Abstract
Communicating genetic risk information in ways that maximize understanding and promote health is increasingly important given the rapidly expanding availability and capabilities of genomic technologies. A well-developed literature on risk communication in general provides guidance for best practices, including presentation of information in multiple formats, attention to framing effects, use of graphics, sensitivity to the way numbers are presented, parsimony of information, attentiveness to emotions, and interactivity as part of the communication process. Challenges to communicating genetic risk information include deciding how best to tailor it, streamlining the process, deciding what information to disclose, accepting that communications may have limited influence, and understanding the impact of context. Meeting these challenges has great potential for empowering individuals to adopt healthier lifestyles and improve public health, but will require multidisciplinary approaches and collaboration.
Keywords: risk communication, risk assessment, personalized medicine, genome-wide association, whole-genome sequencing
INTRODUCTION
Genetic risk communication will rapidly broaden in scope and practice as emerging genomic technologies allow more and more individuals to access information about their own genetic makeup, either through their physicians or outside of the medical system. Although communicating about genetic information is currently the special province of medical geneticists and genetic counselors, the limited number of these professionals is unlikely to satisfy the demand for services as genomic medicine expands (49, 50, 79), particularly for information about common disorders such as diabetes or heart disease. In the future, genetic risk information for common disorders will be interpreted, communicated, and utilized in the context of clinical presentation, medical history, and family history, as well as other known risk factors that take into account the entirety of the patient, making primary care a likely home base for the communication of genetic risk information. However, clinicians of all specialties will need to be well versed in the nuances of this communication. The shift in responsibility for genetic risk communication away from genetics specialists should not be surprising, as there is a natural transition in medicine for practices once limited to specialists to be adopted by generalists. For example, family practitioners and general internists have replaced specialists in the routine care of children and adults with Down’s syndrome (25), adults with diabetes, and children with asthma. These types of transitions are sometimes driven by a lack of access to specialists (54).
Although genetic information is unique and identifiable and has implications for blood relatives, genetic risk factors only rarely provide information that is inherently different from that provided by other risk predictors commonly used in health care. The deterministic aspects of most genetic tests are not inherently different from those when early evidence of a medical syndrome is discovered through screening. And the susceptibility aspects of genetic testing are not significantly different from those of common risk factors such as age, blood pressure, smoking status, cholesterol, and family history (34). We also maintain that genetic risk prediction is only rarely qualitatively different from prediction based on other medical risks. A growing body of evidence suggests that the lay public views genetic information about common disorders the same way it views other medical information, and feels it should be treated similarly (28, 91). In rare cases where some types of genetic information could lead to stigmatization or discrimination, it has been suggested that the health care system’s precedent for treatment of psychiatric information may be followed (34).
In a 1999 review article titled “Communicating Genetic Risk Information,” Marteau (71) speculated that those seeking to communicate genetic risk for common disorders might learn from existing methods of communicating other health information such as diagnoses, treatment, and prediction of illness. In that spirit, we first discuss risk communication in medicine, including some examples of tools to quantify disease risk and how they have been used. Second, we provide a synopsis of best practices in risk communication from the literature on both genetic and nongenetic risk assessment. Third, we address some of the opportunities and challenges specific to genetic risk communication. The goals of this review are to provide insight from empirical work on improving genetic risk communication and to address challenges facing the field in the era of genomic medicine.
THE BASICS OF RISK COMMUNICATION
We are bombarded by numerical probabilities on a daily basis. We might learn that there is a 30% chance of a thunderstorm for the upcoming weekend, or that our favorite football team is twice as likely to win if they play at home than if they play on the road. In a similar manner, health-related risks are frequently communicated as numerical probabilities: A surgeon might summarize the risk of adverse outcomes from a procedure as 1-2%, and a primary care physician might inform a patient with diabetes that his or her risk of heart attack and stroke is nearly doubled. Furthermore, decisions are (and are expected to be) made based on these numerical risk figures-for example, choosing whether to cancel an outdoor activity owing to the chance of a thunderstorm, or choosing whether to proceed with surgery. It is important to realize that an individual brings a lifetime of experience to how he or she perceives, interprets, understands, or even ignores these figures (36).
There is a tremendous amount of literature about risk communication, ranging from how the general public understands the probabilities associated with weather forecasts (58), general health risks (77), and adverse events during therapy (93) to how to communicate genetic risk information (71). Literature on risk communication is abundant (36), but its diversity poses a challenge to synthesis because the various disciplines do not typically overlap (77). The goal of this section is to review selected examples of risk calculators and risk prediction in medicine, providing precedents and principles for incorporating genetic information into medical care.
Risk Calculators in Medicine
The identification of risk factors for disease is one of the cornerstones of epidemiology. Risk can be assessed from factors that are nonmodifiable, such as genetics and demographics, as well as those that are modifiable, such as environment and lifestyle. Family history, although commonly thought of as a proxy for heritable factors, also has shared environmental components. For example, parents and children are more likely to share risky behaviors, such as cigarette smoking, and to share exposure to a given environment. Historically, family history has been considered an imprecise marker for both genetic and environmental risk; nonetheless, early prediction models utilized family history as a surrogate genetic marker to identify individuals at high risk for a given disease.
The Framingham Heart Study (68) was designed to explore hypertension and cardiovascular disease based on the premise that cardiovascular events could be at least partially attributable to lifestyle, environmental factors, and inheritance (26). Over several decades, this study gradually established many risk factors for cardiovascular disease, including smoking, obesity, physical inactivity, hypertension, and hyperlipidemia. The Framingham risk score (FRS) was devised as a way to combine lifestyle and clinical characteristics into a single quantifiable risk for coronary heart disease events. First validated in men in 1982 and further codified in 1998, the FRS quantifies the likelihood of a coronary heart event over a 10-year period based on smoking status, blood pressure, cholesterol levels, and history of hypertension (42). The score has since been refined to calculate intervals other than 10 years and has been validated in women and other populations (24). The FRS was initially devised to help physicians identify high-risk patients who might benefit from more rigorous preventative strategies or lifestyle changes, but it is now available to the general public through the Internet. Other cardiovascular risk prediction methods utilize features not in the FRS, such as C-reactive protein (Reynolds risk score, developed from the Framingham cohort) and family history of cardiovascular disease (ASSIGN, developed from the Scottish Heart Health Extended Cohort) (85, 105).
After the success of the FRS, similar risk prediction models were developed for other diseases, with the focus shifting from a population-based strategy to personalized medicine, including genetic information. The Gail model, first published in 1989, was developed to help physicians counsel women about the risk of breast cancer (37). This model utilizes demographics, past medical history, and family history data to calculate five-year and lifetime absolute risks of breast cancer. It also estimates a woman’s risk compared with the risk of the average women of the same demographics but without the subject’s medical or family history. The Gail model considers first-degree relatives (mother, sisters, and daughters) to be significant. Like the FRS, the Gail model was originally developed as a way for clinicians to identify high-risk women for further screening strategies. The Gail model is now readily accessible to the general public via websites and has been utilized in the assessment of cost-effectiveness strategies for screening methods, such as mammography. The Gail model was developed prior to the discovery of the increased breast cancer risk associated with the BRCA1 and BRCA2 genes, but it uses family history as a proxy measure to identify patients with increased genetic susceptibility.
In 2008, the World Health Organization devised a risk calculator for osteoporosis called the Fracture Risk Assessment Tool (FRAX) (61). This calculator was designed to help physicians detect high-risk patients who would benefit from preventative treatment or rigorous screening. Unlike the FRS and the Gail model, this calculator was initially designed for online use. It utilizes demographics, body mass index, medical history (steroid exposure, history of fracture, rheumatoid arthritis), risky habits (smoking, drinking), family history (parental fractured hip), and biomarkers (femoral neck bone mineral density) to calculate the probability of a major osteoporotic or hip fracture over 10 years. FRAX also includes diverse geographic and ethnic options to further personalize the risk.
Risk calculators used in clinical practice have evolved to include diverse data on demographics, ethnicity, medical history, lifestyle factors, environmental exposure, family history, and biomarkers. Although personalized genetic data are not yet commonly utilized in the risk calculators that are employed on a large scale, it is likely that genetic data will soon be incorporated into existing risk calculators to further refine risk estimation as we learn more about genetic risk factors for common disorders and as genetic testing becomes widely accessible to the public.
Risk Prediction in Complex Diseases
Complex polygenic diseases, such as diabetes, inflammatory bowel disease, and rheumatoid arthritis, are notoriously difficult to predict. This is due partially to the influence of many genes and to large and heterogeneous gene-gene and gene-environment interactions (59). Genome-wide association studies are currently the most common way of identifying single-nucleotide polymorphisms (SNPs) related to risk of a particular disease. These studies compare many thousands of SNPs in diseased cases against those of healthy controls to identify SNPs conferring increased or decreased risk. They typically require many thousands of patients for a reliable risk prediction model. Despite advances in the identification of genetic markers for disease risk, however, our fundamental knowledge of genetic contribution to disease risk has much room for improvement. In the best-studied complex polygenic diseases, such as diabetes, macular degeneration, and Crohn’s disease, it is estimated that less than 50% of heritability is explained by currently identified SNPs (70).
Risk prediction models often group factors as either nonmodifiable (age, sex, ethnicity, location, and genetics) or modifiable (lifestyle factors such as obesity and physical activity). Biomarkers are often utilized to detect preclinical or subclinical manifestations. These biomarkers are typically common tests (such as the use of blood glucose levels for predicting diabetes) but are sometimes novel (such as the use of serum adiponectin levels for predicting diabetes).
In a 2011 review that identified 46 risk prediction models in type 2 diabetes (13), nearly every model included family history, but only 5 included genetic information in the form of genetic risk scores from SNPs. According to this article, the discriminatory accuracy of models using genetics was only modestly improved compared with those using family history alone. Even in large genome-wide association studies, many SNPs significantly linked to diabetes have modest odds ratios compared with modifiable risk factors such as obesity.
As risk prediction models have evolved, the intended audience has also changed. The original risk prediction models were aimed at physicians to help them identify high-risk patients for preventative strategies or preventative research studies. More recently, these models have been made available to the general public, usually via online risk tools or genetic susceptibility panels that utilize no clinical information. In the near future, clinical, demographic, environmental, and genetic data will likely be combined to offer more precise risk prediction directly to consumers/patients. These methods may be strictly informative or financially motivated through tie-ins with health promotion companies, whereas other models will continue to emphasize screening strategies, prevention efforts, and the modifiability of risk for a given disease.
How Well Do Individuals Understand Risk Information?
Numerical probabilities are challenging to communicate effectively to the lay public and even to trained medical clinicians owing to the wide range of scientific and mathematical expertise and personal experiences that affect risk perception and understanding (95). Therefore, a one-size-fits-all approach is likely to be ineffective. Weather reports are a great example. Meteorologists across the country daily communicate the numerical chance of a weather event in the same format, and studies have demonstrated that the general public frequently misunderstands these reports. For example, the public tends to misconstrue the probability of precipitation, such as an 80% chance of rain today, as being deterministic, referring to the proportion of area that will be rained on or the proportion of time it will actually rain (58). In the same manner, patients often do not take the risk figures presented to them at face value, adopt them as true, or even recall them over time (9, 12, 67, 80). Studies of genetic counseling have demonstrated that patients tend to rely more heavily upon personal experience and personal theories of risk than upon communicated risk figures in interpreting and understanding their own genetic risk information (74). Even after a thorough explanation of risk figures, patients in one multicenter study of cancer genetic counseling did not fully understand their risk of developing cancer, sometimes incorrectly underestimating their risk after counseling (9).
Why is risk information so difficult to understand? The psychological, social, and spiritual aspects of a person’s life impact how risk is integrated into the individual’s complex network of belief systems and life experiences (98, pp. 208--12). Consider a person who has a family history of Huntington’s disease, with this person’s mother and five of six maternal aunts and uncles having the disease. This counselee may disregard the counseling that he or she has a 50% risk of having the mutation because his or her real-life experience seems to indicate a much higher risk. This scenario demonstrates two key factors that may influence risk perception: (a) a concept known as representativeness, which means that an individual applies what happened in a small sample (often a personal experience) to a larger group, including themselves, and (b) the general difficulty in understanding numerical values and probability (101, pp. 125--37). Table 1 summarizes additional factors that impact risk perception and understanding.
Table 1.
| Factor | Description |
|---|---|
| Individual factors | |
| Cognitive/emotional traits |
Personality traits such as optimism versus pessimism, risk-taking attitudes, and preference for numerical format of risk figures |
| Numeracy | The ability to understand numerical values and probability (the numerical equivalent of literacy) |
| Consequences | The range of consequences related to the risk information; consequences can be positive, negative, life altering, neutral, etc. |
| Uncertainty and the need to reduce uncertainty |
The uncertainty associated with risk figures and the emotional need to reduce this uncertainty |
| Experiential factors | |
| A priori beliefs | Initial beliefs about risk level |
| Availability | Prior experiences, i.e., real-life experiences that are cognitively “available” to the client when the risk is presented |
| Representativeness | Inferences from a small sample (e.g., a family) to a larger group (e.g., a specific population) |
| Other factors | |
| Anchoring | Bias introduced by the first concept or risk figure introduced |
| Binarization | The tendency to simplify risk information and reorient it toward the possible outcomes rather than the likelihood of those outcomes. (i.e., viewing numerical risk in two categories---50/50, present/absent, will/will not happen---regardless of the probability presented) |
| Complexity | The generally complex nature of risk figures, particularly multiple related risk figures presented together or in sequence |
Sometimes the explanation for why people do not understand risk information is simply that the communication was unclear. Risk is commonly communicated as a single-event probability: “With this medication, there is a 30% to 50% risk of a bad side effect.” Yet this statement of risk does not specify a reference class. In this situation, the risk communicator may intend for the reference class to be the group of all patients on this medication, but an individual patient may think of his or her day-to-day life as the reference class (i.e., this bad side effect will happen on 30% to 50% of the days). A frequency statement makes the reference class clear: Of 10 patients on this medication, 3 to 5 of them will report experiencing this bad side effect (40). Risk communicators are encouraged to present and frame numerical risks in multiple ways in order to attempt to navigate the complexity associated with understanding risk information.
Strategies for Presenting Risk Information
The broader risk communication literature provides several evidence-based strategies for presenting risk information, which are summarized in this section.
Present risk information in multiple formats, but avoid qualitative modifiers
Individuals differ in their preference for the format of numerical risk information, and it is therefore important not to assume that any single technique is best in communicating numerical probability. For example, some people may prefer that risk be presented as a percentage (25%), whereas others may prefer a proportion (25 in 100), a population comparison (25 in 100 compared with 5 in 100 for the general population), or even gambling odds (3 to 1) (51). Thus, experts suggest that risk figures be presented in multiple numerical formats if possible (“there is a 25% chance of x, or a 25 in 100 chance of x”). Additionally, it is generally best to communicate risk in terms of absolute risk rather than relative risk, as individuals tend to be influenced by relative risk information (36). Many individuals prefer that risk figures be presented in qualitative words, such as high or low, but using terms like these typically allows much greater latitude for interpretation than a quantitative format does (73). When using numbers, risk communicators should strive to ensure that the numbers are presented in a format easily understandable to individuals with low numeric literacy (numeracy) (36).
Be conscious of framing biases, and frame risk in multiple ways
The framing of a risk figure is an important consideration. Equivalent risks can be framed in terms of a positive/gain or a negative/loss, and differences in framing can lead to differences in risk perception. For example, a study of Swiss physicians and patients regarding perception of the treatment benefit of a hypothetical new drug showed that this perception differed among both doctors and patients who received equivalent risk information in the following formats: survival proportions, mortality proportions, relative mortality reduction, and all three presentations of risk (83). This study highlights two important points: (a) Physicians (not just patients) were susceptible to framing bias, and (b) presenting information about the drug in terms of absolute mortality risk led to the least biased perceptions of the benefit when a combination of three risk formats was used as the reference.
To attempt to circumvent framing effects, it may help to communicate risk information in both positive and negative ways. For example, a 60% risk of developing a condition also means a 40% risk of not developing the condition, or, out of 100 patients with these risk factors, 60 of them will develop the condition and 40 will not (82).
Use pictures, but choose carefully
Graphics in risk communication not only are widely used but have been shown to improve understanding of risk, particularly for those with lower levels of education and numeracy (38, 100, 107). In general, pictographs with 100 boxes have been found to be the best method for communicating percentages, and they are good at communicating both verbatim information and “the gist” of the information (53). Pictographs directly translate percentages into discrete visual units and better communicate the part-to-whole ratio. They can also help to reduce the influence of anecdotal information on how patients interpret risk information (35). One study of graphics found that patients preferred risk information to be presented via multiple graphics (29); however, this finding may simply be due to a cognitive perception that more graphics means the data are more accurate.
Pictographs can be easily created by making a table of 100 boxes and shading in the boxes corresponding to a particular percentage. Pictograph generators are also available on the Internet for public use (e.g., http://www.iconarray.com).
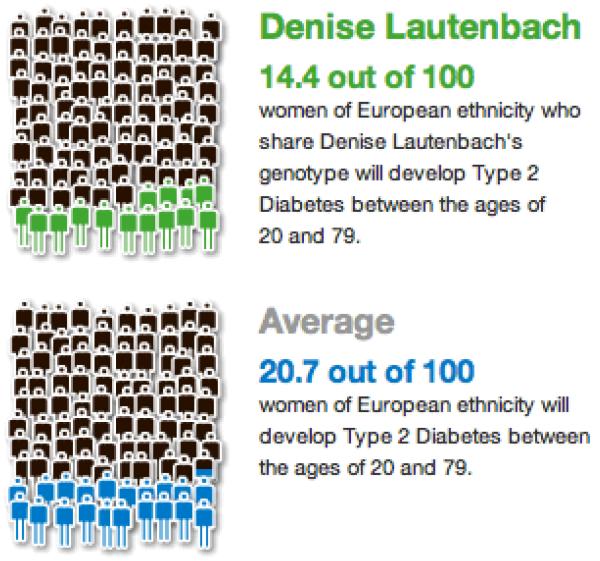
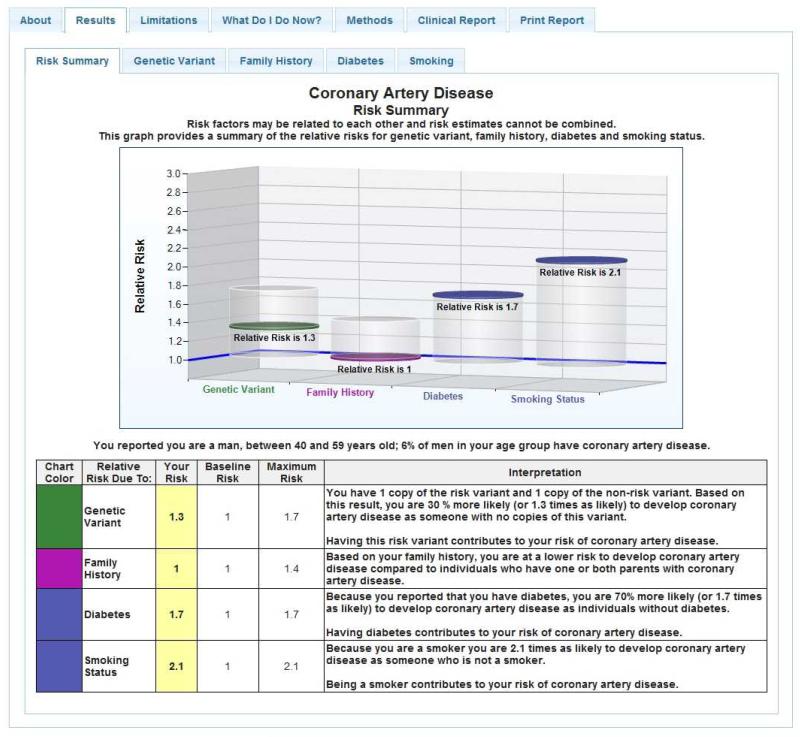
Several companies and research studies in genetic risk communication currently utilize graphics, and it is worth noting and building on the work already done. For example, 23andMe uses pictographs of 100 cartoon people with some shaded to depict percentages (Figure 1). The Coriell Personalized Medicine Collaborative has used graphics to communicate relative risk figures, providing estimates of disease prevalence as a reference point (Figure 2). In this study, Coriell chose to present risk as relative risk in order to consistently report risk figures across multiple diseases and across genetic and nongenetic factors (96). Coriell also presents competing environmental risks that allow recipients to contextualize genetic risk more appropriately.
Figure 1.
Sample pictographs from 23andMe results. © 23andMe, Inc. 2013
Figure 2.
Sample risk summary from the Coriell Institute for Medical Research.
Denominators matter!
Can you visualize 1,000 people in a room? What about 100,000? Most people, particularly those with low numeracy, have trouble doing so. One study of medical risk communication found that individuals, particularly those with low numeracy, often incorrectly recalled or simply disregarded risk figures with large denominators (greater than 5,000); the authors suggested that ratios with smaller denominators (from 50 to 100) are easier to understand and visualize and therefore are more suitable for communicating risk information to patients (39). Using consistent denominators when comparing risks presented as ratios reduces the need for individuals to perform a mental calculation to compare the magnitudes of those risks (84). Also, individuals with low numeracy frequently neglect the denominator when comparing risk figures and focus only on the numerator (known as denominator neglect). For example, when asked which risk figure is higher, individuals commonly say that that 4:100 is higher than 2:50 (because 4 is greater than 2), although in fact these ratios are equivalent (39).
Less is more
Experts in risk communication suggest keeping graphics simple and assuming low numeracy to streamline information and emphasize the most important parts of the message (95). High-level statistical concepts, such as confidence intervals, should generally be avoided because people tend to either accept or reject numerical information without cognitively adjusting for its overall quality (84).
Be aware that factual risk information is often distorted by emotions
Using numbers in risk communication gives the impression of a highly rational process, but emotions matter a great deal. Individuals process risk information both cognitively and affectively, and the emotional aspect plays a role in how they understand, adopt, and integrate the risk information and then make decisions based on it. It is important to realize that factual risk information may take second place to “gut feelings,” the views of acquaintances, cultural beliefs, past experiences, and a priori perceived risks (95).
Engage recipients in the process
Engagement and interactivity increase both patients’ understanding of their disease risk and the likelihood of changing their behavior to reduce their risk (32). Online risk assessment tools offer great potential for the field of risk communication in this regard. These tools can quickly process information on patients’ lifestyles, family histories, and genetics to calculate their risk for a given disease using a risk prediction model. They also allow individuals to participate in and interact with the process by inputting their own risk factors (demographics, exposures, habits, etc.) and manipulating results to see how altering certain behaviors might change their risk and potential outcomes. Online risk assessments additionally enhance the relevance of information by allowing messages to be tailored to patients’ individual characteristics. Message tailoring may be helpful in risk communication interventions where behavior change is the desired outcome (60).
Summary
Current models of risk calculation and prediction for common disorders and the evidence-based literature on how to best communicate numerical probabilities provide a framework for communicating genetic risk information. The next section highlights some of the opportunities and challenges associated with genetic risk communication that have emerged through empirical work on the impact of genetic risk assessments for common disorders.
CHALLENGES TO COMMUNICATING INFORMATION IN A RAPIDLY EVOLVING FIELD
The rapid pace of genetic discoveries and the evolution of genomic technologies present both opportunities and challenges for the incorporation of genetic risk information into medicine. Since 2000, our research group has studied the behavioral and psychosocial impact (48) of apolipoprotein E (APOE) genotyping to estimate Alzheimer’s disease (AD) susceptibility as part of the Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study. APOE genotype and AD risk assessment serve as an excellent model for translational trials in disclosing and communicating genomic information because the disease is common (affecting more than 5 million Americans) and because the APOE ε4 allele (carried by 20% or more of most populations) is a well-established and robust predictor of AD risk (2, 22). Moreover, the APOE ε4 allele is neither necessary nor sufficient to develop AD, making APOE a stronger representative of the types of genetic markers that have been identified in the past decade through genome-wide association studies. Although genetic risk assessment is moving quickly away from single-gene, single-disease risk assessment, many of the research questions and challenges addressed in the REVEAL Study are relevant to genetic risk profiling for multiple diseases, and show how it can have myriad effects on emotional well-being, disease beliefs, and health behaviors (88). The following section addresses opportunities and challenges associated with the communication of genetic risk information, using findings from the REVEAL Study as examples. Table 2 summarizes the research subjects and randomization all four REVEAL Study trials.
Table 2.
Characteristics of the research subjects and randomization in the four Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study trials
| Subjects | Randomized study armsa | ||
|---|---|---|---|
| Comparison | Intervention | ||
| REVEAL I (2000--2003) |
Cognitively normal adults with a first-degree relative with AD |
Genotype nondisclosure (AD risk excluding APOE) |
Genotype disclosure (AD risk including APOE) |
| REVEAL II (2003--2006) |
Same as REVEAL I, but including older adults and targeted recruitment of African Americans |
Standard protocol (in-person educational session, extended counseling); Genotype disclosure (AD risk including APOE) |
Condensed protocol (mailed educational brochure, abbreviated counseling); Genotype disclosure (AD risk including APOE) |
| REVEAL III (2006--2009) |
Cognitively normal adults with or without a family history of AD |
Condensed protocol; Genotype disclosure (AD risk including APOE) |
Condensed protocol; Genotype disclosure (AD risk including APOE, plus APOE-associated risk of cardiovascular disease) |
| In-person risk disclosure | Telephone risk disclosure | ||
| REVEAL IV (2010--2013) |
Persons with a diagnosis of mild cognitive impairment |
Standard protocol; Genotype nondisclosure (AD risk excluding APOE) |
Standard protocol; Genotype disclosure (AD risk including APOE) |
In REVEAL I, II, and IV, subjects were 1:2 randomized for the comparison:intervention assignment. In REVEAL III, subjects were double randomized (factorial design) to receive either Alzheimer’s disease (AD) risk information alone or AD risk plus APOE-associated cardiovascular risk information and to receive either in-person risk disclosure or telephone risk disclosure.
Tailoring Communication to Patient Profiles
Numeric risk estimation based on an individual’s genetic profile is a cornerstone of personalized genetic medicine. The challenges in creating such estimates are often understated. The validity of the susceptibility loci used in some models has been questioned (57), and it can be difficult to identify situations where different markers represent the same DNA sequences. Polygenic risk models that attempt to adjust for these factors are limited in number, and these have often not been validated in populations other than Caucasians; thus, the associations between single markers and disease risk are frequently exaggerated (8). Even when genetic risk assessment focuses on a well-established marker for disease, like APOE as a marker for AD risk, personalizing risk estimates can be contentious. Questions arise such as whether to adjust for demographic factors (such as sex, race, and education) or environmental factors (18), and inferences must be made about the incidence of disease (23).
Genetic risk information for common conditions is currently available to the general public only through consumer-oriented personal genomics companies, and the introduction of genetic risk information for common complex diseases into clinical care is likely to be slow. Understanding who wants genetic risk information and their reasons for wanting it may help clinicians tailor communication to maximize its relevance and satisfy patients’ needs. Interest in genetic risk information is affected by individual beliefs, interpersonal relationships, and social and environmental factors. Nevertheless, trends suggest that the following groups may be more likely to seek genetic risk assessments for common disorders: women (33, 89), Caucasians (1, 89), individuals with a family history of disease (90), and adults with more education (30, 86). The last group is particularly notable given the complexity of genetic risk information. The public often scores slightly better than random chance on genetic knowledge scales (17, 92). Moreover, the way genetic information is commonly communicated compounds these challenges; some personal genomics company websites, for instance, are written at a grade-15 education level (63).
Understanding the reasons for pursuing a genetic risk assessment for common disorders may also help maximize the relevance of tailoring communication. In the REVEAL Study, most participants endorsed multiple reasons for seeking genetic risk assessment for AD (mean = 7.2), including the need to arrange personal affairs, curiosity, and the need to prepare one’s family for the possibility of AD (89). Although obtaining information about prevention is the most popular perceived benefit of a genetic risk assessment for AD (19), these findings highlight that individuals who seek genetic susceptibility testing may not consider direct medical benefits their only objective. In fact, most research on genetic risk assessments has found that patients perceive benefits to testing that are unrelated to that testing, whether the context is targeted BRCA1/2 testing to identify breast/ovarian cancer risk (5, 14) or general genome screening to identify risk for multiple common conditions (41).
Conducting Genetic Testing Safely in an Abbreviated Manner
Genetics providers already report feeling strained by current patient care demands and are streamlining their practices (104). Currently, there are approximately 1,500 certified clinical geneticists and 3,000 certified genetic counselors practicing in the United States (3, 4). To compensate, a number of recent studies have used nonspecialists (e.g., a health educator) or have tried alternative formats (e.g., the Internet) to communicate genetic risk information (10, 62, 75). Whether such methods compromise the ability of patients to understand or cope with the information is unclear. The second REVEAL Study trial was one of the few studies to explore modifications to pre-test education as well as risk disclosure. Participants randomized to a “condensed protocol” arm were mailed an educational brochure to replace an in-person educational session, and subject-led question-and-answer time replaced structured genetic-counselor-led discussion prior to genotyping. Analyses found that, in addition to including one fewer in-person encounter, the condensed protocol reduced clinician time from 77 minutes to 34 minutes. On measures of information recall and understanding, participants receiving condensed education performed no worse than participants receiving extended education (87). Furthermore, preliminary analyses suggest that when test results were disclosed by a genetic counselor, subjects receiving the condensed protocol reported no greater post-test anxiety, depression, or test-related distress at one year after disclosure than did subjects receiving the original extended protocol. However, when test results were communicated by a nongeneticist physician, APOE ε4 carriers receiving the condensed protocol had greater test-related distress scores at six weeks after disclosure. Further work will need to explore whether differences between physician and genetic-counselor disclosures are due to differences in training, differences in rapport (genetic counselors also acted as study coordinators and were younger and female, whereas physicians were older and male), or other factors. Cautious approaches are also warranted given that patients frequently forget key pieces of information shortly after education or disclosure of test results (31, 81). Nevertheless, results suggest that educational burdens may be reduced without affecting psychological outcomes or understanding (47).
The third REVEAL Study trial examined another way of streamlining genetic risk disclosure by comparing telephone disclosure against in-person disclosure. Preliminary analyses suggest that, although there were no differences between disclosure methods in terms of anxiety, those receiving results via telephone had higher scores on scales of depression and test-related distress one to six weeks after disclosure. Results suggest that telephone disclosure may not address patients’ short-term psychological needs as well as in-person disclosure does, and patients of concern (e.g., those with elevated depression prior to testing) may benefit from face-to-face discussions when receiving results. At the same time, all scores on psychological outcomes were well below clinical cutoffs for concern, and measures of information recall and understanding may have been better among those who received telephone disclosure. Similar, if not more favorable, results have been reported from initial work related to the use of videoconferencing technologies during risk disclosure (43, 66, 108, 109).
Should Incidental Information Be Disclosed?
The advent of genome sequencing technologies has led to an emerging debate about whether and (if so) how to disclose genetic risk information incidental to the original purpose of testing. A primary aim of the third REVEAL Study trial was to examine this question empirically by disclosing an unexpected element during risk assessment for AD: that the APOE ε4 allele is also associated with increased risk for cardiovascular disease. Preliminary analyses suggest that this additional information may actually help individuals cope with indications of increased risk for disease and may motivate them to make additional improvements to health behaviors. Results suggest that disclosure of incidental genetic information can have unexpected benefits with respect to coping and disease prevention (20, 21).
How might communicating genetic risk information facilitate behavior change? Chao and colleagues (16) used data from the first REVEAL Study trial to examine this issue empirically. Subjects who learned they were APOE ε4 positive were more likely than those who learned they were APOE ε4 negative or those who had received a nongenetic risk assessment to report an AD-specific health behavior change. In fact, approximately half (52%) of those who learned they had an increased genetic risk of AD reported a health behavior change based on their genetic test results, such as changes made to medications, vitamins, diet, or exercise. Vernarelli and colleagues (99) explored these findings further, focusing on dietary supplement use in the second REVEAL Study trial. Subjects who were APOE ε4 positive were nearly five times more likely to report a change in dietary supplement use compared with subjects who were ε4 negative.
Results from the REVEAL Study have also shown that genetic susceptibility testing may affect insurance purchasing. In the first REVEAL Study trial, APOE ε4-positive subjects were almost six times more likely to report altering their long-term care insurance coverage compared with those who received a nongenetic risk assessment (106). Similarly, in the second REVEAL Study trial, subjects with one APOE ε4 allele were 2.3 times more likely to increase their long-term care insurance coverage or report that they plan to do so compared with those with an APOE ε3/ε3 genotype (97). Use of self-reported outcomes, examination of self-selected populations, and examination of an emotionally charged condition like AD limit the generalizability of behavioral findings, but REVEAL Study data support the possibility that genetic risk information can motivate behavior change.
Such findings run counter to data from other reports on genetic susceptibility testing, however. Communicating an increased genetic risk for lung cancer can motivate short-term smoking cessation, but the effects disappear over time (27, 94). High-penetrance mutations associated with various forms of cancer often increase screening rates, but genetic tests examining markers that have moderate to weak associations with common complex conditions have shown little ability to motivate behaviors such as smoking cessation, dietary changes, or improved physical activity (76). Thus, general pessimism surrounds the idea that genetic risk information will evolve into an effective tool for health promotion.
Considered against REVEAL Study findings, however, the inconsistencies suggest that the field may be addressing the wrong question. It may be a question not of whether genetic risk information can motivate health behavior changes, but rather under what conditions it might do so. Some experts have suggested that more effective approaches to communicating genetic risk information would capitalize on the ways this information differs from other types of risk information. Group-level interventions are frequently more successful than individual-level behavioral interventions, suggesting that greater benefits might accrue from tailoring genetic risk communication to families rather than individuals (76). Individuals receiving genetic risk information already seem to understand the familial implications: Of the more than 80% of REVEAL Study subjects who reported telling others their genetic test results, 64% reported telling a family member (6). Such interventions have not been tested but represent fertile ground for future work as genetic information becomes more commonplace.
Accepting the Limited Ability of Genetic Testing to Change Perceptions
Genetic profiling can change perceptions and reduce uncertainty about disease risk (64), but it is important to keep in mind that it may not be the most important determinant of them. In 2010, Linnenbringer and colleagues (69) analyzed REVEAL Study participants who accurately recalled their communicated risk estimate and found that nearly half believed their actual risk was different from what they were told. Among those who disagreed with the given lifetime risk estimate, the majority (69%) reported that they thought their risk was higher than what was disclosed. Those who perceived a greater baseline AD risk were more likely to believe their actual risk was greater than the risk indicated by the genetic risk assessment. These findings build on prior work examining nongenetic risk assessments, family history analyses, and genetic tests for deterministic mutations associated with cancer that showed that risk perceptions are resistant to change (7, 52, 102). Genetic susceptibility information is not the sole determinant of risk perceptions, and individuals integrate genetic test results into existing beliefs about disease and well-being.
What Is the Impact of Using Genetic Risk Markers to Communicate About “Imminent Risk”?
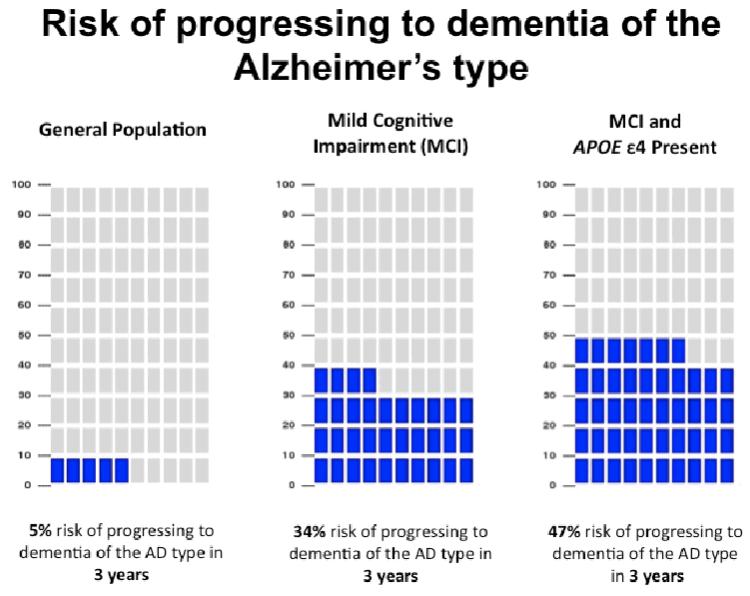
The first three REVEAL Study trials examined disclosure of genetic risk information to asymptomatic adults, the vast majority of whom were unlikely to develop AD until well into the future. The fourth REVEAL Study trial is currently under way, examining the safety and impact of genetic risk disclosure among individuals who already have mild cognitive impairment and are more imminently at risk of developing AD. Figure 3 provides an example of a pictograph set used for risk presentation in this trial. The trial will also offer insight into how individuals respond to genetic risk information in the context of phenotypic information (65).
Figure 3.
Sample risk result pictograph set from the fourth Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study trial. Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment.
Impact of the REVEAL Study on Understanding of Genetic Risk Communication
The REVEAL Study was the first study of genetic susceptibility testing for common complex diseases to empirically evaluate issues in genetic risk communication using randomized clinical trials. Although this study examined these topics in the context of single-gene testing to determine risk for a specific disease, several key points may be generalizable when considering the impact of communicating genomic risk information from more sophisticated technologies (e.g., SNP arrays that examine markers for multiple conditions, whole-genome and whole-exome sequencing). First, many people are interested in this information even if there is nothing they can do to reduce their risk. Although health care professionals may value medical utility and clinical actionability as important reasons for offering a genetic susceptibility test, we must recognize that the public may attach substantial personal utility to such information. Second, communicating an increased genetic risk for a terrifying, incurable, adult-onset disease can be done safely by modifying protocols developed for deterministic conditions like Huntington’s disease, and protocols can even be streamlined without compromising the understanding or psychological well-being of test recipients who volunteer for such studies. Finally, the way individuals interpret genetic risk information is complex and often unexpected. The fact that many REVEAL Study participants did not take the provided information at face value and the way in which the disclosure of incidental cardiovascular disease associations motivated behavior change and improved coping are just two examples of the complex ways individuals internalize genetic risk information.
During the REVEAL Study, several direct-to-consumer companies arose offering the public the opportunity to obtain genetic risk information for hundreds of diseases or conditions at once, thus making the REVEAL Study particularly relevant to the controversial discussion that arose (55). Numerous commentators have raised questions about the ethics and regulation of direct-to-consumer personal genomics services (11), the potential for misunderstanding or psychological harm, and the potential for such information to increase unnecessary medical costs (78). However, these companies have also created novel ways for communicating vast amounts of genetic risk information, utilizing the Internet as a large-scale avenue for disclosure, and thus they provide a great opportunity to study the impact of communicating this type of information. Partnerships between these private companies and academic institutions have stimulated multiple research initiatives across the United States to study how individuals respond to, understand, and utilize hundreds of pieces of genetic risk information (15, 66a), including the Impact of Personal Genomics (PGen) Study to measure the pre- and post-test impact of personal genomics services. Studies to date suggest that learning this type of risk information does not result in short-term changes in psychological health, diet, or exercise behavior or the use of screening tests (10) but may modestly influence risk perception and worry (56). Other high-profile recent and ongoing research endeavors to further elucidate how the public understands and utilizes genetic risk information include the Coriell Personalized Medicine Collaborative (62) and the Multiplex Initiative led by scientists at the National Human Genome Research Institute (75). Results from the Coriell Personalized Medicine Collaborative indicate that early adopters of such a service are motivated by their own curiosity and to find out information that directly benefits their own health (41) and that participants have a reasonable understanding of the genomic risk information they received (62).
RISK COMMUNICATION TO PROMOTE POSITIVE HEALTH OUTCOMES
Investigators around the world are examining novel methods for communicating health risk information to promote positive health outcomes. Although genetic information may or may not be sufficient to promote positive behavior changes on its own (72), it is becoming increasingly relevant when considering an individual’s risk of disease. Experts in risk communication have identified problems with accurate risk communication among both patients and physicians. For example, authors of a New Zealand study of cardiovascular disease risk communication identified that telling a person who smokes that he or she has a 10% risk of a cardiovascular event is unlikely to motivate the person to stop smoking unless the person is also told what the absolute risk of a cardiovascular event would be after quitting smoking (103). Similarly, telling a person who smokes that his or her risk of a cardiovascular event is twice that of nonsmokers is relatively unhelpful unless the absolute risk of a cardiovascular event in nonsmokers is also communicated. In this study, the authors developed a program for communicating cardiovascular disease risk called Your Heart Forecast, which incorporates both absolute and relative risk information to advise patients of their risk of cardiovascular disease over time, how that risk compares with that of other individuals of the same age and of different ages, and how risk may be modified as risk factors change over time (103). Unlike Your Heart Forecast, empirical studies of genetic risk communication over the past decade have examined whether genetic risk information influences behavior change but have generally focused on communicating a single risk figure, rather than showing how risk may be lowered as behavior changes. This may be due to an early and now-dissipating perception that genetic risk information alone may be highly deterministic and thus powerful enough to influence behavior.
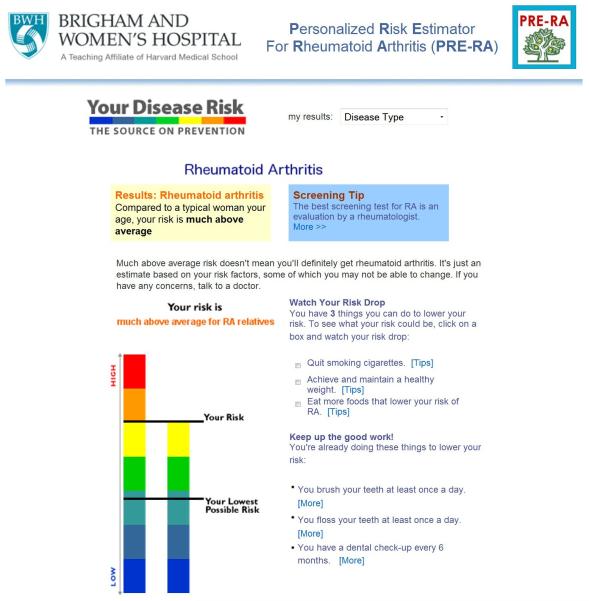
Another example of using genetics to instigate behavior change is the ongoing Personalized Risk Estimator for Rheumatoid Arthritis (PRE-RA) Family Study. This study is funded by the National Institutes of Health to examine the behavioral impact of communicating a risk prediction model for rheumatoid arthritis. The risk prediction model incorporates both nonmodifiable (age, sex, family history, and genetic risk score) and modifiable (smoking, periodontitis, and fish intake) risk factors. It also utilizes biomarkers by testing for autoantibodies associated with rheumatoid arthritis. Unlike risk calculators aimed at physicians for prevention or treatment, the PRE-RA risk tool is modeled after Your Disease Risk (http://www.yourdiseaserisk.wustl.edu), an easily navigable online interface, and is aimed directly at participants to identify behaviors that they can modify to change their own risk for rheumatoid arthritis (Figure 4).
Figure 4.
Sample results from the Personalized Risk Estimator for Rheumatoid Arthritis (PRE-RA) Family Study risk calculator.
PREPARING FOR THE FUTURE
The era of genomic medicine has arrived (44). Laboratories around the country are offering Clinical Laboratory Improvement Amendments (CLIA)--certified whole-genome and whole-exome sequencing as a clinical service (46). Furthermore, access to sequencing is expected to become increasingly available to the general public via personal genomics companies. As the cost of DNA sequencing decreases, the technology will become even more accessible.
As the genomic era makes available vast amounts of genetic data on each individual, a need emerges to effectively communicate information about hundreds of genetic risk factors to individuals or their health care providers, as well as to prioritize important results to disclose if returning all results is not feasible (45). For these purposes, current models of genetic counseling and genetic risk communication are simply not scalable.
We believe that whole-genome and whole-exome sequencing will be used in many ways, including in two distinct and complementary situations. In generally healthy patients, physicians will use the results to gain insight into future health risks and to inform prevention and surveillance efforts, a category we refer to as general genomic medicine. In patients presenting with a family history or symptoms of a disease, physicians will use the results to interrogate particular sets of genes known to be associated with the disease in question, a category we refer to as disease-specific genomic medicine. How these differing contexts affect the communication of risk information derived from sequencing is an open question and the focus of ongoing research. Three of the authors of this article (D.M.L., K.D.C., and R.C.G.) are part of a team of more than 40 investigators currently conducting a project to develop a process to integrate whole-genome sequencing into clinical medicine and explore the impact on health outcomes in both primary and specialty care settings. In the MedSeq Project, we are randomizing physicians and their patients to receive clinically meaningful information derived from whole-genome sequencing as opposed to information from current standards of care (National Institutes of Health grant U01HG006500, ClinicalTrials.gov ID NCT01736566). Data from the MedSeq Project will provide critical insight about ways to communicate the wealth of risk information derived from DNA sequencing to enhance clinical care and maximize patient understanding and well-being.
This review highlights the need to draw on the collective wisdom gained through multidisciplinary perspectives when considering the communication of genetic risk information, whether from a single genetic marker or from hundreds of risk figures derived from genome sequencing data. As we embark on a new era of estimating and communicating genetic risk information, we should not only build on our past experiences, but also collaborate with risk communication colleagues in various disciplines to develop methods that can help integrate vast amounts of genetic risk information with nongenetic risk information to improve health outcomes.
SUMMARY POINTS.
Communication of genetic risk information, integration of such information into individualized health care, and the consequences of both will rapidly broaden in scope and practice, as current and emerging technologies allow more individuals and their health care providers to access information about genetic makeup.
Communication of genetic risk information may be best practiced with insight into how individuals understand, perceive, and make decisions based on risk information daily, including both health- and non-health-related risk information and both genetic and nongenetic health risk information.
Evidence-based strategies for presenting all types of risk information include (a) presenting risk information in multiple formats while avoiding qualitative modifiers, (b) being conscious of framing biases and framing risk in multiple ways, (c) using carefully chosen graphics, (d) using a small denominator (from 50 to 100) when possible, (e) remembering that less is more, (f) paying attention to emotions that may influence perception and adoption of risk figures, and (g) engaging recipients in communication.
The REVEAL Study was the first study of genetic susceptibility testing for a common complex disease to utilize randomized trials in communicating genetic risk information, and some of these findings may be generalizable when considering the impact of communicating genomic risk information for other conditions.
Investigators in the field of genetic risk communication should not only build on past experiences but also collaborate with risk communication colleagues in various disciplines to develop methods that can best achieve positive health outcomes.
ACKNOWLEDGMENTS
We thank Rachel Miller for assistance in writing this article. The authors are supported by National Institutes of Health grants R01HG002213, U01HG006500, K24AG027841, R01HG005092, F32HG006993, P60AR047782, R01AR049880, and K24AR052403.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Alford SH, McBride CM, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Participation in genetic testing research varies by social group. Public Health Genomics. 2011;14:85–93. doi: 10.1159/000294277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Assoc. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2012;8:131–68. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Am. Board Genet. Couns. (ABGC) About ABGC. 2013. http://www.abgc.net/About_ABGC/GeneticCounselors.asp. [Google Scholar]
- 4.Am. Board Med. Genet. Number of certified specialists in genetics. 2011. http://www.abmg.org/pages/resources_certspecial.shtml. [Google Scholar]
- 5.Armstrong K, Weiner J, Weber B, Asch DA. Early adoption of BRCA1/2 testing: who and why. Genet. Med. 2003;5:92–98. doi: 10.1097/01.GIM.0000056829.76915.2A. [DOI] [PubMed] [Google Scholar]
- 6.Ashida S, Koehly LM, Roberts JS, Hiraki S, Green RC. Disclosing the disclosure: factors associated with communicating the results of genetic susceptibility testing for Alzheimer’s disease. J. Health Commun. 2009;14:768–84. doi: 10.1080/10810730903295518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audrain-McGovern J, Hughes C, Patterson F. Effecting behavior change: awareness of family history. Am. J. Prev. Med. 2003;24:183–89. doi: 10.1016/s0749-3797(02)00592-5. [DOI] [PubMed] [Google Scholar]
- 8.Berg JS, Adams M, Nassar N, Bizon C, Lee K, et al. An informatics approach to analyzing the incidentalome. Genet. Med. 2012;15:36–44. doi: 10.1038/gim.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorvatn C, Eide GE, Hanestad BR, Øyen N, Havik OE, et al. Risk perception, worry and satisfaction related to genetic counseling for hereditary cancer. J. Genet. Couns. 2007;16:211–22. doi: 10.1007/s10897-006-9061-4. [DOI] [PubMed] [Google Scholar]
- 10.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N. Engl. J. Med. 2011;364:524–34. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boddington P. The ethics and regulation of direct-to-consumer genetic testing. Genome Med. 2009;1:71. doi: 10.1186/gm71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. Fam. Cancer. 2006;5:61–75. doi: 10.1007/s10689-005-2577-1. [DOI] [PubMed] [Google Scholar]
- 13.Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol. Rev. 2011;33:46–62. doi: 10.1093/epirev/mxq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappelli M, Surh L, Humphreys L, Verma S, Logan D, et al. Psychological and social determinants of women’s decisions to undergo genetic counseling and testing for breast cancer. Clin. Genet. 1999;55:419–30. doi: 10.1034/j.1399-0004.1999.550605.x. [DOI] [PubMed] [Google Scholar]
- 15.Caulfield T, Ries NM, Ray PN, Shuman C, Wilson B. Direct-to-consumer genetic testing: good, bad or benign? Clin. Genet. 2010;77:101–5. doi: 10.1111/j.1399-0004.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 16.Chao S, Roberts JS, Marteau TM, Silliman RA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL Study. Alzheimer Dis. Assoc. Disord. 2008;22:94–97. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen KD, Jayaratne TE, Roberts JS, Kardia SLR, Petty EM. Understandings of basic genetics in the United States: results from a national survey of black and white men and women. Public Health Genomics. 2010;13:467–76. doi: 10.1159/000293287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen KD, Roberts JS, Royal CDM, Fasaye G-A, Obisesan T, et al. Incorporating ethnicity into genetic risk assessment for Alzheimer’s disease: the REVEAL Study experience. Genet. Med. 2008;10:207–14. doi: 10.1097/GIM.0b013e318164e4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen KD, Roberts JS, Uhlmann WR, Green RC. Changes to perceptions of the pros and cons of genetic susceptibility testing after APOE genotyping for Alzheimer disease risk. Genet. Med. 2011;13:409–14. doi: 10.1097/GIM.0b013e3182076bf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen KD, Roberts JS, Uhlmann WR, Whitehouse PJ, Obisesan T, et al. How does pleiotropic information affect health behavior changes? Initial results from the REVEAL study, a randomized trial of genetic testing for Alzheimer’s disease risk; Presented at Am. Coll. Med. Genet. Genomics Annu. Clin. Genet. Meet; Albuquerque, NM. 2010.Mar, pp. 24–28. [Google Scholar]
- 21.Christensen KD, Roberts JS, Uhlmann WR, Whitehouse PJ, Obisesan T, et al. The psychological impact of learning cardiovascular disease associations during APOE genotyping to determine risk for Alzheimer’s disease: initial findings from the REVEAL Study. Alzheimer’s Dement. 2010;6:S96. [Google Scholar]
- 22.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a “thrifty” allele? Ann. Hum. Genet. 1999;63:301–10. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 23.Cupples LA, Farrer LA, Sadovnick AD, Relkin NR, Whitehouse PJ, Green RC. Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: the REVEAL Study. Genet. Med. 2004;6:192–96. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–87. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 25.Davidson MA. Primary care for children and adolescents with Down syndrome. Pediatr. Clin. N. Am. 2008;55:1099–111. doi: 10.1016/j.pcl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Dawber TR, Meadors GF, Moore FE., Jr. Epidemiological approaches to heart disease: the Framingham Study. Am. J. Public Health Nation’s Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Viron S, Van der Heyden J, Ambrosino E, Arbyn M, Brand A, Van Oyen H. Impact of genetic notification on smoking cessation: systematic review and pooled-analysis. PLoS ONE. 2012;7:e40230. doi: 10.1371/journal.pone.0040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diergaarde B, Bowen DJ, Ludman EJ, Culver JO, Press N, Burke W. Genetic information: special or not? Responses from focus groups with members of a health maintenance organization. Am. J. Med. Genet. A. 2007;143A:564–69. doi: 10.1002/ajmg.a.31621. [DOI] [PubMed] [Google Scholar]
- 29.Dolan JG, Iadarola S. Risk communication formats for low probability events: an exploratory study of patient preferences. BMC Med. Inform. Decis. Mak. 2008;8:14. doi: 10.1186/1472-6947-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donovan KA, Tucker DC. Knowledge about genetic risk for breast cancer and perceptions of genetic testing in a socio-demographically diverse sample. J. Behav. Med. 2000;23:15–36. doi: 10.1023/a:1005416203239. [DOI] [PubMed] [Google Scholar]
- 31.Eckert SL, Katzen H, Roberts JS, Barber M, Ravdin LD, et al. Recall of disclosed apolipoprotein E genotype and lifetime risk estimate for Alzheimer’s disease: the REVEAL Study. Genet. Med. 2006;8:746–51. doi: 10.1097/01.gim.0000250197.44245.a3. [DOI] [PubMed] [Google Scholar]
- 32.Emmons KM, Wong M, Puleo E, Weinstein N, Fletcher R, Colditz G. Tailored computer-based cancer risk communication: correcting colorectal cancer risk perception. J. Health Commun. 2004;9:127–41. doi: 10.1080/10810730490425295. [DOI] [PubMed] [Google Scholar]
- 33.Esplen MJ, Madlensky L, Aronson M, Rothenmund H, Gallinger S, et al. Colorectal cancer survivors undergoing genetic testing for hereditary non-polyposis colorectal cancer: motivational factors and psychosocial functioning. Clin. Genet. 2007;72:394–401. doi: 10.1111/j.1399-0004.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 34.Evans JP, Burke W. Genetic exceptionalism. Too much of a good thing? Genet. Med. 2008;10:500–1. doi: 10.1097/gim.0b013e31817f280a. [DOI] [PubMed] [Google Scholar]
- 35.Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: Is a picture worth a thousand statistics? Med. Decis. Making. 2005;25:398–405. doi: 10.1177/0272989X05278931. [DOI] [PubMed] [Google Scholar]
- 36.Fischhoff B, Brewer NT, Downs JS, editors. Rep., US Food Drug Adm. Silver Spring; MD: 2011. Communicating risks and benefits: an evidence-based user’s guide; p. 234. [Google Scholar]
- 37.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl. Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Retamero R, Galesic M. How to reduce the effect of framing on messages about health. J. Gen. Intern. Med. 2010;25:1323–29. doi: 10.1007/s11606-010-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Retamero R, Galesic M. Using plausible group sizes to communicate information about medical risks. Patient Educ. Couns. 2011;84:245–50. doi: 10.1016/j.pec.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Gigerenzer G, Galesic M. Why do single event probabilities confuse patients? BMJ. 2012;2012(344):e245. doi: 10.1136/bmj.e245. [DOI] [PubMed] [Google Scholar]
- 41.Gollust SE, Gordon ES, Zayac C, Griffin G, Christman MF, et al. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15:22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon T, Kannel WB. Multiple risk functions for predicting coronary heart disease: the concept, accuracy, and application. Am. Heart J. 1982;103:1031–39. doi: 10.1016/0002-8703(82)90567-1. [DOI] [PubMed] [Google Scholar]
- 43.Gray J, Brain K, Iredale R, Alderman J, France E, Hughes H. A pilot study of telegenetics. J. Telemed. Telecare. 2000;6:245–47. doi: 10.1258/1357633001935329. [DOI] [PubMed] [Google Scholar]
- 44.Green ED, Guyer MS. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–13. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 45.Green RC, Berg JS, Berry GT, Biesecker LG, Dimmock DP, et al. Exploring concordance and discordance for return of incidental findings from clinical sequencing. Genet. Med. 2012;14:405–10. doi: 10.1038/gim.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green RC, Rehm HL, Kohane I. Clinical genome sequencing. In: Ginsburg GS, Willard HW, editors. Genomic and Personalized Medicine. 2nd ed. Elsevier; San Diego: 2012. pp. 102–22. [Google Scholar]
- 47.Green RC, Roberts JS, Chen CA, Whitehouse PJ, Relkin NR, et al. Comparing the impact of a condensed versus extended protocol for disclosure of APOE to relatives of patients with AD: the REVEAL Study. Alzheimer’s Dement. 2007;3:S184. [Google Scholar]
- 48.Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N. Engl. J. Med. 2009;361:245–54. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guttmacher AE, McGuire AL, Ponder B, Stefansson K. Personalized genomic information: preparing for the future of genetic medicine. Nat. Rev. Genet. 2010;11:161–65. doi: 10.1038/nrg2735. [DOI] [PubMed] [Google Scholar]
- 50.Guttmacher AE, Porteous ME, McInerney JD. Educating health-care professionals about genetics and genomics. Nat. Rev. Genet. 2007;8:151–57. doi: 10.1038/nrg2007. [DOI] [PubMed] [Google Scholar]
- 51.Hallowell N, Statham H, Murton F, Green J, Richards M. “Talking about chance”: the presentation of risk information during genetic counseling for breast and ovarian cancer. J. Genet. Couns. 1997;6:269–86. doi: 10.1023/A:1025624221369. [DOI] [PubMed] [Google Scholar]
- 52.Harle C, Padman R, Downs J. AMIA Annual Symposium Proceedings. Am. Med. Inform. Assoc.; Bethesda, MD: 2008. The impact of web-based diabetes risk calculators on information processing and risk perceptions; pp. 283–87. [PMC free article] [PubMed] [Google Scholar]
- 53.Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ. Couns. 2008;73:448–55. doi: 10.1016/j.pec.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Hoagwood K, Kelleher KJ, Feil M, Comer DM. Treatment services for children with ADHD: a national perspective. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:198–206. doi: 10.1097/00004583-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Hudson K, Javitt G, Burke W, Byers P. ASHG statement on direct-to-consumer genetic testing in the United States. Obstet. Gynecol. 2007;110:1392–95. doi: 10.1097/01.AOG.0000292086.98514.8b. [DOI] [PubMed] [Google Scholar]
- 56.James KM, Cowl CT, Tilburt JC, Sinicrope PS, Robinson ME, et al. Impact of direct-to-consumer predictive genomic testing on risk perception and worry among patients receiving routine care in a preventive health clinic. Mayo Clin. Proc. 2011;86:933–40. doi: 10.4065/mcp.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janssens AC, van Duijn CM. Genome-based prediction of common diseases: advances and prospects. Hum. Mol. Genet. 2008;17:R166–73. doi: 10.1093/hmg/ddn250. [DOI] [PubMed] [Google Scholar]
- 58.Joslyn S, Nadav-Greenberg L, Nichols RM. Probability of precipitation assessment and enhancement of end-user understanding. Bull. Am. Meteorol. Soc. 2009;90:185. [Google Scholar]
- 59.Jostins L, Barrett JC. Genetic risk prediction in complex disease. Hum. Mol. Genet. 2011;20:R182–88. doi: 10.1093/hmg/ddr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Julian-Reynier C, Welkenhuysen M, Hagoel L, Decruyenaere M, Hopwood P. Risk communication strategies: state of the art and effectiveness in the context of cancer genetic services. Eur. J. Hum. Genet. 2003;11:725–36. doi: 10.1038/sj.ejhg.5201037. [DOI] [PubMed] [Google Scholar]
- 61.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008;19:385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keller MA, Gordon ES, Stack CB, Gharani N, Sill CJ, et al. Coriell Personalized Medicine Collaborative®: a prospective study of the utility of personalized medicine. Pers. Med. 2010;7:301–17. doi: 10.2217/pme.10.13. [DOI] [PubMed] [Google Scholar]
- 63.Lachance CR, Erby LAH, Ford BM, Allen VCJ, Kaphingst KA. Informational content, literacy demands, and usability of websites offering health-related genetic tests directly to consumers. Genet. Med. 2010;12:304–12. doi: 10.1097/GIM.0b013e3181dbd8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.LaRusse S, Roberts JS, Marteau TM, Katzen H, Linnenbringer EL, et al. Genetic susceptibility testing versus family history-based risk assessment: impact on perceived risk of Alzheimer disease. Genet. Med. 2005;7:48–53. doi: 10.1097/01.gim.0000151157.13716.6c. [DOI] [PubMed] [Google Scholar]
- 65.Lautenbach DM, Chen CA, Cupples LA, Roberts JS, Petersen RC, Green RC. Using APOE to predict “imminent” risk of Alzheimer’s disease conversion among patients with MCI: the REVEAL Study. Alzheimer’s Dement. 2010;6:S194–95. [Google Scholar]
- 66.Lea DH, Johnson JL, Ellingwood S, Allan W, Patel A, Smith R. Telegenetics in Maine: successful clinical and educational service delivery model developed from a 3-year pilot project. Genet. Med. 2005;7:21–27. doi: 10.1097/01.gim.0000151150.20570.e7. [DOI] [PubMed] [Google Scholar]
- 67.Lerman C, Croyle RT, Tercyak KP, Hamann H. Genetic testing: psychological aspects and implications. J. Consult. Clin. Psychol. 2002;70:784–97. doi: 10.1037//0022-006x.70.3.784. [DOI] [PubMed] [Google Scholar]
- 68.Levy D, Brink S. A Change of Heart: How the People of Framingham, Massachusetts, Helped Unravel the Mysteries of Cardiovascular Disease. Knopf; New York: 2005. [PubMed] [Google Scholar]
- 69.Linnenbringer E, Roberts JS, Hiraki S, Cupples LA, Green RC. “I know what you told me, but this is what I think”: perceived risk of Alzheimer disease among individuals who accurately recall their genetics-based risk estimate. Genet. Med. 2010;12:219–27. doi: 10.1097/GIM.0b013e3181cef9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marteau TM. Communicating genetic risk information. Br. Med. Bull. 1999;55:414–28. doi: 10.1258/0007142991902466. [DOI] [PubMed] [Google Scholar]
- 72.Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst. Rev. 2010;2010:CD007275. doi: 10.1002/14651858.CD007275.pub2. [DOI] [PubMed] [Google Scholar]
- 73.Mazur DJ, Hickam DH. Patients’ interpretations of probability terms. J. Gen. Intern. Med. 1991;6:237–40. doi: 10.1007/BF02598968. [DOI] [PubMed] [Google Scholar]
- 74.McAllister M. Personal theories of inheritance, coping strategies, risk perception and engagement in hereditary non-polyposis colon cancer families offered genetic testing. Clin. Genet. 2003;64:179–89. doi: 10.1034/j.1399-0004.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 75.McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Putting science over supposition in the arena of personalized genomics. Nat. Genet. 2008;40:939–42. doi: 10.1038/ng0808-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McBride CM, Wade CH, Kaphingst KA. Consumers’ views of direct-to-consumer genetic information. Annu. Rev. Genomics Hum. Genet. 2010;11:427–46. doi: 10.1146/annurev-genom-082509-141604. [DOI] [PubMed] [Google Scholar]
- 77.McComas KA. Defining moments in risk communication research: 1996-2005. J. Health Commun. 2006;11:75–91. doi: 10.1080/10810730500461091. [DOI] [PubMed] [Google Scholar]
- 78.McGuire AL, Burke W. Raiding the medical commons: an unwelcome side effect of direct-to-consumer personal genome testing. JAMA. 2008;300:2669–71. doi: 10.1001/jama.2008.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McPherson E, Zaleski C, Benishek K, McCarty CA, Giampietro PF, et al. Clinical genetics provider real-time workflow study. Genet. Med. 2008;10:699–706. doi: 10.1097/gim.0b013e318182206f. [DOI] [PubMed] [Google Scholar]
- 80.Meiser B, Halliday JL. What is the impact of genetic counseling in women at increased risk of developing hereditary breast cancer? A meta-analytic review. Soc. Sci. Med. 2002;54:1463–70. doi: 10.1016/s0277-9536(01)00133-2. [DOI] [PubMed] [Google Scholar]
- 81.Michie S, French D, Allanson A, Bobrow M, Marteau TM. Information recall in genetic counselling: a pilot study of its assessment. Patient Educ. Couns. 1997;32:92–100. doi: 10.1016/s0738-3991(97)00068-2. [DOI] [PubMed] [Google Scholar]
- 82.O’Doherty K, Suthers GK. Risky communication: pitfalls in counseling about risk, and how to avoid them. J. Genet. Couns. 2007;16:409–17. doi: 10.1007/s10897-006-9077-9. [DOI] [PubMed] [Google Scholar]
- 83.Perneger TV, Agoritsas T. Doctors and patients’ susceptibility to framing bias: a randomized trial. J. Gen. Intern. Med. 2011;26:1411–17. doi: 10.1007/s11606-011-1810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff. 2007;26:741–48. doi: 10.1377/hlthaff.26.3.741. [DOI] [PubMed] [Google Scholar]
- 85.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–19. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 86.Roberts JS, Barber M, Brown TM, Cupples LA, Farrer LA, et al. Who seeks genetic susceptibility testing for Alzheimer’s disease? Findings from a multisite, randomized clinical trial. Genet. Med. 2004;6:197–203. doi: 10.1097/01.gim.0000132688.55591.77. [DOI] [PubMed] [Google Scholar]
- 87.Roberts JS, Chen CA, Uhlmann WR, Green RC. Effectiveness of a condensed protocol for disclosing APOE genotype and providing risk education for Alzheimer’s disease. Genet. Med. 2012;14:742–48. doi: 10.1038/gim.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Green RC. Genetic risk assessment for adult children of people with Alzheimer’s disease: the Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study. J. Geriatr. Psychiatr. Neurol. 2005;18:250–55. doi: 10.1177/0891988705281883. [DOI] [PubMed] [Google Scholar]
- 89.Roberts JS, LaRusse SA, Katzen H, Whitehouse PJ, Barber M, et al. Reasons for seeking genetic susceptibility testing among first-degree relatives of people with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2003;17:86–93. doi: 10.1097/00002093-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Ropka ME, Wenzel J, Phillips EK, Siadaty M, Philbrick JT. Uptake rates for breast cancer genetic testing: a systematic review. Cancer Epidemiol. Biomark. Prev. 2006;15:840–55. doi: 10.1158/1055-9965.EPI-05-0002. [DOI] [PubMed] [Google Scholar]
- 91.Sanderson SC, Waller J, Humphries SE, Wardle J. Public awareness of genetic influence on chronic disease risk: Are genetic and lifestyle causal beliefs compatible? Public Health Genomics. 2011;14:290–97. doi: 10.1159/000294280. [DOI] [PubMed] [Google Scholar]
- 92.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299:1320–34. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- 93.Siegel CA. Lost in translation: helping patients understand the risks of inflammatory bowel disease therapy. Inflamm. Bowel Dis. 2010;16:2168–72. doi: 10.1002/ibd.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smerecnik C, Grispen JEJ, Quaak M. Effectiveness of testing for genetic susceptibility to smoking-related diseases on smoking cessation outcomes: a systematic review and meta-analysis. Tobacco Control. 2012;21:347–54. doi: 10.1136/tc.2011.042739. [DOI] [PubMed] [Google Scholar]
- 95.Spiegelhalter D, Pearson M, Short I. Visualizing uncertainty about the future. Science. 2011;333:1393–400. doi: 10.1126/science.1191181. [DOI] [PubMed] [Google Scholar]
- 96.Stack CB, Gharani N, Gordon ES, Schmidlen T, Christman MF, Keller MA. Genetic risk estimation in the Coriell Personalized Medicine Collaborative. Genet. Med. 2011;13:131–39. doi: 10.1097/GIM.0b013e318201164c. [DOI] [PubMed] [Google Scholar]
- 97.Taylor DH, Jr, Cook-Deegan RM, Hiraki S, Roberts JS, Blazer DG, Green RC. Genetic testing for Alzheimer’s and long-term care insurance. Health Aff. 2010;29:102–8. doi: 10.1377/hlthaff.2009.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uhlmann WR, Schuette JL, Yashar B, editors. A Guide to Genetic Counseling. 2nd ed. Wiley-Blackwell; Hoboken, NJ: 2009. p. 624. [Google Scholar]
- 99.Vernarelli JA, Roberts JS, Hiraki S, Cupples LA, Green RC. Effect of Alzheimer’s disease genetic risk disclosure on dietary supplement use. Am. J. Clin. Nutr. 2010;91:1402–7. doi: 10.3945/ajcn.2009.28981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waters EA, Weinstein ND, Colditz GA, Emmons K. Formats for improving risk communication in medical tradeoff decisions. J. Health Commun. 2006;11:167–82. doi: 10.1080/10810730500526695. [DOI] [PubMed] [Google Scholar]
- 101.Weil J. Oxf. Monogr. Med. Genet. Oxford Univ. Press; Oxford, UK: 2000. Psychosocial Genetic Counseling; p. 41. [Google Scholar]
- 102.Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons KM. Colon cancer: risk perceptions and risk communication. J. Health Commun. 2004;9:53–65. doi: 10.1080/10810730490271647. [DOI] [PubMed] [Google Scholar]
- 103.Wells S, Kerr A, Eadie S, Wiltshire C, Jackson R. “Your Heart Forecast”: a new approach for describing and communicating cardiovascular risk? Heart. 2010;96:708–13. doi: 10.1136/hrt.2009.191320. [DOI] [PubMed] [Google Scholar]
- 104.Wham D, Vu T, Chan-Smutko G, Kobelka C, Urbauer D, Heald B. Assessment of clinical practices among cancer genetic counselors. Fam. Cancer. 2010;9:459–68. doi: 10.1007/s10689-010-9326-9. [DOI] [PubMed] [Google Scholar]
- 105.Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93:172–76. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zick CD, Mathews CJ, Roberts J, Cook-Deegan R, Pokorski RJ, Green RC. Genetic testing for Alzheimer’s disease and its impact on insurance purchasing behavior. Health Aff. 2005;24:483–90. doi: 10.1377/hlthaff.24.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Improving understanding of adjuvant therapy options by using simpler risk graphics. Cancer. 2008;113:3382–90. doi: 10.1002/cncr.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zilliacus EM, Meiser B, Lobb EA, Kelly PJ, Barlow-Stewart K, et al. Are videoconferenced consultations as effective as face-to-face consultations for hereditary breast and ovarian cancer genetic counseling? Genet. Med. 2011;13:933–41. doi: 10.1097/GIM.0b013e3182217a19. [DOI] [PubMed] [Google Scholar]
- 109.Zilliacus EM, Meiser B, Lobb EA, Kirk J, Warwick L, Tucker K. Women’s experience of telehealth cancer genetic counseling. J. Genet. Couns. 2010;19:463–72. doi: 10.1007/s10897-010-9301-5. [DOI] [PubMed] [Google Scholar]