Abstract
Patients with advanced chronic kidney disease nearing dialysis but without pre-established access almost uniformly initiate dialysis with a temporary central venous catheter. These catheters are associated with high rates of infection and flow disturbances, requiring removal and subsequent replacement. Many of these patients might be candidates for peritoneal dialysis (PD), but because of the absence of prior catheter placement, the default initial modality is hemodialysis. Recent reports, however, have demonstrated the feasibility of initiating PD urgently despite the late referral for access placement. Urgent-start PD clinical pathways require a unique infrastructure and treatment approach. This article reviews the salient features required to establish an urgent-start PD program.
Keywords: Urgent initiation, interventional radiology, infrastructure, outcomes, vascular access, prescription
In patients with advanced chronic kidney disease, dialysis is most ideally initiated in an elective manner with a previously established permanent dialysis access. Despite all efforts to plan for dialysis initiation, 80% of incident hemodialysis (HD) patients initiate dialysis with a central venous catheter (CVC). Moreover, patients presenting late in the course of their disease are almost universally initiated on in-center HD with a CVC (1).
The concept of urgently initiating peritoneal dialysis (PD) in the late-presenting patient has gained increasing attention in the United States. Recent studies have demonstrated successful urgent initiation of PD without the need for temporary vascular access (2,3). Urgent-start PD programs have been developed as quality improvement initiatives to avoid temporary vascular catheters in unplanned patients who are otherwise acceptable PD candidates. By offering PD to late-presenting patients, physicians can give them a fuller set of options to consider for the initiation of dialysis and may be able to avoid temporary catheters.
Compared with elective PD initiation, urgent-start PD requires establishment of infrastructure and clinical pathways to deal with its unique logistical nuances. Those logistics include a mechanism for rapid PD catheter placement; early initiation of PD therapy with a modified prescription; and adequate staffing, nursing, and space.
In this article, we review several key infrastructure requirements of urgent-start PD programs and recommendations that can assist in operationalizing an urgent-start PD capability. We also review published clinical reports of urgent-start PD programs.
Discussion
General Concept and Definition of Urgent-Start PD
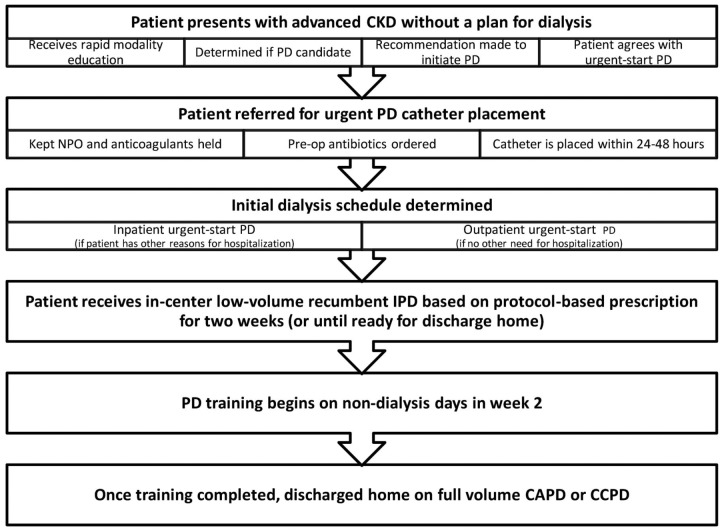
Urgent-start PD can generally be defined as initiation of PD in the unplanned incident end-stage renal disease patient before the traditional waiting period of 2 or more weeks after PD catheter placement. The traditional waiting period has been recommended in part to minimize the risk of catheter-related complications, specifically pericatheter or incisional leaks, and to allow for training of patients before they start PD at home. Urgent-start PD refers to the more rapid initiation of PD therapy, either in an outpatient PD unit or in an inpatient setting, under a modified protocol to avoid the potential complications associated with early initiation of PD. Figure 1 summarizes the urgent-start clinical pathway from patient presentation until discharge home.
Figure 1 —
Urgent-start clinical pathway for peritoneal dialysis (PD), from patient presentation until discharge on home therapy. CKD = chronic kidney disease; NPO = no intake by mouth; IPD = intermittent PD; CAPD = continuous ambulatory PD; CCPD = continuous cycling PD.
Preparing for an Urgent-Start PD Program
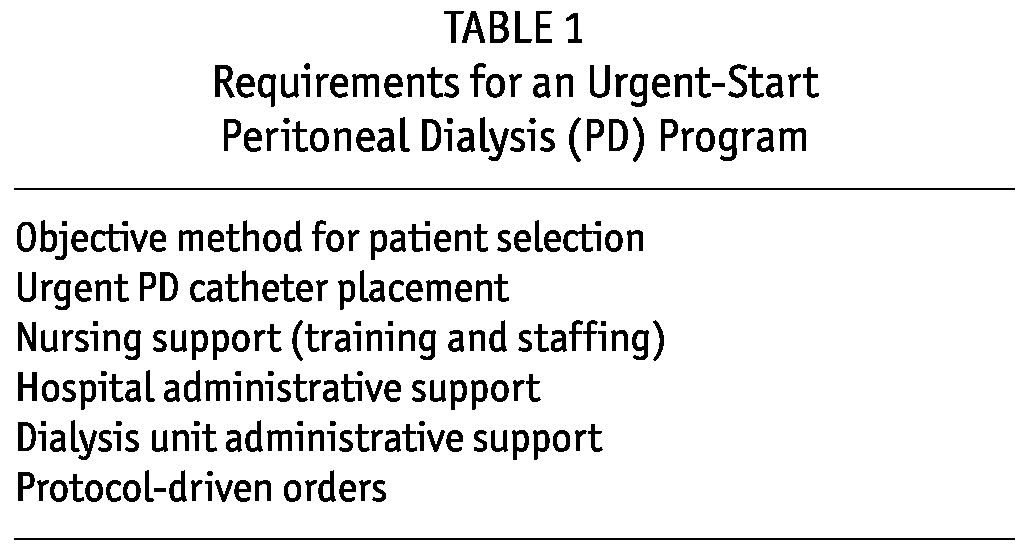
Table 1 lists several key requirements that need to be met to ensure success for an urgent-start PD program. The subsections that follow provide details for each requirement.
TABLE 1.
Requirements for an Urgent-Start Peritoneal Dialysis (PD) Program
Patient Selection: Patients considered for urgent-start PD might be relatively new to the caregivers, have symptoms of uremia, and require expedited options for education and catheter placement. Rarely is there time for full-options education or home visits. The patients often need a quick decision on how to initiate dialysis, and so the evaluating providers are required to make a decision on candidacy for PD.
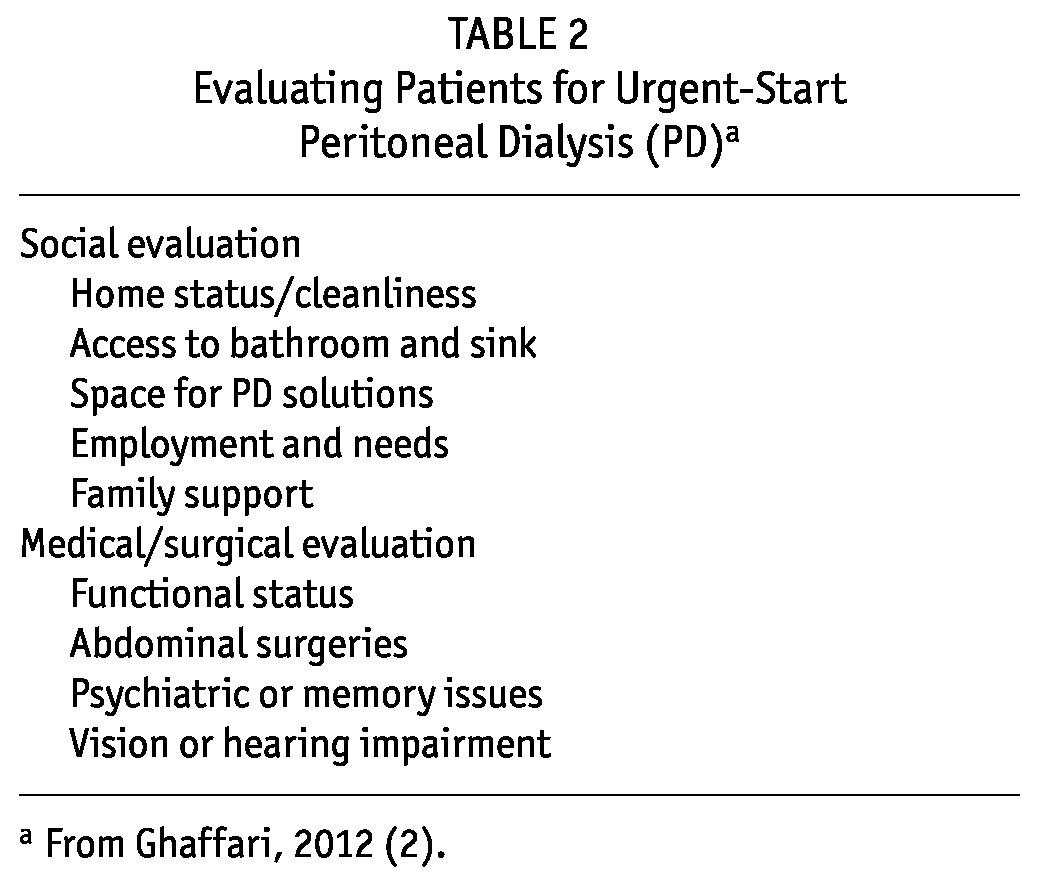
When patients are being evaluated for PD candidacy, they can be asked to clarify their home situation and living environment and to indicate the support that they do or do not have at home. The patient should be questioned about the availability of a toilet in the home for disposing of dialysate and a space for storage of supplies, their ability to lift dialysate bags, the presence of pets, and a general assessment of cleanliness in the home. An assessment of visual acuity and manual dexterity can be made at the bedside. The abdomen should be inspected to determine whether any pre-existing abdominal factors, such as extensive previous abdominal surgeries or large hernias, would complicate initiation of PD (Table 2).
TABLE 2.
Evaluating Patients for Urgent-Start Peritoneal Dialysis (PD)a
The assessment can be completed in part or in full by an options educator, nurse, case manager, social worker, fellow-in-training, or treating nephrologist using a standardized questionnaire. Once the caregivers have made an initial assessment and determined that the patient is a PD candidate, a formal recommendation to initiate dialysis with PD can be offered. The medical team making the recommendation to initiate urgent-start PD can emphasize the importance of avoiding temporary catheters to avoid morbidity and to reduce the total number of procedures involved with dialysis therapy (4-6).
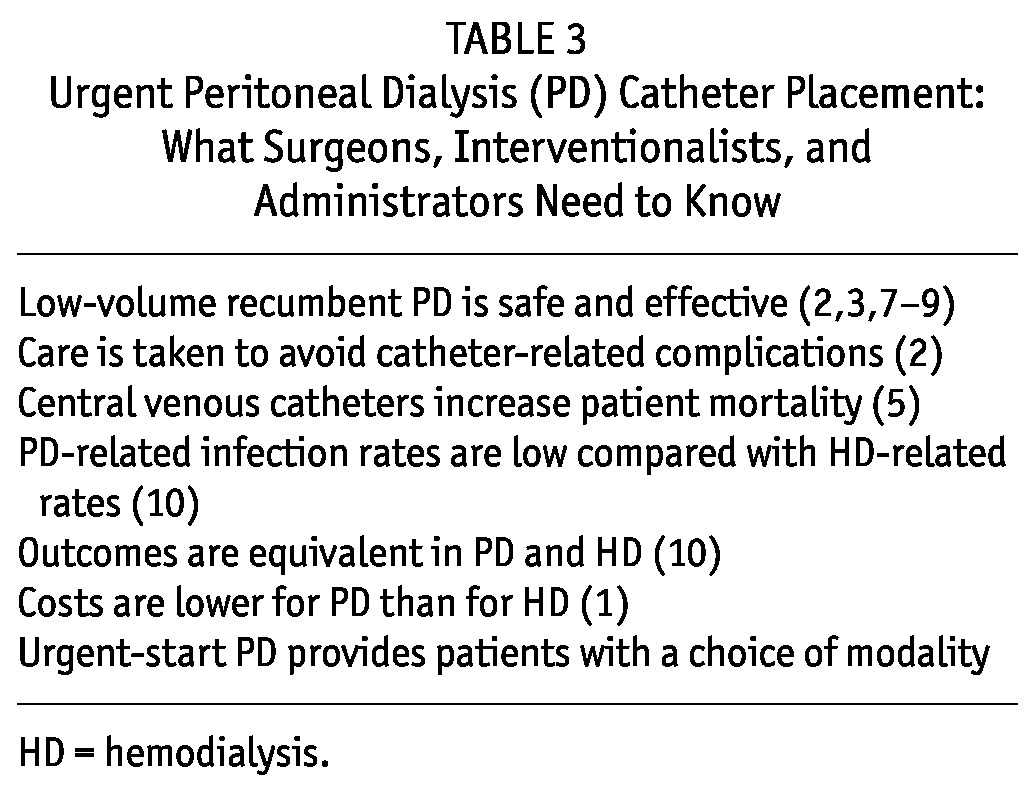
Catheter Placement Techniques and Catheter Management: The most important factor for urgently starting patients on PD and avoiding CVCs is being able to obtain a PD catheter quickly. Surgically placed catheters and catheters placed by interventionists have been used by urgent-start PD programs (2,3). Surgeons, interventionists, and administrators should be educated about the concept of urgent-start PD, the advantages of having an urgent-start program, the rationale for avoiding temporary vascular access, and the need for a quick response to a request for PD catheter placement in an urgent setting (Table 3). Surgeons can elect to create a purse-string suture at the rectus sheath to provide an additional barrier against dialysate leaks. In uremic patients who are more unstable and whose preoperative clearance may be hampered by uremia, acidosis, or volume or electrolyte abnormalities, it is acceptable to provide a temporary non-tunneled CVC for HD or continuous renal replacement therapy until they are stabilized, after which a PD catheter can be placed and the CVC removed to minimize exposure.
TABLE 3.
Urgent Peritoneal Dialysis (PD) Catheter Placement: What Surgeons, Interventionalists, and Administrators Need to Know
Catheters placed by interventional nephrologists or radiologists allow for the rapid initiation of PD and may offer several logistical efficiencies by obviating the need for surgical consultation, scheduling of an operating room, general anesthesia, and a recovery room (2,11,12). Although some interventional nephrologists or radiologists have little experience with placing PD catheters, that skill is readily obtainable. Literature and courses can familiarize the operators with the appropriate techniques (13,14). Several studies suggest that catheters placed under radiologic guidance have technical outcomes comparable to those placed using an open or basic laparoscopic technique (15-17). Whether to place a catheter by the percutaneous approach or by the surgical approach should depend on local experience and the established mechanism for catheter placement at the individual site.
In preparation for catheter placement, patients should be kept fasting overnight or at for least 6 hours before the procedure. The medication list should be reviewed with particular attention to anticoagulants (which should be withheld) and coagulopathies (which should be corrected). Preoperative prophylactic antibiotics are recommended.
Before the procedure, the patient can be bathed with an antiseptic soap. The patient should be instructed to void completely, and if bladder dysfunction is present, a Foley catheter can be placed to ensure full drainage of the bladder. Ideally, the expected catheter exit site should be marked by the nephrologist before catheter placement. The placement of the catheter bandage should allow access to the transfer set for initializing dialysis. The bandage applied at the time of catheter placement should be semi-occlusive. It should be kept dry and left in place for the initial 7 days. At day 7, the bandage is changed and left in place for an additional 7 days (18).
Patients should be instructed to leave the bandage on and to avoid showers or immersion bathing until the catheter insertion site has fully healed. Sponge bathing around the site is acceptable until it has fully healed. Any exposed catheter should be securely taped to the abdomen when the patient is at home, and patients can be instructed on taping procedures, handwashing, and the basic components of the catheter to ensure that the catheter remains capped at all times.
Patients should be on a bowel regimen to avoid constipation. They should be instructed to report any fever, chills, abdominal pain, soiling or bleeding at the catheter dressing site, or constipation.
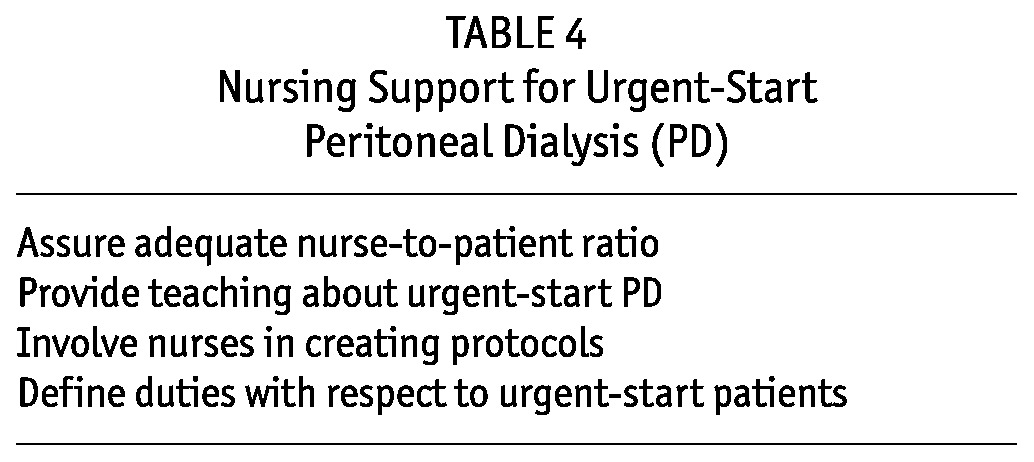
Nursing Support: Urgent-start outpatient PD programs require new clinical pathways for patients within a PD unit, which will increase the nursing responsibilities during outpatient intermittent PD (IPD) treatments (Table 4). Units with several PD nurses are better suited for expanding treatment options to include urgent-start PD. A single PD nurse in the unit may have primary responsibility for urgent patients, including initially helping them to assume the recumbent position, connecting them to the cycler, giving them instructions for the session, and intermittently checking on them during treatment. The urgent patient typically does not require fulltime one-to-one nursing. However, a call button or bell should be available for calling the nursing staff if needed. At the discretion of staff and depending on patient condition, some patient education can occur during the initial IPD sessions. If the patient needs to sit up or stand, the nursing staff should initiate a full drain and prevent such movement until the effluent has finished draining.
TABLE 4.
Nursing Support for Urgent-Start Peritoneal Dialysis (PD)
The initial nursing assessment of the patient focuses on uremic symptoms and volume status. The assessment can help to determine whether the dialysis treatments should be daily or intermittent; it can also determine the tonicity of the dialysis solutions used for the exchanges. At initiation of urgent-start PD, the medication list is reviewed, particularly to address medications indicated for residual kidney diuresis and preservation of kidney function: diuretics and inhibitors of the reninangiotensin system. Initiation or continuation of such medications is recommended and should be reviewed with the medical team (19).
Depending on the patient’s state of uremia and their own availability, the nursing staff may elect to begin general training in PD concepts right away or to defer training (usually 1 - 2 weeks) until the condition of the patient has adequately improved.
As mentioned in the preceding subsection, especially in the immediate postoperative period after catheter placement, staff should initiate the patient on a bowel regimen, if needed to avoid constipation that may interfere with catheter flow.
With the initiation of PD, the drain bags should be carefully inspected for postoperative blood that presents as hemoperitoneum. If hemoperitoneum is observed, heparin can be added to the subsequent dialysate exchanges until clearing is noted. During the subsequent treatment sessions, the initial effluent should be inspected to rule out persistent blood or cloudy fluid. The abdomen should be examined for any signs of catheter bleeding, dialysate leak, or tenderness.
Hospital Component: Urgent-start PD programs should allow for the option of initiation of PD either in the hospital or in an outpatient setting. The hospital setting may be indicated for patients with more advanced uremia or concurrent medical issues. The outpatient setting may be used for the initiation of urgent, but not emergent, treatments. For the hospitalized patient, PD must be initiated with hospital staff educated on the concepts of urgent-start PD, the restrictions of infused dialysate volumes, and the need for strict adherence to the recumbent position during exchanges. Hospital beds can be used for the exchanges, and staff can position the cycler next to the bed or perform manual exchanges at bedside. Compared with patients in the outpatient setting, hospitalized patients can have extended treatment times, with fewer time restrictions.
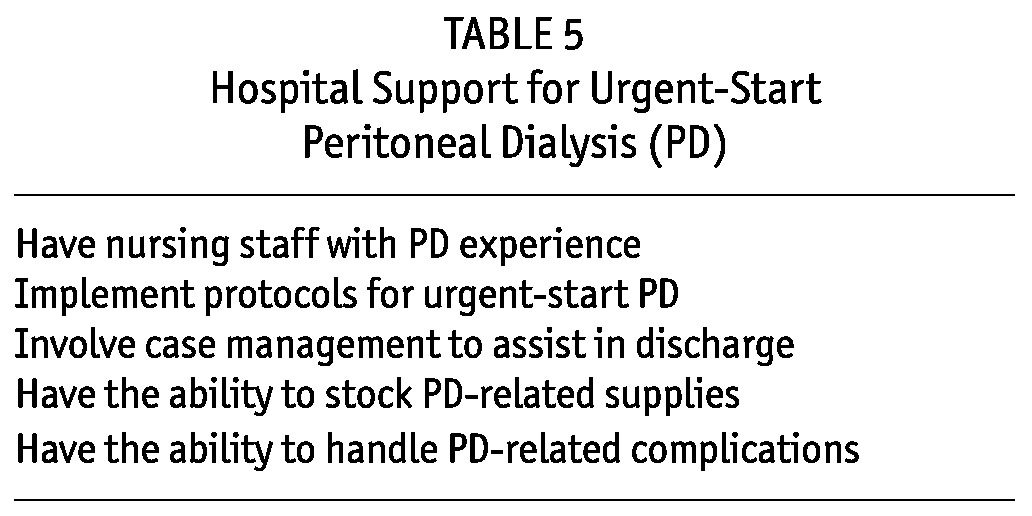
Although inpatient initiation of urgent-start PD is feasible, it requires a dedicated staff of nurses who routinely perform PD; many floor and intensive care nurses have little relevant experience (Table 5). To avoid complications and delay in patient care, a process should be developed for transferring the care of the urgent-start PD patient from the inpatient setting to the outpatient setting. The transfer can be accomplished by a renal case manager familiar with urgent-start PD or through communication between the PD inpatient and outpatient nursing staff.
TABLE 5.
Hospital Support for Urgent-Start Peritoneal Dialysis (PD)
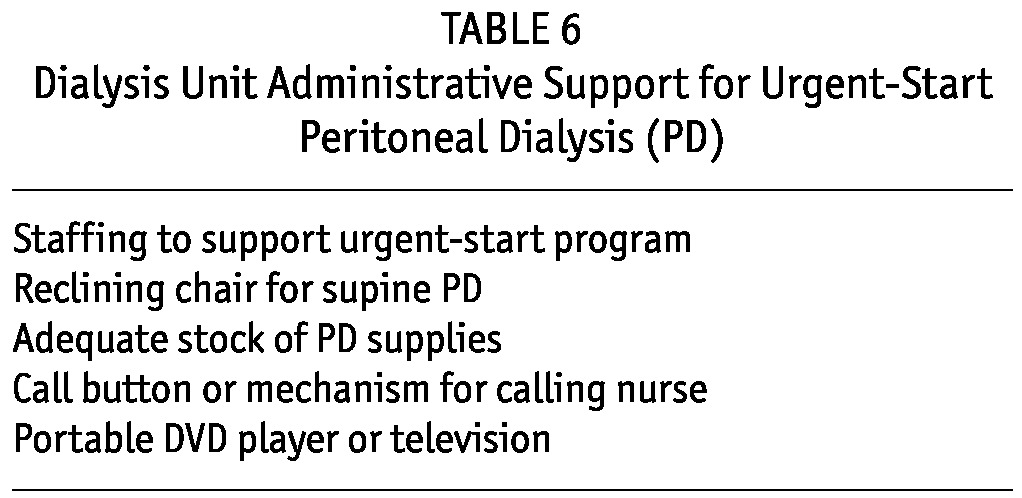
Dialysis Unit Administrative Support—Space and Material Requirements: For the outpatient initiation of PD, units must be able to perform exchanges in patients in the supine position. Stretchers or reclining chairs allow for such exchanges. Adequate space, protocols, materials, staffing, and nurse training are needed for a successful program. Buy-in by nurses and dialysis unit administration is needed, and the staff should be educated on the benefits to patients and to the PD program (Tables 3 and 6). Dedicated education on managing such patients should also be provided to outpatient nurses.
TABLE 6.
Dialysis Unit Administrative Support for Urgent-Start Peritoneal Dialysis (PD)
The outpatient PD unit that will accept the urgent-start patients should have at least 2 PD training rooms. The rooms are needed for managing an established PD program, clinics, training of new patients, and intermittent occupation by patients requiring urgent-start PD. Space must be adequate for the placement of a reclining chair (preferably automated) or a stretcher. Space is also needed for easy access to a cycler near the patient. In addition, an adequate stock of PD supplies (solutions, mini-caps, etc.) should be available for in-center PD treatments. If the existing PD program is already at capacity without an option to expand the number of training rooms, urgent-start PD can be performed in a HD chair adjacent to a HD unit with PD nurse access.
During urgent-start PD in an outpatient program, the patient should have access to a call button or other mechanism to contact the treating nurse for any issue that may arise. A television monitor or portable DVD player allows the patient to watch educational videos on PD and end-stage renal disease or to be entertained during the treatment.
Prescription: After PD catheter placement, patients should be evaluated for uremic symptoms to determine if dialysis should be initiated within 24 hours or whether a longer interim period is possible to allow for catheter healing. If the clinician determines that PD can be initiated within several days to several weeks of PD catheter placement, complications may be minimized. However, if dialysis must be initiated urgently, that, too, can be successfully accomplished.
Initiating dialysis soon after catheter placement risks leakage of the dialysate into subcutaneous tissue. To minimize that possibility, PD using lower dwell volumes is initiated and continued only while the patient is in the recumbent position. If the patient is not overtly uremic, IPD schedules allow for more gradual and incremental initiation of dialysis and minimize any risk of leakage on non-dialysis days. Intermittent PD can be performed 3 - 5 days per week either in the hospital setting or in the outpatient setting. Because the patient is new to PD and untrained, the initial PD prescription is administered by trained medical staff for the initial 2 weeks of therapy. After 2 weeks, to allow for catheter healing and improvement of uremic symptoms, the patient is then trained for home therapy and catheter care.
To initiate urgent-start PD, the nephrology staff must determine the dwell volume and number of exchanges to be delivered during the IPD session. Certain volume recommendations have been published previously (2). Patients with a smaller body surface area (BSA) of 1.65 m2 or less can be initiated on dwell volumes of 500 mL. Dwell volume can be increased to 750 mL in larger patients with a BSA of 1.65 - 1.8 m2, and a 1-L dwell volume can be used in patients with a BSA exceeding 1.8 m2. The number of cycler exchanges can be determined on the basis of clinical judgment of uremic symptoms and assessment of underlying residual kidney function. Prescribed daily exchanges have varied between 4 and 6, and total treatment time can be 5 - 8 hours per session (2). Alternative prescriptions have been described (7,8,17). Tidal regimens that allow for a reservoir volume of dialysate can reduce cycler alarms during low-volume exchanges. Kinetic modeling of various IPD regimens used for urgent-start PD has been described (20).
Emergent Starts: Patients presenting with a truly emergent need for dialysis (hyperkalemia, metabolic acidosis, or volume overload) can be treated with temporary vascular access placement, followed by several sessions of HD or continuous renal replacement therapy to gain control of the situation. However, once the patient is stabilized and ready for discharge, the temporary catheter can be discontinued, reducing the risk of subsequent infection, and the patient can enter the urgent-start PD clinical pathway—a bimodal approach.
Several publications have demonstrated the value of a dedicated nurse or nurse practitioner for specific modality education of the emergent-start patient who initiates dialysis with a temporary vascular catheter (9,10). Patients with a CVC typically transition to chronic dialysis in the outpatient setting and continue on long-term HD. After specific and intensified options education, many of those patients can elect to pursue therapy at home. The patient with a temporary CVC must be told that, if maintained on HD, they will require additional procedures to convert the temporary catheter to a tunneled catheter and then undergo surgery on the arm for permanent vascular access. On the other hand, if the patient has chosen PD, a single additional procedure for a PD catheter, followed by the home therapy option, will ensue.
Transition for Urgent-Start to Training for Home: At the end of 2 weeks of recumbent IPD, the completion of formal training for home therapy can occur. Often the formal training time required is considerably shortened because patients have had ample time to learn during the urgent-start period. Self-care of the catheter exit site with the application of exit-site antibiotic prophylaxis is initiated. Patients can be trained to initiate either continuous ambulatory PD or automated PD therapy at home.
Review of Urgent-Start PD Reports
Several recent publications have reported on urgent-start PD programs (2,3). One program utilized urgent PD catheter placement by interventional radiologists and the other relied on surgical placement. Ghaffari (2) reported on 90-day infectious and mechanical outcomes in 18 patients initiated on dialysis using an urgent PD pathway. The mechanical complication that occurred most often was an early dialysate leak. Of the 18 patients, 4 had minor leaks requiring cessation of treatment and rescheduling for the following day; 2 had major leaks requiring catheter replacement. In the Casaretto et al. report (3), 11 patients with surgically placed catheters initiated urgent PD. No leaks were reported. The authors concluded that urgent-start PD offers a viable alternative, in appropriately chosen patients, that allows for early initiation of PD without temporary vascular access followed by HD. Other reports in the literature document success in more rapid initiation of PD in late-referred patients (7,21,22).
Conclusions
Peritoneal dialysis can be initiated urgently in the late-presenting patient with advanced chronic kidney disease. Traditionally, initiation of PD has been deferred for at least 2 weeks after catheter placement to allow for healing of the catheter insertion site and tissue growth into the Dacron cuffs of the catheter. As a result, many late-presenting patients were not felt to be candidates for PD and instead received temporary vascular access catheters followed by initiation of urgent HD.
Experience suggests that PD can be initiated urgently, in appropriately selected patients, and that urgent-start PD is a viable option for the late-presenting patient. Several recent publications have documented the success of urgent-start PD programs. Key infrastructure requirements need to be met to provide an organized and protocol-driven approach to the delivery of dialysis. Those infrastructure requirements, once met, allow for a more uneventful transition from pre-dialysis to the initiation of PD and from subsequent training to discharge and home therapy.
Allowing for the initiation of PD in the late-presenting patient avoids temporary vascular access, requires only a single procedure for both urgent and long-term access, gives the patient the lifestyle opportunities afforded by home dialysis, might better preserve residual kidney function, and allows for a gentle, incremental dialysis initiation.
Disclosures
AG is a speaker for DaVita and Baxter Healthcare. VK is a speaker for DaVita. SG is an employee of Baxter Healthcare Corporation.
References
- 1. United States, Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, US Renal Data System (USRDS). USRDS 2012 Annual Data Report. Bethesda, MD: USRDS; 2012: 172 [Google Scholar]
- 2. Ghaffari A. Urgent-start peritoneal dialysis: a quality improvement report. Am J Kidney Dis 2012; 59:400–8 [DOI] [PubMed] [Google Scholar]
- 3. Casaretto A, Rosario R, Kotzker WR, Pagan-Rosario Y, Groenhoff C, Guest S. Urgent-start peritoneal dialysis: report from a US private nephrology practice. Adv Perit Dial 2012; 28:102–6 [PubMed] [Google Scholar]
- 4. Vats HS. Complications of catheters: tunneled and nontunneled. Adv Chronic Kidney Dis 2012; 19:188–94 [DOI] [PubMed] [Google Scholar]
- 5. Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis 2005; 46:501–8 [DOI] [PubMed] [Google Scholar]
- 6. Oliver MJ, Verrelli M, Zacharias JM, Blake PG, Garg AX, Johnson JF, et al. Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 2012; 27:810–16 [DOI] [PubMed] [Google Scholar]
- 7. Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant 2006; 21(Suppl 2):ii56–9 [DOI] [PubMed] [Google Scholar]
- 8. Jo YI, Shin SK, Lee JH, Song JO, Park JH. Immediate initiation of CAPD following percutaneous catheter placement without break-in procedure. Perit Dial Int 2007; 27:179–83 [PubMed] [Google Scholar]
- 9. Rioux J, Cheema H, Bargman JM, Watson D, Chan CT. Effect of an in-hospital chronic kidney disease education program among patients with unplanned urgent-start dialysis. Clin J Am Soc Nephrol 2011; 6:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanko J, Jastrzebski J, Nieva C, White L, Li G, Zalunardo N. Dedication of a nurse to educating suboptimal haemodialysis starts improved transition to independent modalities of renal replacement therapy. Nephrol Dial Transplant 2011; 26:2302–8 [DOI] [PubMed] [Google Scholar]
- 11. Brunier G, Hiller JA, Drayton S, Pugash RA, Tobe SW. A change to radiological peritoneal dialysis catheter insertion: three-month outcomes. Perit Dial Int 2010; 30:528–33 [DOI] [PubMed] [Google Scholar]
- 12. Reddy C, Dybbro PE, Guest S. Fluoroscopically guided percutaneous peritoneal dialysis catheter placement: single center experience and review of the literature. Ren Fail 2010; 32:294–9 [DOI] [PubMed] [Google Scholar]
- 13. Abdel-Aal AK, Gaddikeri S, Saddekni S. Technique of peritoneal catheter placement under fluoroscopic guidance. Radiol Res Pract 2011; 2011:141707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henderson S, Brown E, Levy J. Safety and efficacy of percutaneous insertion of peritoneal dialysis catheters under sedation and local anaesthetic. Nephrol Dial Transplant 2009; 24:3499–504 [DOI] [PubMed] [Google Scholar]
- 15. Medani S, Shantier M, Hussein W, Wall C, Mellotte G. A comparative analysis of percutaneous and open surgical techniques for peritoneal catheter placement. Perit Dial Int 2012; 32:628–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenthal MA, Yang PS, Liu IL, Sim JJ, Kujubu DA, Rasgon SA, et al. Comparison of outcomes of peritoneal dialysis catheters placed by fluoroscopically guided percutaneous method versus directly visualized surgical method. J Vasc Interv Radiol 2008; 19:1202–7 [DOI] [PubMed] [Google Scholar]
- 17. Voss D, Hawkins S, Poole G, Marshall M. Radiological versus surgical implantation of first catheter for peritoneal dialysis: a randomized non-inferiority trial. Nephrol Dial Transplant 2012; 27:4196–204 [DOI] [PubMed] [Google Scholar]
- 18. Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, et al. on behalf of the EBPG Expert Group on Peritoneal Dialysis. European best practice guidelines for peritoneal dialysis. 3 Peritoneal access. Nephrol Dial Transplant 2005; 20(Suppl 9):ix8–12 [DOI] [PubMed] [Google Scholar]
- 19. National Kidney Foundation (NKF). Guideline 3: preservation of residual kidney function. In: Clinical Practice Guidelines for Peritoneal Dialysis Adequacy, Update 2006. New York, NY: NKF; 2006: 150 [Google Scholar]
- 20. Guest S, Akonur A, Ghaffari A, Sloand J, Leypoldt JK. Intermittent peritoneal dialysis: urea kinetic modeling and implications of residual kidney function. Perit Dial Int 2012; 32:142–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koch M, Kohnle M, Trapp R, Haastert B, Rump LC, Aker S. Comparable outcome of acute unplanned peritoneal dialysis and hemodialysis. Nephrol Dial Transplant 2012; 27:375–80 [DOI] [PubMed] [Google Scholar]
- 22. Lobbedez T, Lecouf A, Ficheux M, Henri P, Hurault de Ligny B, Ryckelynck JP. Is rapid initiation of peritoneal dialysis feasible in unplanned dialysis patients? A single-centre experience. Nephrol Dial Transplant 2008; 23:3290–4 [DOI] [PubMed] [Google Scholar]