Abstract
♦ Objective: The objective of our research was to summarize and review evidence supporting a causal relationship between exit-site infection and peritonitis in peritoneal dialysis (PD) patients.
♦ Data Sources: We undertook a qualitative review of studies retrieved from MEDLINE, EMBASE, and PubMed, and supplemented that process with a hand search of references and abstracts in the literature.
♦ Study Selection: Our quality criteria were based on the Paediatric Risk of Mortality guidelines, definitions, and recommendations from the International Society for Peritoneal Dialysis (ISPD), and the Bradford Hill criteria for causality. All identified abstracts were reviewed for content. Of 776 abstracts, 59 were selected for full-text evaluation, and 22 of those met the ISPD criteria for good-quality research in PD-related infections. Of the 22 eligible studies, 9 met the study’s quality criteria and were included in the summative analysis. No articles reported sufficient data for a quantitative analysis.
♦ Data Extraction: Information on study design, study population characteristics, definitions, peritonitis rates, exit-site care protocol, exit-site treatment protocol, follow-up period, potential bias, and outcomes was extracted. Criteria for including data in the final study were determined using ISPD guidelines.
♦ Data Synthesis: Of the 9 included studies, 8 suggested that a history of exit-site infection increased the risk for subsequent peritonitis. Of those studies, 3 met 5 causality criteria, 4 met 4 causality criteria, and 1 met 3 causality criteria.
♦ Conclusions: The literature provides weak evidence to support a causal relationship between exit-site infection and subsequent peritonitis. Few criteria for causation were met. We were unable to attribute causation and could assume an association only. The exclusion of studies focusing on PD-related tunnel infections may be viewed as both a strength and a limitation of the present work.
Keywords: Exit-site infection, catheter infection, peritonitis, infectious complications, causality, Bradford Hill
Catheter-related infections are the most common, and serious, of all complications associated with chronic peritoneal dialysis (PD). Although infection rates have declined in recent years, bacterial and fungal infections continue to be the leading cause of technique failure and mortality in PD patients (1-7).
It has long been assumed that a bacterial (or fungal) infection around the catheter exit site [“exit-site infection” (ESI)] will lead to tracking along the catheter path and a predisposition to peritonitis. As a result, the International Society for Peritoneal Dialysis (ISPD) and other leading authorities have recommended measures to help prevent, detect, and aggressively treat ESIs (3-7). We questioned whether the literature supports only a clinical association between ESI and peritonitis or whether the data are sufficient to establish causality. The distinction is important to those interested in developing novel or innovative strategies to reduce the incidence of peritonitis, particularly if aggressive strategies to reduce or manage ESIs inadvertently increase the risk of peritonitis by an alternative mechanism.
In the present study, we used robust epidemiologic criteria (Bradford Hill criteria) to make a distinction between causation and association (8). The Bradford Hill criteria outline the conditions that should ideally be fulfilled to establish a causal relationship between two events. In the absence of those criteria, an association only and not causality must be assumed.
Association and causation are epidemiologic concepts. The term “association” describes any relationship between 2 or more variables without attributing cause and effect. Such variables might be associated indirectly through other important characteristics of the patient or the environment. In contrast, the term “causation” implies that changes in (or exposure to) variable A directly causes changes in variable B. In medicine, causation can rarely be proven; however, certain characteristics can be sought to support causality.
The objective of the present study was to systematically review the literature and to summarize the evidence supporting a causal relationship between ESI and peritonitis.
Methods
We used a 3-step process: literature search, data quality assessment, and data extraction.
Literature Search and Eligibility Criteria
We undertook a literature search with the help of a specialist librarian. Three medical databases (MEDLINE, EMBASE, PubMed) were systematically searched, and relevant reference lists and published abstracts were subsequently hand-searched (Figure 1). Search terms included “peritoneal dialysis” AND “peritonitis” AND 1 of 3 terms associated with ESIs [“catheter-related infections” OR “catheterization/ae” OR “(exit site adj4 infection*).mp”]. Determination of tunnel infections was felt to be highly dependent on both screening and diagnostic protocols within units, and therefore “tunnel infection” was not included as a search term. All identified abstracts were screened, and selected full texts were reviewed to ensure that they met the eligibility criteria: inclusion of information about ESIs and peritonitis, and specificity to PD patients.
Figure 1 —
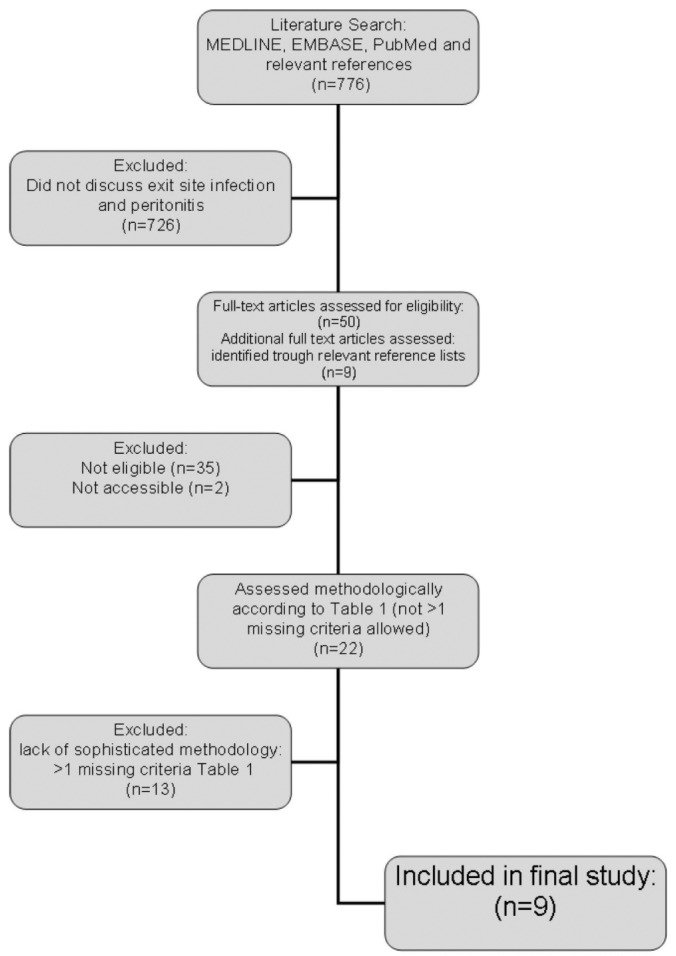
Literature search.
Data Quality Assessment
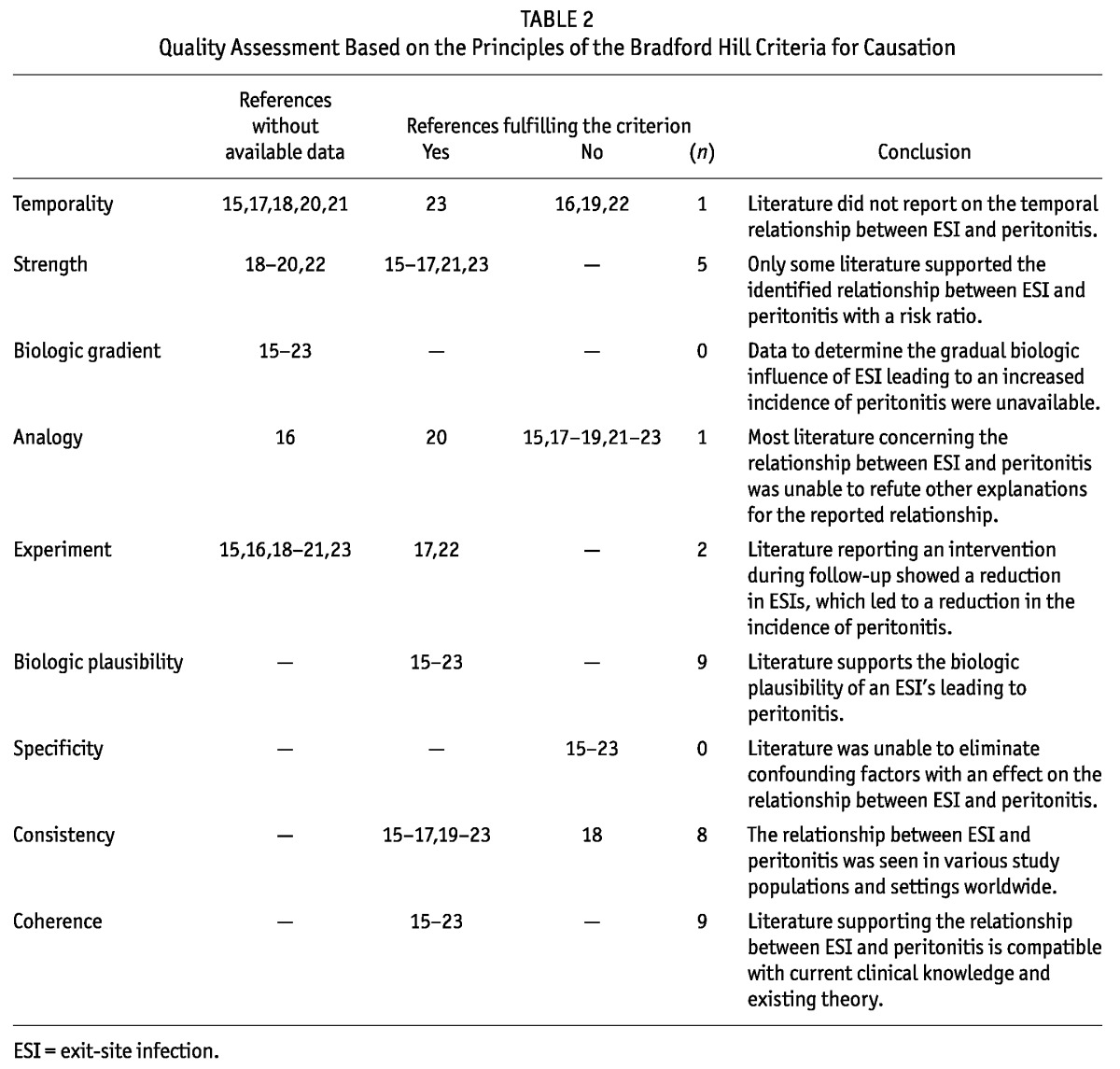
Eligible studies underwent a quality assessment using criteria specific to our question. The criteria were developed in two steps. In the first step, we used the ISPD definitions of good-quality research on PD-related infections (Table 1) to determine criteria for “adequate quality” (5). We then identified which question-specific data elements were required to meet each of the 9 Bradford Hill criteria for causation (8) (Table 2).
TABLE 1.
Variables for Quality Assessment of the Literature Reporting a Relationship Between Exit-Site Infection and Peritonitis

TABLE 2.
Quality Assessment Based on the Principles of the Bradford Hill Criteria for Causation
Data Extraction
In the final step, demographic data, publication data, and risk results were all extracted using a systematic approach. All data were extracted by a single observer (ATNVD).
Results
Identification of Qualitative Evidence
The literature search found a total of 776 papers. Reference lists were hand-searched and an additional 9 papers were identified. We excluded 726 papers because they did not report data pertaining to both ESIs and peritonitis rates. Papers focusing solely on tunnel infections or nasal carriage were excluded (9-14). We assessed the full texts of the remaining 59 articles for eligibility. After assessment, an additional 37 papers were excluded because they did not contain information relevant to the relationship between ESIs and peritonitis.
Table 2 sets out our criteria for adequate quality. Those criteria were applied to all 22 papers (15-36) that reported relevant information. Among those 22 papers, only 9 (15-23) met sufficient criteria to be included in the final analysis (Figure 1, Table 1).
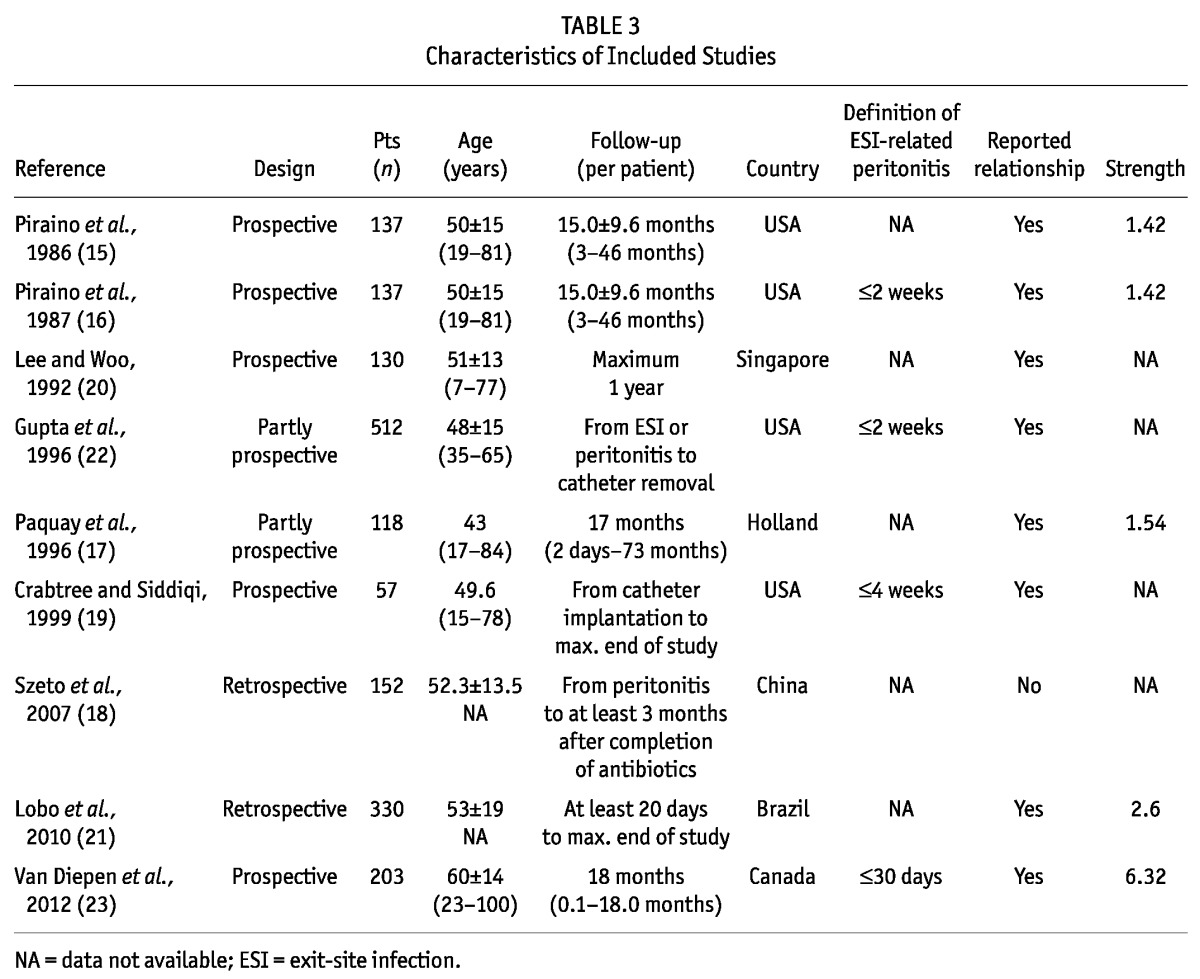
Study Characteristics
Table 3 summarizes the baseline characteristics of the 9 studies. Although all the studies reported peritonitis rates (range: 0.38 - 1.36 episodes per patient-year), only 5 of the 9 (19-23) met the reporting standards recommended by the ISPD guidelines (5). The incidence of ESI varied broadly between studies, with an overall trend of declining rates over time: studies published before 1990 reported 1.02 episodes per patient-year (15,16), and studies from subsequent years reported 0.20 - 0.80 episodes per patient-year (17-23).
TABLE 3.
Characteristics of Included Studies
Bradford Hill Criteria for Causation
Of the 9 studies (15-23) that met our criteria for a full qualitative review, 8 (15-17,19-23) reported a temporal relationship between ESI and peritonitis, and 1 study (18) reported no relationship between ESI and recurrent or relapsing peritonitis. Table 3 shows the results of the quality assessment.
The criterion of biologic feasibility was considered fulfilled because it is widely accepted that organisms can track along implanted devices. In PD patients, the organisms would track along the catheter wall between the exit site and the peritoneum. Clear evidence of a temporal relationship was missing in 3 reports (16,19,22) that did not have a clear definition of ESI-related peritonitis. In those 3 studies, it was unclear whether peritonitis preceded, occurred simultaneously with, or presented after ESI. Sufficient data were given in 5 studies (15-17,21,23) to allow for derivation of the relative risk ratio of peritonitis after ESI compared with peritonitis without ESI (range: 1.4 - 6.3). Other studies (18-20,22) did not include sufficient data for a calculation of a risk ratio from their results. Only 1 study reported whether an individual’s hazard of peritonitis declined over time (23).
Causation criteria suggest that other possible explanations for the identified relationship should be sought and, to establish causality, excluded. Of the studies reviewed, 7 (15,17,19-22,23) discussed other explanations for the relationship, but were unable to support or refute the relative importance of those explanations. The study by Lee et al. (20) controlled for the overrepresentation of patients with diabetes in their population. Only 1 study (16) did not discuss any alternative conclusions.
Any intervention that has an impact on one variable and effects a change in another (for example, a treatment that reduces the incidence of ESI that reduces peritonitis) can support a determination of causality between ESI and subsequent peritonitis. In 2 studies (17,22), an intervention may have affected the ESI incidence. In one of those studies (17), the authors reported results before and after the introduction of the Y-set in combination with an intensified antibiotic treatment protocol for ESI; in the other (22), the exitsite prophylaxis protocols were changed. Both studies showed a decline in both the ESI rate and the peritonitis rate, suggesting causality. (Ideally an attempt would have been made to withdraw therapy and see a return to baseline infection rates, but taking that action was not feasible.)
Overall, the literature was consistent across the various study populations and study settings. Because 4 studies did not report the strength of the observed association, consistency across the estimated risk was not established. Nor was it possible to comment on the presence or absence of a biologic gradient (akin to dose-response) by searching for a graduated risk in either the number of ESIs or the risk attribution.
Discussion and Conclusions
We used the Bradford Hill criteria for causation (8) to evaluate whether the literature was able to establish causality between ESI and subsequent peritonitis. According to our strict qualitative review, the data supported an association, but were insufficient to demonstrate a causal relationship. The presence of an association questions (a) whether the relationship depends on the individual (a patient with an ESI has an inherent immunologic risk, placing him or her at higher risk of peritonitis) or (b) whether the ESI itself directly compromises the patient and predisposes the individual to a newly increased risk of peritonitis. We believe that the answer is important because it can inform the development of novel strategies to reduce peritonitis. If the answer is (a), then novel strategies will target identification of the patient at risk and apply patient-level interventions; if the answer is (b), novel treatments will target ESI prevention.
We were surprised to find that the literature did not establish a causal relationship between ESI and subsequent peritonitis. We suggest that further work is required in the field. We recommend wider adoption of the recent ISPD guidelines for research into infections (5) and the strict inclusion of a time interval in papers reporting an association between ESI and any related peritonitis episodes. Published articles should include a clear definition of ESI-related peritonitis, support the results with a numeric estimate of the risk strength, and distinguish between ESI and tunnel infection if possible. We also raise the question of whether, as PD physicians, we need to reconsider the correctness of the assumption that ESI leads directly to subsequent peritonitis and to investigate phenotype in patients in whom ESI leads to subsequent peritonitis. Researchers might then be able to identify the nature of important contributing factors.
Disclosures
In the past 3 years, SVJ has received speaker honoraria from Amgen Canada.
Acknowledgments
The authors acknowledge Raymond T. Krediet, MD, for his support and valuable advice. Anouk T.N. van Diepen received funding from the Dutch Kidney Foundation (Nierstichting Nederland: KSBS 10.0047).
References
- 1. Keogh AM. Complications in peritoneal dialysis: peritonitis and exit site infections. Perit Dial Int 1996; 16(Suppl 1):S464–7 [PubMed] [Google Scholar]
- 2. Piraino B. Infectious complications of peritoneal dialysis. Perit Dial Int 1997; 17(Suppl 3):S15–18 [PubMed] [Google Scholar]
- 3. Nessim SJ. Prevention of peritoneal dialysis-related infections. Semin Nephrol 2011; 31:199–212 [DOI] [PubMed] [Google Scholar]
- 4. Bender FH, Bernardini J, Piraino B. Prevention of infectious complications in peritoneal dialysis: best demonstrated practices. Kidney Int Suppl 2006; 103:S44–54 [DOI] [PubMed] [Google Scholar]
- 5. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. on behalf of the International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]
- 6. Piraino B. Insights on peritoneal dialysis-related infections. Contrib Nephrol 2009; 163:161–8 [DOI] [PubMed] [Google Scholar]
- 7. Thodis E, Passadakis P, Ossareh S, Panagoutsos S, Vargemezis V, Oreopoulos DG. Peritoneal catheter exit-site infections: predisposing factors, prevention and treatment. Int J Artif Organs 2003; 26:698–714 [DOI] [PubMed] [Google Scholar]
- 8. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965; 58:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plum J, Sudkamp S, Grabensee B. Results of ultrasound-assisted diagnosis of tunnel infections in continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1994; 23:99–104 [DOI] [PubMed] [Google Scholar]
- 10. Lye WC, Leong SO, van der Straaten J, Lee EJ. Staphylococcus aureus CAPD-related infections are associated with nasal carriage. Adv Perit Dial 1994; 10:163–5 [PubMed] [Google Scholar]
- 11. Davies SJ, Ogg CS, Cameron JS, Poston S, Noble WC. Staphylococcus aureus nasal carriage, exit-site infection and catheter loss in patients treated with continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1989; 9:61–4 [PubMed] [Google Scholar]
- 12. Luzar MA, Coles GA, Faller B, Slingeneyer A, Dah GD, Briat C, et al. Staphylococcus aureus nasal carriage and infection in patients on continuous ambulatory peritoneal dialysis. N Engl J Med 1990; 322:505–9 [DOI] [PubMed] [Google Scholar]
- 13. Scalamogna A, Castelnovo C, De Vecchi A, Ponticelli C. Exit-site and tunnel infections in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 1991; 18:674–7 [DOI] [PubMed] [Google Scholar]
- 14. Sewell CM, Clarridge J, Lacke C, Weinman EJ, Young EJ. Staphylococcal nasal carriage and subsequent infection in peritoneal dialysis patients. JAMA 1982; 248:1493–5 [PubMed] [Google Scholar]
- 15. Piraino B, Bernardini J, Sorkin M. The influence of peritoneal catheter exit-site infections on peritonitis, tunnel infections, and catheter loss in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1986; 8:436–40 [DOI] [PubMed] [Google Scholar]
- 16. Piraino B, Bernardini J, Sorkin M. A five-year study of the microbiologic results of exit site infections and peritonitis in continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1987; 10:281–6 [DOI] [PubMed] [Google Scholar]
- 17. Paquay YC, Jansen JA, Goris RJ, Hoitsma AJ. Long-term clinical experience with continuous ambulatory peritoneal dialysis: access-related problems. J invest Surg 1996; 9:81–93 [DOI] [PubMed] [Google Scholar]
- 18. Szeto CC, Chow KM, Kwan BC, Law MC, Chung KY, Yu S, et al. Staphylococcus aureus peritonitis complicates peritoneal dialysis: review of 245 consecutive cases. Clin J Am Soc Nephrol 2007; 2:245–51 [DOI] [PubMed] [Google Scholar]
- 19. Crabtree JH, Siddiqi RA. Dialysis catheter infection related peritonitis: incidence and time dependent risk. ASAIO J 1999; 45:574–80 [PubMed] [Google Scholar]
- 20. Lee GS, Woo KT. Infection in continuous ambulatory peritoneal dialysis (CAPD): aetiology, complications and risk factors. Ann Acad Med Singapore 1992;21:354–60 [PubMed] [Google Scholar]
- 21. Lobo JV, Villar KR, de Andrade Júnior MP, Bastos Kde A. Predictor factors of peritoneal dialysis-related peritonitis. J Bras Nefrol 2010; 32:156–64 [Erratum in: J Bras Nefrol 2010; 32:332] [PubMed] [Google Scholar]
- 22. Gupta B, Bernardini J, Piraino B. Peritonitis associated with exit site and tunnel infections. Am J Kidney Dis 1996; 28:415–19 [DOI] [PubMed] [Google Scholar]
- 23. van Diepen AT, Tomlinson GA, Jassal SV. The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clin J Am Soc Nephrol 2012; 7:1266–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cleper R, Davidovits M, Kovalski Y, Samsonov D, Amir J, Krause I. Peritonitis in a pediatric dialysis unit: local profile and implications. Isr Med Assoc J 2010; 12:348–52 [PubMed] [Google Scholar]
- 25. Kofteridis DP, Valachis A, Perakis K, Maraki S, Daphnis E, Samonis G. Peritoneal dialysis-associated peritonitis: clinical features and predictors of outcome. Int J Infect Dis 2010; 14:e489–93 [DOI] [PubMed] [Google Scholar]
- 26. Szeto CC, Kwan BC, Chow KM, Law MC, Pang WF, Chung KY, et al. Recurrent and relapsing peritonitis: causative organisms and response to treatment. Am J Kidney Dis 2009; 54:702–10 [DOI] [PubMed] [Google Scholar]
- 27. Chiu MC, Tong PC, Lai WM, Lau SC. Peritonitis and exit-site infection in pediatric automated peritoneal dialysis. Perit Dial Int 2998; 28(Suppl 3):S179–82 [PubMed] [Google Scholar]
- 28. Boehm M, Vécsei A, Aufricht C, Mueller T, Csaicsich D, Arbeiter K. Risk factors for peritonitis in pediatric peritoneal dialysis: a single-center study. Pediatr Nephrol 2005; 20:1478–83 [DOI] [PubMed] [Google Scholar]
- 29. Szeto CC, Chow KM, Leung CB, Wong TY, Wu AK, Wang AY, et al. Clinical course of peritonitis due to Pseudomonas species complicating peritoneal dialysis: a review of 104 cases. Kidney Int 2001; 59:2309–15 [DOI] [PubMed] [Google Scholar]
- 30. Furth SL, Donaldson LA, Sullivan EK, Watkins SL. on behalf of the North American Pediatric Renal Transplant Cooperative Study. Peritoneal dialysis catheter infections and peritonitis in children: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 2000; 15:179–82 [DOI] [PubMed] [Google Scholar]
- 31. Szabo T, Siccion Z, Izatt S, Vas SI, Bargman J, Oreopoulos DG. Outcome of Pseudomonas aeruginosa exit-site and tunnel infections: a single center’s experience. Adv Perit Dial 1999; 15:209–12 [PubMed] [Google Scholar]
- 32. Bunke M, Brier ME, Golper TA. Pseudomonas peritonitis in peritoneal dialysis patients: the Network #9 Peritonitis Study. Am J Kidney Dis 1995; 25:769–74 [DOI] [PubMed] [Google Scholar]
- 33. Levy M, Balfe JW, Geary D, Fryer-Keene S, Bannatyne R. Exit-site infection during continuous and cycling peritoneal dialysis in children. Perit Dial Int 1990; 10:31–5 [PubMed] [Google Scholar]
- 34. Abraham G, Savin E, Ayiomamitis A, Izatt S, Vas SI, Mathews RE, et al. Natural history of exit-site infection (ESI) in patients on continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1088; 8:211–16 [Google Scholar]
- 35. Nolph KD, Prowant B, Serkes KD, Morgan LM, Pyle WK, Hiatt MP, et al. A randomized multicenter clinical trial to evaluate the effects of an ultraviolet germicidal system on peritonitis rate in continuous ambulatory peritoneal dialysis. Perit Dial Int 1985; 5:19–24 [Google Scholar]
- 36. Kim D, Tapson J, Wu G, Khanna R, Vas SI, Oreopoulos DG. Staph aureus peritonitis in patients on continuous ambulatory peritoneal dialysis. Trans Am Soc Artif Intern Organs 1984; 30:494–7 [PubMed] [Google Scholar]


