diagnostic potential. Contact Dermatitis. 2005;53:335–338. doi: 10.1111/j.0105-1873.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- 37.Kraft D, Roth A, Mischer P, et al. Specific and total serum IgE measurements in the diagnosis of penicillin allergy. A long term follow-up study. Clin Allergy. 1977;7:21–28. doi: 10.1111/j.1365-2222.1977.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 38▪▪.Johansson SG, Adedoyin J, van Hage M, et al. False-positive penicillin immunoassay: an unnoticed common problem. J Allergy Clin Immunol. 2013;132:235–237. doi: 10.1016/j.jaci.2012.11.017. This study highlights the importance of the high number (up to 26%) of false positives associated with the ImunoCAP in-vitro assay for penicillin allergy. [DOI] [PubMed] [Google Scholar]
- 39.Leysen J, Sabato V, Verweij MM, et al. The basophil activation test in the diagnosis of immediate drug hypersensitivity. Expert Rev Clin Immunol. 2011;7:349–355. doi: 10.1586/eci.11.14. [DOI] [PubMed] [Google Scholar]
- 40.Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100:586–591. doi: 10.1016/s0091-6749(97)70159-3. [DOI] [PubMed] [Google Scholar]
- 41.Torres MJ, Ariza A, Fernandez J, et al. Role of minor determinants of amoxicillin in the diagnosis of immediate allergic reactions to amoxicillin. Allergy. 2010;65:590–596. doi: 10.1111/j.1398-9995.2009.02245.x. [DOI] [PubMed] [Google Scholar]
- 42.Kranke B, Aberer W. Skin testing for IgE-mediated drug allergy. Immunol Allergy Clin North Am. 2009;29:503–516. doi: 10.1016/j.iac.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Romano A, Torres MJ, Fernandez J, et al. Allergic reactions to ampicillin. Studies on the specificity and selectivity in subjects with immediate reactions. Clin Exp Allergy. 1997;27:1425–1431. [PubMed] [Google Scholar]
- 44.Romano A, Bousquet-Rouanet L, Viola M, et al. Benzylpenicillin skin testing is still important in diagnosing immediate hypersensitivity reactions to penicillins. Allergy. 2009;64:249–253. doi: 10.1111/j.1398-9995.2008.01874.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a determinant in penicillin skin testing. Int Arch Allergy Immunol. 2010;152:313–318. doi: 10.1159/000288284. [DOI] [PubMed] [Google Scholar]
- 46.Torres MJ, Ariza A, Mayorga C, et al. Clavulanic acid can be the component in amoxicillin-clavulanic acid responsible for immediate hypersensitivity reactions. J Allergy Clin Immunol. 2010;125:502–505. e2. doi: 10.1016/j.jaci.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 47▪.Prematta T, Shah S, Ishmael FT. Physician approaches to beta-lactam use in patients with penicillin hypersensitivity. Allergy Asthma Proc. 2012;33:145–151. doi: 10.2500/aap.2012.33.3526. A survey of clinicians and their use of antibiotics in patients with a history of penicillin allergy. Providers were unfamiliar with the safety of prescribing penicillin in patients with a history of maculopapular rash, and the low cross-reactivity of monobactams and carbapenems in penicillin allergic individuals. Its importance is that it underscores the need for educating clinicians about antibiotic allergy. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez T, Torres MJ, R-Pena R, et al. Decrease of selective immuno-globulin E response to amoxicillin despite repeated administration of benzyl-penicillin and penicillin V. Clin Exp Allergy. 2005;35:1645–1650. doi: 10.1111/j.1365-2222.2005.02378.x. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan TJ, Wedner HJ, Shatz GS, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68:171–180. doi: 10.1016/0091-6749(81)90180-9. [DOI] [PubMed] [Google Scholar]
- 50.Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162:822–826. doi: 10.1001/archinte.162.7.822. [DOI] [PubMed] [Google Scholar]
- 51.Bittner A, Greenberger PA. Incidence of resensitization after tolerating penicillin treatment in penicillin-allergic patients. Allergy Asthma Proc. 2004;25:161–164. [PubMed] [Google Scholar]
- 52.Travassos AR, Pacheco D, Antunes J, et al. The importance of patch tests in the differential diagnosis of adverse drug reactions. An Bras Dermatol. 2011;86 (4 Suppl 1):S21–S23. doi: 10.1590/s0365-05962011000700004. [DOI] [PubMed] [Google Scholar]
- 53.Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–2225. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 54.Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644–1648. doi: 10.1111/j.1398-9995.2009.02058.x. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalo-Garijo MA, Rodriguez-Nevado I, de Argila D. Patch tests for diagnosis of delayed hypersensitivity to cephalosporins. Allergol Immunopathol (Madr) 2006;34:39–41. doi: 10.1157/13084227. [DOI] [PubMed] [Google Scholar]
- 56.Pereira N, Canelas MM, Santiago F, et al. Value of patch tests in clindamycin-related drug eruptions. Contact Dermatitis. 2011;65:202–207. doi: 10.1111/j.1600-0536.2011.01942.x. [DOI] [PubMed] [Google Scholar]
- 57.Perrin-Lamarre A, Petitpain N, Trechot P, et al. Glycopeptide-induced cutaneous adverse reaction: results of an immunoallergic investigation in eight patients. Ann Dermatol Venereol. 2010;137:101–105. doi: 10.1016/j.annder.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80–90. [PubMed] [Google Scholar]
- 59.Kano Y, Hirahara K, Mitsuyama Y, et al. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy. 2007;62:1439–1444. doi: 10.1111/j.1398-9995.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 60.Nagao-Dias AT, Teixeira FM, Coelho HL. Diagnosing immune-mediated reactions to drugs. Allergol Immunopathol (Madr) 2009;37:98–104. doi: 10.1016/s0301-0546(09)71112-7. [DOI] [PubMed] [Google Scholar]
- 61.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 62.Lucena MI, Molokhia M, Shen Y, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Donohue J, Oien KA, Donaldson P, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717–720. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donaldson PT, Daly AK, Henderson J, et al. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J Hepatol. 2010;53:1049–1053. doi: 10.1016/j.jhep.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 65▪.Carr DF, Chaponda M, Jorgensen AL, et al. Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-infected population. Clin Infect Dis. 2013;56:1330–1339. doi: 10.1093/cid/cit021. A recent finding of HLA-C*04:01 carriage as a risk factor for nevirapine-induced SJS/TEN in a Malawian HIV cohort, from 117 patients with nevirapine hypersensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪.Phillips E, Bartlett JA, Sanne I, et al. Associations between HLA-DRB1*0102, HLA-B*5801, and hepatotoxicity during initiation of nevirapine-containing regimens in South Africa. J Acquir Immune Defic Syndr. 2013;62:e55–e57. doi: 10.1097/QAI.0b013e31827ca50f. A recent study highlighting the first HLA associations with nevirapine hepatotoxicity in a black South African population with particular note of the homology between HLA-DRB1*01:02 and a previously described association with NVP-associated hepatotoxicity in whites (HLA-DRB1*01:01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan J, Guo S, Hall D, et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011;25:1271–1280. doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Likanonsakul S, Rattanatham T, Feangvad S, et al. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Res Ther. 2009;6:22. doi: 10.1186/1742-6405-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chantarangsu S, Mushiroda T, Mahasirimongkol S, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19:139–146. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- 70.Vitezica ZG, Milpied B, Lonjou C, et al. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–541. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]
- 71.Littera R, Carcassi C, Masala A, et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006;20:1621–1626. doi: 10.1097/01.aids.0000238408.82947.09. [DOI] [PubMed] [Google Scholar]
- 72.Martin AM, Nolan D, James I, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–99. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 73▪.Garcia Nunez I, BarasonaVillarejo MJ, et al. Diagnosis of patients with immediate hypersensitivity to beta-lactams using retest. J Investig Allergol Clin Immunol. 2012;22:41–47. A large retrospective study that underlines the importance of approaches to diagnose and manage β-lactam allergy. [PubMed] [Google Scholar]
- 74▪.Sagar PS, Katelaris CH. Utility of penicillin allergy testing in patients presenting with a history of penicillin allergy. Asia Pac Allergy. 2013;3:115–119. doi: 10.5415/apallergy.2013.3.2.115. A recent study of 128 patients with recorded penicillin allergy undergoing IDT and 3-day oral challenge. Fifteen percent of the population had true penicillin allergy with 47% detected via oral challenge. This study highlights the utility of prolonged oral challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498–1501. doi: 10.1111/j.1365-2222.2011.03837.x. [DOI] [PubMed] [Google Scholar]
- 76.Rerkpattanapipat T, Chiriac AM, Demoly P. Drug provocation tests in hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2011;11:299–304. doi: 10.1097/ACI.0b013e328348a4e9. [DOI] [PubMed] [Google Scholar]
- 77.Padial A, Antunez C, Blanca-Lopez N, et al. Nonimmediate reactions to beta-lactams: diagnostic value of skin testing and drug provocation test. Clin Exp Allergy. 2008;38:822–828. doi: 10.1111/j.1365-2222.2008.02961.x. [DOI] [PubMed] [Google Scholar]
- 78.Borch JE, Bindslev-Jensen C. Full-course drug challenge test in the diagnosis of delayed allergic reactions to penicillin. Int Arch Allergy Immunol. 2011;155:271–274. doi: 10.1159/000320384. [DOI] [PubMed] [Google Scholar]
- 79▪▪.Hjortlund J, Mortz CG, Skov PS, et al. One-week oral challenge with penicillin in diagnosis of penicillin allergy. Acta Derm Venereol. 2012;92:307–312. doi: 10.2340/00015555-1254. A large study of 405 patients demonstrated that an extended 7-day oral challenge with penicillin detected an additional 20% of patients who would have been missed with a single dose challenge as suggested by the European Network for Drug Allergy. [DOI] [PubMed] [Google Scholar]
- 80.Richter AG, Wong G, Goddard S, et al. Retrospective case series analysis of penicillin allergy testing in a UK specialist regional allergy clinic. J Clin Pathol. 2011;64:1014–1018. doi: 10.1136/jcp.2010.088203. [DOI] [PubMed] [Google Scholar]
- 81▪.Picard M, Paradis L, Nguyen M, et al. Outpatient penicillin use after negative skin testing and drug challenge in a pediatric population. Allergy Asthma Proc. 2012;33:160–164. doi: 10.2500/aap.2012.33.3510. A study addressing the reuse of β-lactam antibiotics following de-labeling that found that almost 1/4 of parents still avoided ‘de-labeled’ antibiotics after negative in-vivo testing. [DOI] [PubMed] [Google Scholar]
- 82.Warrington RJ, Burton R, Tsai E. The value of routine penicillin allergy skin testing in an outpatient population. Allergy Asthma Proc. 2003;24:199–202. [PubMed] [Google Scholar]
- 83.National Patient Safety Agency. [Accessed 1 April 2013];Safety in doses: medication safety incidents in the NHS. 2007 http://www.nrls.npsa.nhs.uk/EasySiteWeb/getresource.axd?AssetID=61392.
- 84.Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol. 2003;24:347–350. doi: 10.1086/502212. [DOI] [PubMed] [Google Scholar]
- 85.del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol. 2007;98:355–359. doi: 10.1016/S1081-1206(10)60882-4. [DOI] [PubMed] [Google Scholar]
- 86.MacLaughlin EJ, Saseen JJ, Malone DC. Costs of beta-lactam allergies: selection and costs of antibiotics for patients with a reported beta-lactam allergy. Arch Fam Med. 2000;9:722–726. doi: 10.1001/archfami.9.8.722. [DOI] [PubMed] [Google Scholar]
- 87.Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000;160:2819–2822. doi: 10.1001/archinte.160.18.2819. [DOI] [PubMed] [Google Scholar]
- 88.Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol. 2006;97:681–687. doi: 10.1016/S1081-1206(10)61100-3. [DOI] [PubMed] [Google Scholar]
- 89.Park MA, McClimon BJ, Ferguson B, et al. Collaboration between allergists and pharmacists increases beta-lactam antibiotic prescriptions in patients with a history of penicillin allergy. Int Arch Allergy Immunol. 2011;154:57–62. doi: 10.1159/000319209. [DOI] [PubMed] [Google Scholar]