Abstract
♦ Background: Kidney transplant failure (TF) is among the leading causes of dialysis initiation. Whether survival is similar for patients treated with peritoneal dialysis (PD) and with hemodialysis (HD) after TF is unclear and may inform decisions concerning dialysis modality selection.
♦ Methods: Between 1995 and 2007, 16 113 adult dialysis patients identified from the US Renal Data System initiated dialysis after TF. A multivariable Cox proportional hazards model was used to evaluate the impact of initial dialysis modality (1 865 PD, 14 248 HD) on early (1-year) and overall mortality in an intention-to-treat approach.
♦ Results: Compared with HD patients, PD patients were younger (46.1 years vs 49.4 years, p < 0.0001) with fewer comorbidities such as diabetes mellitus (23.1% vs 25.7%, p < 0.0001). After adjustment, survival among PD patients was greater within the first year after dialysis initiation [adjusted hazard ratio (AHR): 0.85; 95% confidence interval (CI): 0.74 to 0.97], but lower after 2 years (AHR: 1.15; 95% CI: 1.02 to 1.29). During the entire period of observation, survival in both groups was similar (AHR for PD compared with HD: 1.09; 95% CI: 1.0 to 1.20). In a sensitivity analysis restricted to a cohort of 1865 propensity-matched pairs of HD and PD patients, results were similar (AHR: 1.03; 95% CI: 0.93 to 1.14). Subgroups of patients with a body mass index exceeding 30 kg/m2 [AHR: 1.26; 95% CI: 1.05 to 1.52) and with a baseline estimated glomerular filtration rate (eGFR) less than 5 mL/min/1.73 m2 (AHR: 1.45; 95% CI: 1.05 to 1.98) experienced inferior overall survival when treated with PD.
♦ Conclusions: Compared with HD, PD is associated with an early survival advantage, inferior late survival, and similar overall survival in patients initiating dialysis after TF. Those data suggest that increased initial use of PD among patients returning to dialysis after TF may be associated with improved outcomes, except among patients with a higher BMI and those who initiate dialysis at lower levels of eGFR. The reasons behind the inferior late survival seen in PD patients are unclear and require further study.
Keywords: Hemodialysis, kidney transplantation, kidney allograft loss, survival, US Renal Data System
In the United States, the absolute numbers of incident and prevalent end-stage renal disease (ESRD) patients continue to increase (1). In 2007, 4.1% of US patients initiated dialysis because of a failed kidney allograft, a cause that now rates among the top five leading causes of dialysis initiation (2). Moreover, approximately 16% of US patients wait-listed for kidney transplantation have a history of kidney transplant failure (TF) (2).
Several reasons underlie the significant growth of TF patients within the US dialysis patient population. Advances in kidney transplantation have led to significant improvements in short-term kidney allograft survival; unfortunately, however, equivalent improvements in long-term graft survival have not been realized (3). In addition, with a relatively fixed duration of graft survival, increases in the absolute number of kidney transplantation procedures performed have translated into parallel increases in the number of patients experiencing TF (2). Finally, because of a potentially limited living-donor kidney pool and greater sensitization status for TF patients, repeat kidney transplantation, although associated with improved survival, is performed in only approximately 15%-18% of US TF patients (4,5).
Across both the US and Canadian national registries, high absolute mortality rates have been reported in patients returning to dialysis after TF (6-10). When the survival of those patients is compared with that of patients having ongoing graft function, the annual adjusted death rate is increased by a factor of 3 among patients returning to dialysis after TF (11). Similarly, when the survival of TF patients is compared with that in a cohort of incident, but similarly transplant-eligible dialysis patients wait-listed for kidney transplantation, TF patients have a significantly higher risk of death (6).
Not surprisingly, the risks of cardiovascular complications and infection-related mortality are both considerably higher among TF patients because of the effects of previous and ongoing exposure to immunosuppressive therapy (8,10). Moreover, by virtue of having undergone kidney transplantation, TF patients tend to be younger and to have lower rates of diabetes and fewer comorbidities than transplant-naive dialysis patients (6). Those characteristics have typically been associated with more favorable outcomes on PD among incident dialysis patients (12). Nevertheless, in the United States, most TF patients initiate hemodialysis (HD) after TF. The low rates of PD utilization among TF patients may reflect the general overall trend of declining PD utilization among all incident patients in the United States (13), but it may also reflect a unique concern of increased PD-related infections among TF patients who may be immunocompromised. However, TF patients are also unique among people starting dialysis in that they often have limited pre-dialysis chronic kidney disease care and education, leading to a lack of choice and, possibly, suboptimal dialysis initiation (9).
Preliminary reports suggest that the presence of a failed kidney allograft does not increase the risk of PD-related infectious complications nor compromise PD technique survival (14,15), and yet relatively little is known about the outcomes of TF patients treated with PD and with HD. With limited opportunities for repeat transplantation among TF patients (5), additional strategies that maximize survival and quality of life on dialysis are needed. A critical and contemporary examination of mortality differences by dialysis modality is therefore warranted in this increasing subgroup of ESRD patients. More generally, understanding outcomes by dialysis modality across several key subgroups of patients may be particularly salient in the United States, where recent changes in dialysis reimbursement strategies are likely to incentivize and expand the use of PD by providers (16).
In the present report, our objective was to use data from the US Renal Data System (USRDS) to compare overall survival for HD and PD patients initiating dialysis after kidney TF.
Methods
Study Population and Data Source
The study population included adult patients (≥18 years of age) who began their first treatment between 1995 and 2007 and who initiated dialysis after failure of a first kidney-only transplant. Patients who died or underwent repeat kidney transplantation within 60 days of the TF date were excluded.
The data source for the study was the Standard Analysis Files of the USRDS. To capture comorbidities at the time of kidney TF, patients were included only if a new USRDS 2728 Medical Evidence Form (MEDEVID) was completed within 30 days of dialysis initiation after TF. For TF patients returning to dialysis, a new 2728 form is required only for patients applying for ESRD Medicare coverage. Most of the patients included in the study were therefore those who had become Medicare-ineligible after 3 years of kidney allograft function and who were reapplying for ESRD Medicare.
Information from the RXHIST (treatment history file) and MEDEVID files of the USRDS were used to determine dialysis modality at the time of kidney TF. Patients were included only if both forms were concordant with respect to the modality. The variables collected were age, sex, race or ethnicity, primary modality of dialysis, insurance status, tobacco use, known ischemic heart disease, previous acute myocardial infarction, previous cardiac arrest, congestive heart failure, cerebrovascular disease, peripheral vascular disease, diabetes, use of insulin, diagnosed hypertension, chronic obstructive pulmonary disease, malignancy, documented inability to transfer or ambulate, body mass index, serum creatinine, blood urea nitrogen, serum albumin, and hemoglobin. Glomerular filtration rate (GFR) at dialysis initiation was estimated using the equation derived from the Modification of Diet in Renal Disease study, which is based on age, sex, race, and serum creatinine (17).
We also ascertained selected variables related to the kidney graft from the USRDS United Network for Organ Sharing transplant registration forms. Those variables were living or cadaveric donor, peak panel-reactivity antibody level, year of transplantation, and use of dialysis before transplantation.
Outcome
The primary outcome of interest was mortality from any cause. Secondary outcomes were early (1-year) mortality and mortality among 2-year survivors. Survival was determined from the date of the first dialysis treatment after TF until death. Study subjects were censored if they underwent repeat kidney transplantation or were alive at the end of the observation period (30 September 2007).
Analytical Methods and Statistical Analysis
Continuous variables are reported as means and standard deviations or as medians with first and third quartiles, as appropriate. Categorical variables are described as proportions of the cohort. Characteristics for HD and PD patients were compared using analysis of variance or nonparametric statistics (rank sum tests) and chi-square tests as appropriate.
A multivariable Cox proportional hazards model was constructed to obtain covariate-adjusted measures of the association of initial dialysis modality with rates of death. Dialysis modality was analyzed in an intention-to-treat approach such that, among patients who switched dialysis modality over the course of follow-up, mortality was attributed to the initial modality. Model building was performed based both on univariate testing of all covariates for an association with survival and on forced entry of variables based on a priori knowledge of earlier studies examining the effect of dialysis modality on survival. Prespecified interaction terms with dialysis modality that were explored included age, diabetes, estimated GFR (eGFR) at dialysis initiation, presence or absence of comorbidities, and body mass index (BMI). Observations with missing continuous covariates were excluded from the models; for categorical variables, a missing category was included. The proportional hazards assumption for all confounding variables was tested visually, using log-negative log survival plots; and where appropriate, time-stratified analyses are presented. Statistical analyses were performed using Stata/MP 11 (StataCorp LP, College Station, TX, USA).
Propensity-Matched Sensitivity Analysis
To minimize treatment selection bias, a matched propensity score (PS) analysis was performed. We created a multivariable logistic regression model with dialysis modality as the dependent variable. All of the available covariates were used in creating this model. A predicted probability of treatment (the PS) was calculated for each individual. The predicted probability of the dependent variable (likelihood of HD) from the model represents the PS for each individual and provides an estimate of the probability that an individual was followed. The area under the receiver operating characteristic curve was calculated to assess the predictive ability of the PS model. The HD and PD patients then were matched using “nearest-neighbor PS matching” to create a 1:1 matched data set of PD and HD patients. Because of unobserved heterogeneity between individuals, frailty survival models were used to determine the association between dialysis modality and mortality in the matched data set.
Results
Patient Characteristics
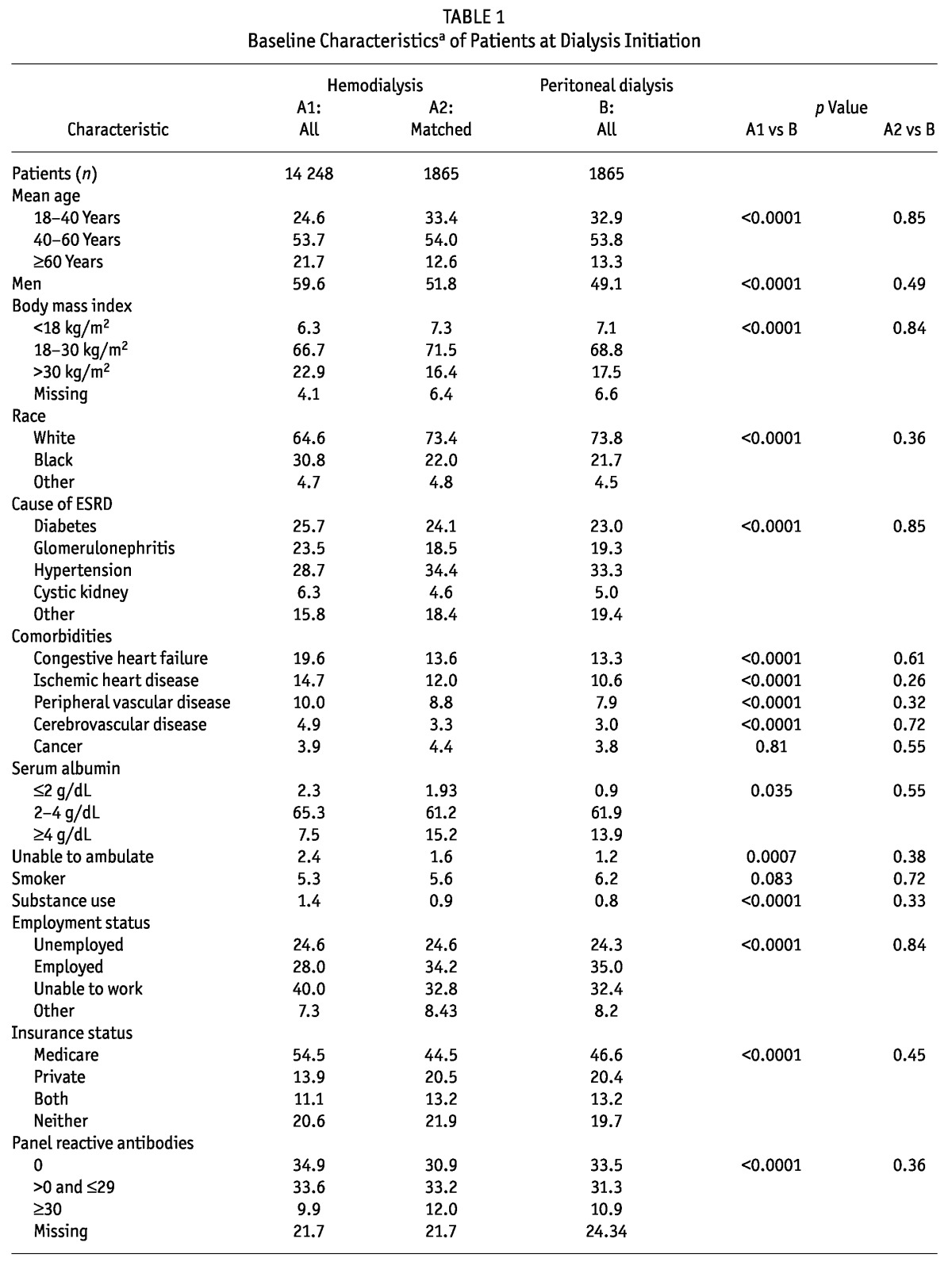
Figure 1 summarizes assembly of the analytic cohort. Among the 41 244 patients who initiated dialysis after TF between 1995 and 2007, 16 113 met the inclusion criteria and were included in the analysis. Table 1 shows the baseline characteristics of the study population. Compared with all the HD patients (n = 14 248, 88%), the PD patients (n = 1865, 12%) were younger, more likely to be white, and less likely to be male (49.1% vs 59.6%); they also tended to have a lower BMI. The most common cause of ESRD in both groups was hypertension. Diabetes mellitus as a cause of ESRD was slightly more common in HD patients (25.7% vs 23.1% in PD patients). The baseline proportion of cardiovascular comorbidities was higher among HD patients. The PD patients had a higher baseline serum albumin, were more likely to ambulate independently and to be employed, and were less likely to have a smoking history. The PD and HD patients initiated dialysis at similar levels of eGFR. The duration of graft function, donor status (living vs deceased), and sensitization status were similar for the PD and HD patients. Patients who received a pre-emptive kidney transplant were more likely to be initiated on PD rather than HD after TF (10.3% vs 7.8%). Over the course of the study, PD utilization continued to decline, with greater use of PD among TF patients during 1995-1998 (15.4%) than during 2003-2007 (11%).
Figure 1 —
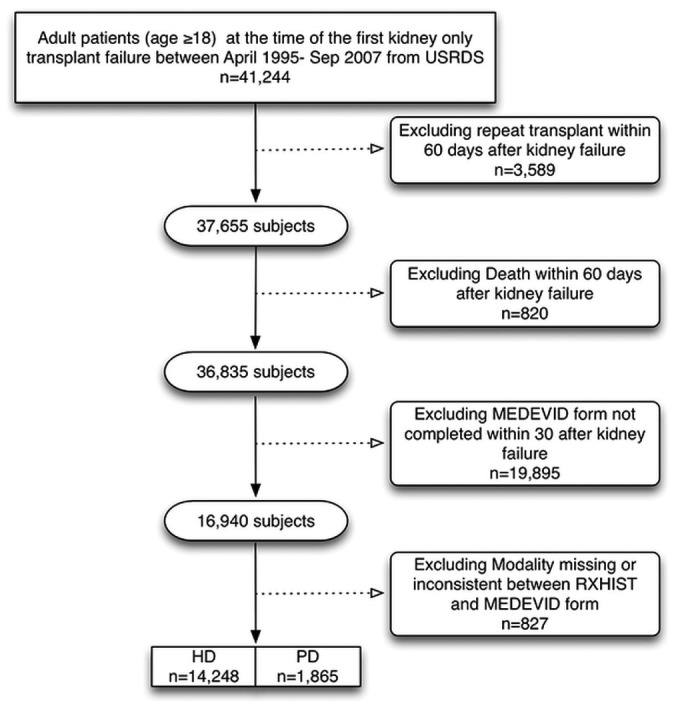
Assembly of the study cohort. USRDS = US Renal Data System; MEDEVID = medical evidence form; RXHIST = treatment history file; HD = hemodialysis; PD = peritoneal dialysis.
TABLE 1.
Baseline Characteristicsa of Patients at Dialysis Initiation
Patient Survival by Dialysis Modality
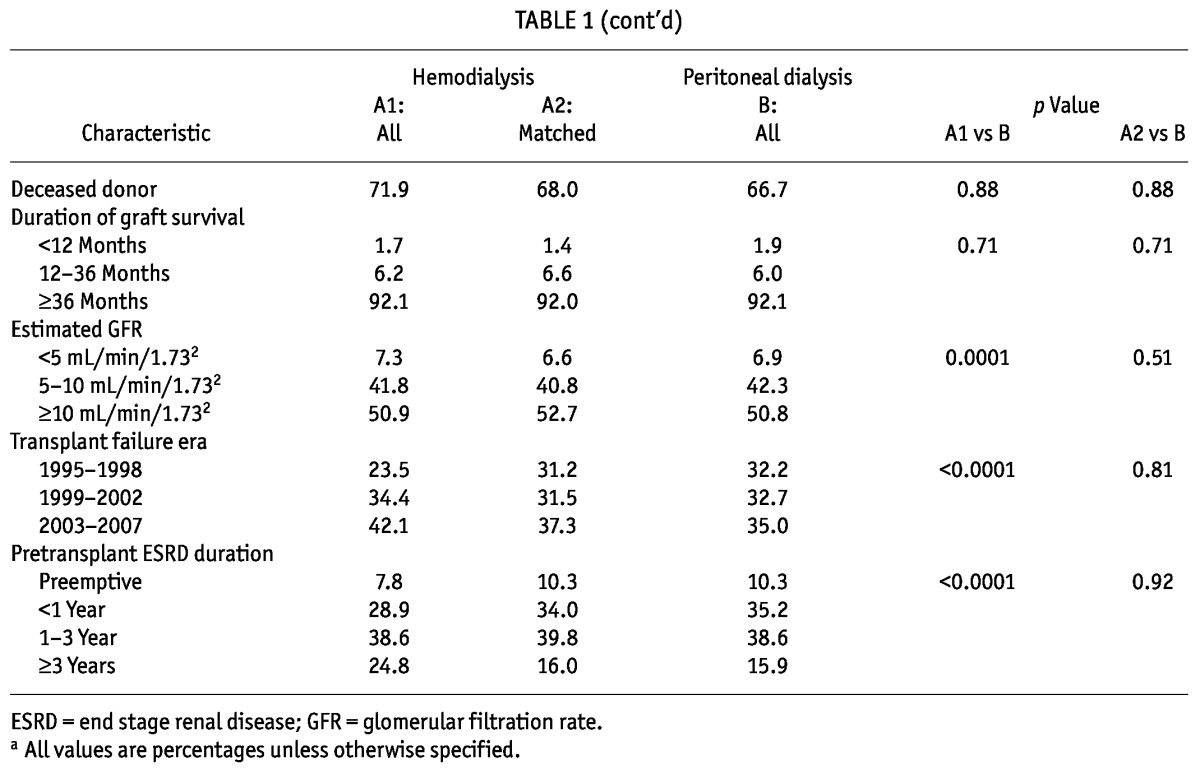
Overall, median follow-up was 2.01 years (interquartile range: 0.90 - 3.96 years). During the study period, 7485 patients died: 6663 on HD (47%) and 822 on PD (44%). Among the 1865 PD patients, 814 remained on PD at the end of year 1, and 347, at the end of year 2.
Table 2 summarizes results from the primary analysis. Relative to HD patients, PD patients had a greater unadjusted overall survival [unadjusted hazard ratio (HR)[PD:HD]: 0.92; 95% confidence interval (CI): 0.85 to 0.99]. After adjustment for available covariates, survival within the first year was greater for PD patients [adjusted HR (AHR)[PD:HD]: 0.85; 95% CI: 0.74 to 0.97], and yet overall, follow-up survival was similar for the HD and PD patients (AHR[PD:HD]: 1.09; 95% CI: 1.0 to 1.20). After 2 years, the risk of mortality was 15% higher for the PD patients (AHR[PD:HD]: 1.15; 95% CI: 1.02 to 1.29).
TABLE 2.
Overall and Time-Dependent Survival in Patients Treated with Either Peritoneal Dialysis or Hemodialysis after Kidney Transplant Failure
Retransplantation Rates by Dialysis Modality
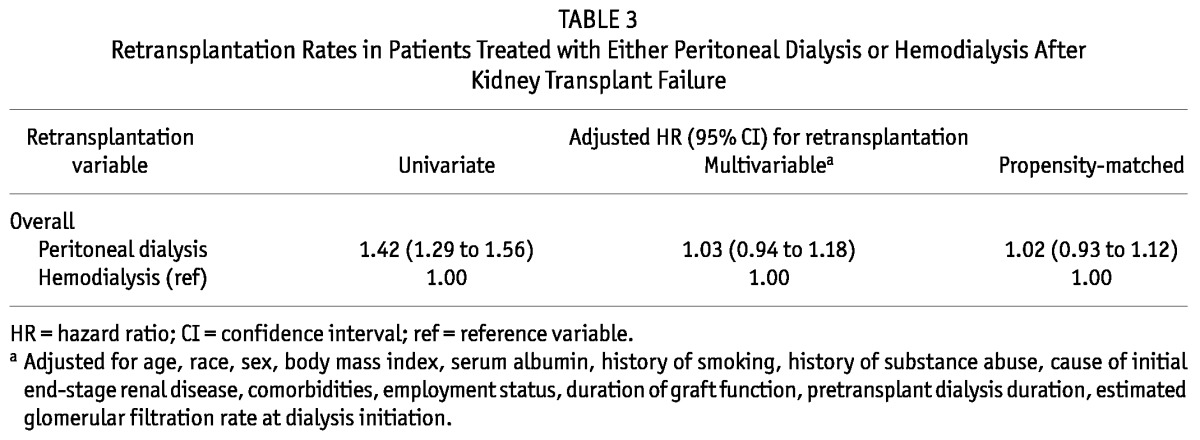
Overall, 3168 patients were retransplanted during study follow-up [2669 on HD (19%), 499 on PD (27%)]. Median time to transplantation was 2 years (interquartile range: 0.92 - 3.7 years). Table 3 shows the crude and multivariable adjusted transplantation rates for the PD patients relative to the HD patients.
TABLE 3.
Retransplantation Rates in Patients Treated with Either Peritoneal Dialysis or Hemodialysis After Kidney Transplant Failure
Propensity-Matched Sensitivity Analysis
The multivariate logistic regression model to derive the PS for choosing PD as the outcome had an area under the receiver operating characteristic curve of 0.91, denoting excellent predictive discrimination with respect to the outcome (1.0 indicates perfect predictive discrimination; 0.5 indicates no ability to discriminate with respect to outcome). Table 1 summarizes the results of the PS matching. After matching, all patient characteristics were similar for the PD and HD patients. Table 2 summarizes the results of the PS survival analyses. Overall survival was similar for the propensity-matched HD and PD patients (HR[PD:HD]: 1.03; 95% CI: 0.93 to 1.14; Figure 2). However, 1-year mortality was lower for the PD patients (HR[PD:HD]: 0.83; 95% CI: 0.69 to 0.99). After 2 years, survival was similar for the PD and HD patients (HR[PD:HD]: 1.11; 95% CI: 0.95 to 1.30).
Figure 2 —
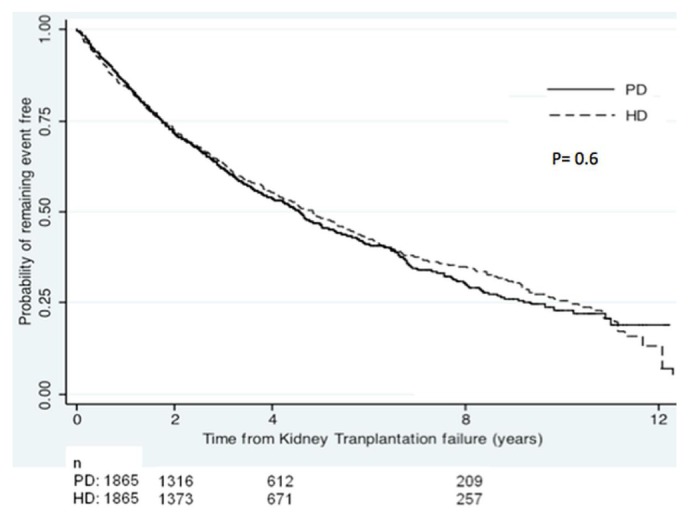
Survival among propensity-matched peritoneal dialysis (PD) and hemodialysis (HD) patients.
Prespecified Interactions
Figure 3 illustrates the results of the prespecified subgroup analyses. Age, diabetes status, and the presence or absence of comorbidities did not significantly modify the relationship between dialysis modality and survival. Among the BMI categories, PD-treated patients with a BMI exceeding 30 kg/m2 had a higher mortality risk (HR[PD:HD]: 1.26; 95% CI: 1.05 to 1.52). However, across other BMI categories, no difference in survival was seen between PD- and HD-treated patients. Among eGFR categories, PD-treated patients who initiated dialysis with an eGFR less than 5 mL/min/1.73 m2 had a 45% greater risk of death than did HD-treated patients who initiated dialysis with an eGFR less than 5 mL/min/1.73 m2 (HR[PD:HD]: 1.45; 95% CI: 1.05 to 1.98).
Figure 3 —
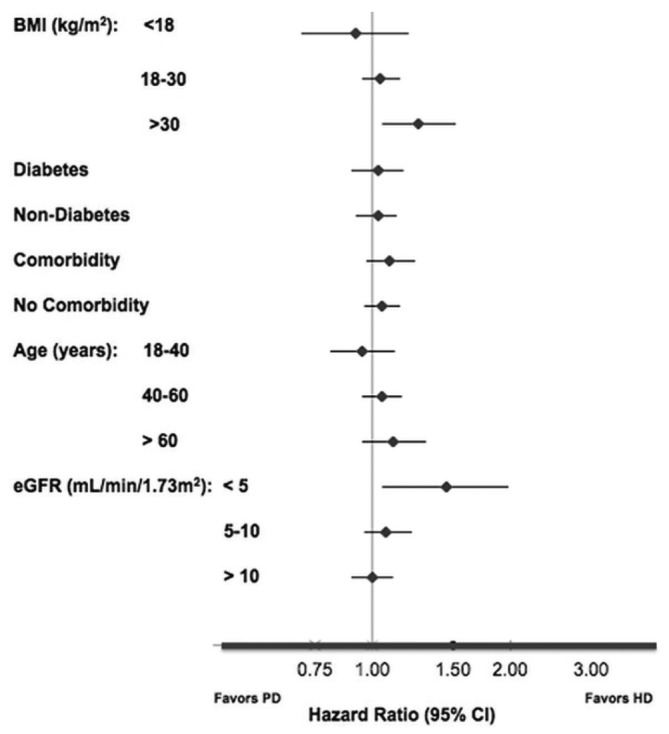
Survival in selected subgroups among propensity-matched peritoneal dialysis (PD) and hemodialysis (HD) patients. BMI = body mass index; eGFR = estimated glomerular filtration rate; CI = confidence interval.
Discussion
In this nationally representative cohort of patients who initiated dialysis after a first kidney TF, we demonstrated that the choice of dialysis modality (HD or PD) did not influence overall patient survival. We also observed a time-dependent survival trend in the association between dialysis modality and risk of death. Within the first year after dialysis initiation, PD treatment was associated with greater survival, and yet among patients with 2 years of follow-up, PD was associated with poorer survival. We also demonstrated that among key subgroups of patients, including those initiating dialysis with an eGFR less than 5 mL/min/1.73 m2 and those with a BMI exceeding 30 kg/m2, PD offered a lower survival rate over the entire course of follow-up. Our primary findings were also consistent in a sensitivity analysis that was restricted to propensity-matched pairs of HD and PD patients.
Our study both extends and supports a similar analysis by our group using data from the Canadian Organ Replacement Register (18). As in the present work, dialysis modality was found not to affect survival in a cohort of 2110 Canadian TF patients. In contrast, the Canadian study demonstrated no time-dependent trend in the relative risks of death by dialysis modality over time. That difference may potentially relate to differences in the general selection criteria for dialysis modalities in the two countries and to limited power to detect time-dependent interactions in a significantly smaller Canadian cohort. Moreover, the Canadian analysis was characterized by a more limited set of confounders, and excluded a cohort of patients who experienced prolonged graft function. Moreover, case-mix adjustment was performed using comorbidities obtained before kidney transplantation and not at the time of TF. It is possible that, in the Canadian analysis, comorbidities accrued over the course of kidney transplantation were underestimated.
As in earlier studies (12,19), we observed a time-dependent trend in the relative mortality risk on PD during the first year after dialysis initiation: the mortality risk was 15% lower with the use of PD than of HD. Patients experiencing TF are at increased risk for septicemia and appear to be most vulnerable within the first 6 months after dialysis initiation, when rates of septicemia appear to be highest (10). The high rates of sepsis may be mediated not only by the effects of immunosuppression, but also by higher rates of incident central venous catheter (CVC) use among TF patients than among transplant-naive dialysis patients (20). We had no information about catheter use among the HD patients; however, in a similar cohort of patients, those treated with HD faced a 70% greater risk of sepsis than did those treated with PD (10). Although case-mix differences between the PD and HD patients may be mediating those differences, a history of kidney TF may be modifying the relationship between dialysis modality and survival. In that regard, greater initial use of PD among TF patients may be one therapeutic strategy to avoid the significantly high infection-related and overall mortality seen among TF patients. In several reports, the use of PD has been associated with a lower risk of infection-related mortality (21,22). Peritoneal dialysis offers the opportunity to avoid HD initiation with a CVC, and treatment-related infections tend to remain localized. Across observational studies, CVC use among incident HD patients is a potent risk factor for infectious complications (23-25). Moreover, incident CVC use was recently demonstrated to be an important determinant of the higher early mortality seen in incident HD patients relative to PD patients (26). Therefore, initial use of PD in TF patients may be associated with a greater early survival advantage for these patients, who are characterized by high rates of infectious complications and high rates of CVC use at dialysis initiation (10,20). Furthermore, as in native kidney disease, incident use of PD in TF patients might, relative to HD use, allow for greater preservation of residual kidney function (27,28). In the present study, we report a novel association between eGFR, dialysis modality, and the risk of death: TF patients who initiated dialysis with an eGFR of less than 5 mL/min/1.73 m2 were observed to have a greater risk of death when treated with PD. Residual kidney function has been demonstrated to affect the survival of PD and HD patients alike (29). It is therefore possible that low levels of residual kidney function at dialysis initiation may be attenuating a survival advantage traditionally associated with the use of PD.
Unfortunately, in the present study, we could not adjust for differential rates of decline in residual kidney function over time. However, independent of dialysis modality, HD and PD patients returning to dialysis after TF both face more rapid loss of residual kidney function than do patients with native kidney disease (30,31). Results of a decision analysis suggest that ongoing continuation of immunosuppression may allow for improved patient survival because of preservation of residual function in a transplanted kidney (32). However, continued immunosuppression must be balanced against the associated infectious and noninfectious risks (33). Whether the rapid loss of residual kidney function among TF patients is further affected by immunosuppression reduction or cessation upon dialysis initiation remains unclear and requires further prospective study.
The increased risk of death that we observed among TF patients after 2 years on PD therapy might possibly be related to an accelerated loss of peritoneal membrane function. In contrast to HD, in which the specifications of the dialyzer are known and can be altered, the peritoneal membrane is a biologic membrane and susceptible to injury. Changes in the morphology of the peritoneal membrane over time—eventually leading to ultrafiltration failure—remain one of the leading causes of technique failure and morbidity among long-term PD patients (34,35). The effects of long-term immunosuppression exposure in exacerbating peritoneal membrane changes remain unclear. Nephrotoxicity is a well-recognized complication of long-term calcineurin inhibition, but “peritoneotoxic” effects—akin to the changes seen in humans over time on PD therapy—have recently been described in animal models of PD with chronic exposure to calcineurin inhibition (35,36). Whether the nature and duration of immunosuppression exposure among TF patients before dialysis initiation (including the use of calcineurin inhibition) affects long-term outcomes on PD requires further study.
Among transplant-naive patients, rates of kidney transplantation have been observed to be higher among those on PD than among those on HD (37). Among TF patients, time to renal transplantation was similar for those being treated with PD or with HD. Any late survival differences that we observed in PD and HD patients are therefore unlikely to be a result of the effects of informative censoring and selected removal of a greater proportion of “healthier” transplant-eligible patients from the PD cohort compared with the HD cohort.
In accord with several studies among incident US patients (38-40), we found that a higher BMI was associated with adverse outcomes in PD patients relative to HD patients. Patients with a BMI above 30 kg/m2 treated with PD faced a 26% greater risk of death. Using registry data from Australia and New Zealand, Badve et al. demonstrated an independent and positive association between BMI and patient survival in 13 947 incident PD patients (15), a finding that was independent of a history of TF. Moreover, BMI was also independently associated with an increased risk of peritonitis, which may be mediating the increased risk of death for these patients. Possible additional reasons include the risks of inadequate dialysis or difficulties with volume management, which may be more challenging in larger PD patients than in similar patients treated with HD.
The findings of the present analysis need to be interpreted within the limitations of the study design. As with all analyses of observational data, the major threat to validity is confounding based on unmeasured facility- and patient-level characteristics that may have affected the relationship between dialysis modality and survival. Patients on PD tended to be younger, with a lower burden of comorbidities and a higher serum albumin. We partially accounted for that bias by performing analyses restricted to patients who survived at least 60 days after dialysis initiation and by conducting a sensitivity analysis restricted to a propensity-matched cohort of HD and PD patients. The propensity-matched analysis did not appreciably change the direction or the magnitude of the results of the main analysis, and it provided an opportunity to confirm the main findings in a population of HD and PD patients well-matched on key confounding variables. As mentioned earlier, we also excluded a significant proportion of patients who did not have a recent MEDEVID form completed at the time of TF. Although that exclusion allowed us to adjust for comorbidities at the time of TF (a strength of the analysis), the ability to extrapolate the present findings to patients experiencing a shorter duration of graft function (who were underrepresented in the analysis) may be limited. Other data, including details of the HD vascular access used at dialysis onset, location of the first dialysis treatment (inpatient or outpatient), receipt of pre-dialysis education, use of transplant nephrectomy, and details about immunosuppression maintenance after TF would have been of interest; however, those details were not available for analysis. In the present study, eGFR was calculated using an equation that has not been well validated among transplant recipients. In addition, our analysis was limited to an intention-to-treat approach, and we therefore could not assess the effect of dialysis modality switches on mortality. Lastly, as with all registry data, large datasets are subject to limitations arising from data surveillance and validity protocols specific to the registry. However, the MEDEVID form has been recently validated: comorbidities are captured with high specificity, but sensitivity may be lacking, with underestimation of comorbidities—particularly among HD patients (41).
Conclusions
Notwithstanding the limitations of the present study, we demonstrated for the first time in a cohort of US dialysis patients that, compared with HD, PD is associated with similar survival among patients initiating dialysis after kidney TF. Our findings support an integrative approach to ESRD care (42), one that encourages PD utilization at the onset of dialysis after TF, followed by repeat transplantation if possible, or a timely transfer to HD where appropriate. Such a strategy stands to maximize overall survival for patients initiating dialysis after TF. Dialysis and transplant practitioners must work closely together if patients are to be empowered to make an informed dialysis modality choice while their smooth transition to dialysis is facilitated. The finding that baseline eGFR at dialysis initiation modifies the relationship between dialysis modality and survival in patients returning to dialysis after TF is novel and should be confirmed in other populations of patients in further prospective studies.
Disclosures
JP has received speaking honoraria from Baxter Healthcare Canada and Amgen Canada and holds an unrestricted educational fellowship from Baxter Healthcare Canada.
References
- 1. Eggers PW. Has the incidence of end-stage renal disease in the USA and other countries stabilized? Curr Opin Nephrol Hypertens 2011; 20:241–5 [DOI] [PubMed] [Google Scholar]
- 2. United States, Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urologic, and Hematologic Diseases, US Renal Data System (USRDS). USRDS 2007 Annual Data Report. Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: US Renal Data System; 2007. [Google Scholar]
- 3. Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant 2011; 11:450–62 [DOI] [PubMed] [Google Scholar]
- 4. Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant 2007; 7:1961–7 [DOI] [PubMed] [Google Scholar]
- 5. Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol 2010; 21:374–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis 2007; 49:294–300 [DOI] [PubMed] [Google Scholar]
- 7. Rao PS, Schaubel DE, Saran R. Impact of graft failure on patient survival on dialysis: a comparison of transplantnaive and post-graft failure mortality rates. Nephrol Dial Transplant 2005; 20:387–91 [DOI] [PubMed] [Google Scholar]
- 8. Gill JS, Abichandani R, Kausz AT, Pereira BJ. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int 2002; 62:1875–83 [DOI] [PubMed] [Google Scholar]
- 9. Gill JS, Abichandani R, Khan S, Kausz AT, Pereira BJ. Opportunities to improve the care of patients with kidney transplant failure. Kidney Int 2002; 61:2193–200 [DOI] [PubMed] [Google Scholar]
- 10. Johnston O, Zalunardo N, Rose C, Gill JS. Prevention of sepsis during the transition to dialysis may improve the survival of transplant failure patients. J Am Soc Nephrol 2007; 18:1331–7 [DOI] [PubMed] [Google Scholar]
- 11. Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation 1998; 66:1651–9 [DOI] [PubMed] [Google Scholar]
- 12. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl 2006; (103):S3–11 [DOI] [PubMed] [Google Scholar]
- 13. Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol 2007; 18:2781–8 [DOI] [PubMed] [Google Scholar]
- 14. Mujais S, Story K. Patient and technique survival on peritoneal dialysis in patients with failed renal allograft: a case-control study. Kidney Int Suppl 2006; (103):S133–7 [DOI] [PubMed] [Google Scholar]
- 15. Badve SV, Hawley CM, McDonald SP, Mudge DW, Rosman JB, Brown FG, et al. on behalf of the ANZDATA Registry PD Working Committee. Effect of previously failed kidney transplantation on peritoneal dialysis outcomes in the Australian and New Zealand patient populations. Nephrol Dial Transplant 2006; 21:776–83 [DOI] [PubMed] [Google Scholar]
- 16. Golper TA, Guest S, Glickman JD, Turk J, Pulliam JP. Home dialysis in the new USA bundled payment plan: implications and impact. Perit Dial Int 2011; 31:12–16 [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–70 [DOI] [PubMed] [Google Scholar]
- 18. Perl J, Hasan O, Bargman JM, Jiang D, Na Y, Gill JS, et al. Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol 2011; 6:582–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20:155–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perl J, Zhang J, Gillespie B, Wikström B, Fort J, Hasegawa T, et al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2012; 27:4464–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aslam N, Bernardini J, Fried L, Burr R, Piraino B. Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol 2006; 1:1226–33 [DOI] [PubMed] [Google Scholar]
- 22. Foley RN, Guo H, Snyder JJ, Gilbertson DT, Collins AJ. Septicemia in the United States dialysis population, 1991 to 1999. J Am Soc Nephrol 2004; 15:1038–45 [DOI] [PubMed] [Google Scholar]
- 23. Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009; 53:475–91 [DOI] [PubMed] [Google Scholar]
- 24. Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE. Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol 2004; 15:1936–42 [DOI] [PubMed] [Google Scholar]
- 25. Moist LM, Trpeski L, Na Y, Lok CE. Increased hemodialysis catheter use in Canada and associated mortality risk: data from the Canadian Organ Replacement Registry 2001-2004. Clin J Am Soc Nephrol 2008; 3:1726–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 2011; 22:1113–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT. on behalf of the NECOSAD Study Group. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62:1046–53 [DOI] [PubMed] [Google Scholar]
- 28. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11:556–64 [DOI] [PubMed] [Google Scholar]
- 29. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 2009; 53:1068–81 [DOI] [PubMed] [Google Scholar]
- 30. Beltrán S, Gavela E, Kanter J, Sancho A, Avila A, Górriz JL, et al. Beginning hemodialysis: do patients with a failed renal transplant start in worse condition? Transplant Proc 2009; 41:2129–31 [DOI] [PubMed] [Google Scholar]
- 31. Davies SJ. Peritoneal dialysis in the patient with a failing renal allograft. Perit Dial Int 2001; 21(Suppl 3):S280–4 [PubMed] [Google Scholar]
- 32. Jassal SV, Lok CE, Walele A, Bargman JM. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: results of a decision analysis. Am J Kidney Dis 2002; 40:178–83 [DOI] [PubMed] [Google Scholar]
- 33. Gregoor PJ, Kramer P, Weimar W, van Saase JL. Infections after renal allograft failure in patients with or without low-dose maintenance immunosuppression. Transplantation 1997; 63:1528–30 [DOI] [PubMed] [Google Scholar]
- 34. Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, et al. on behalf of the EAPOS Group. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol 2003; 14:2948–57 [DOI] [PubMed] [Google Scholar]
- 35. Davies SJ, Mushahar L, Yu Z, Lambie M. Determinants of peritoneal membrane function over time. Semin Nephrol 2011; 31:172–82 [DOI] [PubMed] [Google Scholar]
- 36. van Westrhenen R, Aten J, Hajji N, de Boer OJ, Kunne C, de Waart DR, et al. Cyclosporin a induces peritoneal fibrosis and angiogenesis during chronic peritoneal exposure to a glucose-based, lactate-buffered dialysis solution in the rat. Blood Purif 2007; 25:466–72 [DOI] [PubMed] [Google Scholar]
- 37. Snyder JJ, Kasiske BL, Gilbertson DT, Collins AJ. A comparison of transplant outcomes in peritoneal and hemodialysis patients. Kidney Int 2002; 62:1423–30 [DOI] [PubMed] [Google Scholar]
- 38. Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 2004; 65:597–605 [DOI] [PubMed] [Google Scholar]
- 39. Inrig JK, Sun JL, Yang Q, Briley LP, Szczech LA. Mortality by dialysis modality among patients who have end-stage renal disease and are awaiting renal transplantation. Clin J Am Soc Nephrol 2006; 1:774–9 [DOI] [PubMed] [Google Scholar]
- 40. Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int 2004; 65:2398–408 [DOI] [PubMed] [Google Scholar]
- 41. Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol 2000; 11:520–9 [DOI] [PubMed] [Google Scholar]
- 42. Van Biesen W, Davies S, Lameire N. An integrated approach to end-stage renal disease. Nephrol Dial Transplant 2001; 16(Suppl 6):7–9 [PubMed] [Google Scholar]


