Abstract
♦ Objective: We evaluated the effect of hernias and their surgical or conservative management on peritoneal dialysis (PD) technique survival and residual renal function.
♦ Methods: This 10-year single-center retrospective case-control study (January 2001 - January 2011) compared patient survival, PD technique survival, and residual renal function in patients with a history of abdominal hernias and in a control cohort matched for age and PD vintage.
♦ Results: Of 73 hernias identified in 63 patients (mean age: 55 years; 63% men), umbilical hernias were the most frequent (40%), followed by inguinal (33%), incisional, and epigastric hernias. Some hernias were surgically repaired before (n = 10) or at the time of PD catheter insertion (n = 11), but most (71%) were diagnosed and managed after initiation of PD.
Overall, 49 of 73 (67%) hernias were treated surgically. In 53% of subjects, early postoperative dialysis was not needed; only 7 patients required temporary hemodialysis. The occurrence of a hernia and its treatment did not significantly affect residual renal function. After a hernia diagnosis or repair, 86% of patients were able to continue with PD.
♦ Conclusions: The incidence of abdominal hernia and hernia management in patients on PD do not significantly influence residual renal function or PD technique survival. Timely management of hernias is advisable and does not preclude continuation with PD as a dialysis modality.
Keywords: Hernia, residual renal function, survival
Abdominal wall hernia is a common mechanical complication of peritoneal dialysis (PD), affecting 12% - 37% patients in published series (1-3). Hernias not only cause pain and alter body image, but can also act to sequester dialysate, thereby leading to unpredictable dialysis clearance and ultrafiltration. Studies have identified a low body mass index with muscle wasting (4) and polycystic kidney disease (1) as risk factors for hernia development. Although increased intra-abdominal pressure has not been shown to be a consistent risk factor for hernia incidence (1,5), it may cause progressive enlargement of the hernial sac and lead to the significant recurrence rates seen with this pathology (6-8). For all those reasons, early surgical repair has been advocated.
Despite the high prevalence of hernias in patients on PD, relatively few data on the epidemiology of the condition have been published. Most studies describe outcomes after surgical intervention (2,6,7,9,10). The effect of hernias and their management on PD technique survival remains poorly characterized in the literature. Furthermore, the effect of hernia occurrence and treatment on residual renal function (RRF), which is known to confer significant prognostic benefit in PD, remains unknown (11).
The aim of the present study was to examine the long-term effect of hernias and their management on PD technique survival and to evaluate the impact of hernias on RRF.
Methods
In this retrospective cohort study, we examined the electronic records of all patients receiving PD at our center from 1 January 2001 to 1 January 2011. Patients with a coded diagnosis of hernia either before or after initiation of dialysis constituted the hernia cohort under study. Outcomes in that group were compared with those in a control cohort matched for sex, age (±2 years), and PD vintage (±1 year).
Patient characteristics captured at baseline included age, sex, ethnicity, cause of end-stage renal disease, diagnosis of diabetes mellitus, hypertension, ischemic heart disease (current presence of angina, prior myocardial infarction, or prior coronary intervention with percutaneous or bypass grafting), peripheral vascular disease (clinical or radiologic evidence of aortic or distal arterial atherosclerotic disease), cerebrovascular disease (stroke or transient ischemic attack), prior history of abdominal surgery, smoking history, and body mass index. Other variables captured included the method of peritoneal catheter insertion (that is, percutaneously under local anesthetic or laparotomic under general anesthetic), and the PD modality used at dialysis initiation [continuous ambulatory PD (CAPD) or automated PD (APD)].
In patients with a history of hernia before dialysis initiation, the dialysate exchange volume used at the time of PD treatment start was recorded; for those who developed a hernia while on maintenance PD, the volume used at the time of diagnosis was recorded. Residual renal function was measured in all patients using the mean of urea and creatinine clearances adjusted to body surface area (12) and was recorded at two time points 6 months apart. Time point 1 was within 3 - 6 months of surgical repair or of diagnosis in patients managed conservatively and already established on PD, or within 3 - 6 months of dialysis initiation in patients with a prior diagnosis of hernia. Serum albumin was used as a surrogate marker of nutrition status.
Outcomes of interest included the occurrence of surgical complications, peritonitis within 6 months of surgery, hernia recurrence, patient survival, and PD technique failure (defined as a change to hemodialysis for more than 1 month), and changes in RRF.
Descriptive statistics are expressed as mean ± standard deviation. Continuous and categorical variables were compared using, respectively, the Student t-test and the chi-square or Mann-Whitney U-test. Timeline incidence data were analyzed using a Poisson model. Kaplan-Meier analysis of patient survival was performed on an intention-to-treat basis, censoring for change of dialysis modality, transplantation, dialysis withdrawal, transfer to another center, and loss to follow-up, and analysis of PD technique failure was censored for death, transplantation, transfer to another center, dialysis withdrawal, and loss to follow-up. “Origin” was defined as the date of PD initiation, with comparative adjustment for dialysis vintage in controls to account for lead-time bias. A p value less than 0.05 in two-sided tests was considered statistically significant. Data were analyzed using the SPSS (version 15.0: SPSS, Chicago, IL, USA) and Stata software applications (version 10.1: StataCorp LP, College Station, TX, USA).
Results
During the period under study, 308 patients (mean age: 55.0 ± 16.2 years; 29% with diabetes) were on PD at our center. Within that population, we identified 73 hernias in 63 patients [mean age: 54.5 ± 16.1 years (range: 20 - 88 years)]. Table 1 presents the comparative clinical and demographic characteristics of the study cohorts. The hernia cohort contained a significantly greater proportion of patients with polycystic kidney disease as the cause of ESRD (21% vs 8% in the control group, p = 0.04), a finding in keeping with earlier data (1). A comparatively lower proportion of patients had diabetic nephropathy as their underlying pathology (Table 1).
TABLE 1.
Clinical Characteristics of the Study Population
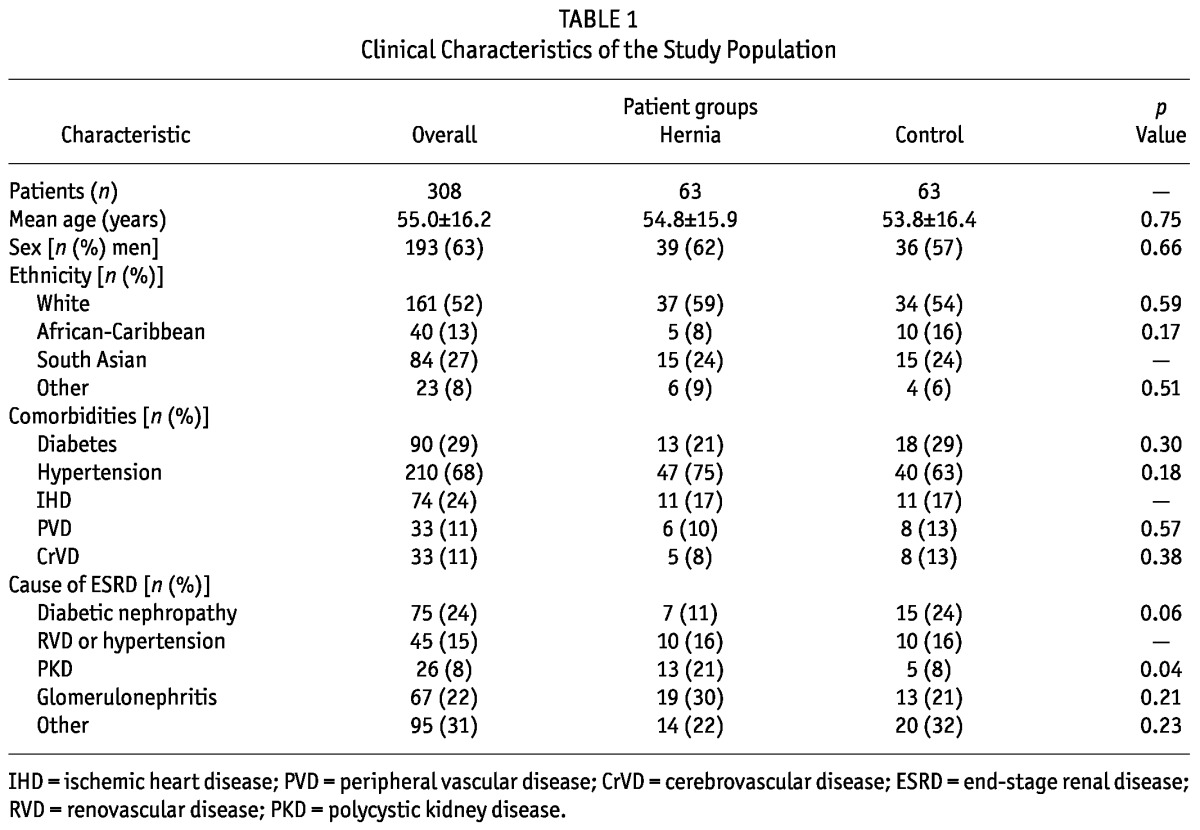
The hernia cohort also showed a significantly greater prevalence of previous abdominal surgery at baseline (19 patients vs 4 patients in the control group, 30% vs 11%, p = 0.001). In 52% of the patients, the prior surgery was related to renal transplantation (graft implantation, n = 7; graft nephrectomy, n = 3; and native nephrectomy, n = 2). The distribution of body mass index was similar in both groups (Figure 1), with no significant difference in mean value (25.9 ± 4.8 kg/m2 vs 26.1 ± 5.4 kg/m2).
Figure 1 —
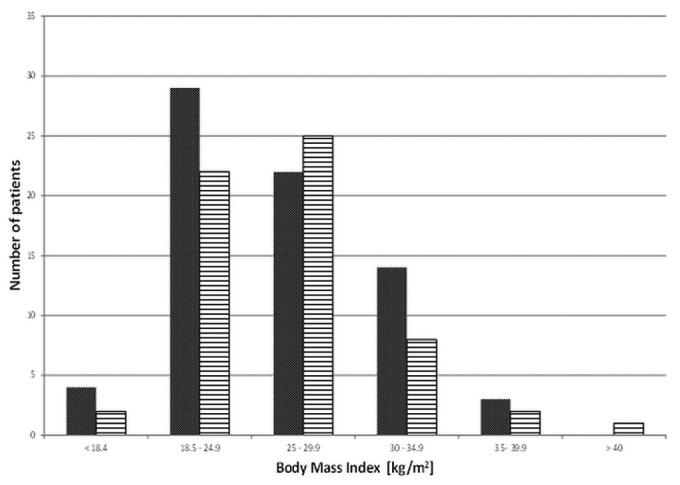
Distribution of body mass index values in the hernia (solid grey bars) and control (horizontally hatched bars) cohorts.
Our center’s policy is that first uncomplicated PD catheters be inserted percutaneously; surgical insertions are used for patients who are very obese, who have had previous pelvic intraperitoneal surgery, or who require simultaneous hernia repair. Of 110 patients, 84 (76%) underwent percutaneous insertion under local anesthesia by appropriately trained nephrologists; the remainder underwent surgical insertion under general anesthesia by surgical teams. More catheters in the hernia cohort were inserted surgically (35% vs 5% in the control group), a finding that may reflect that group’s prior surgical history and concomitant hernia repair at the time of catheter implantation. We observed no differences in the laterality of the exit site in the two groups.
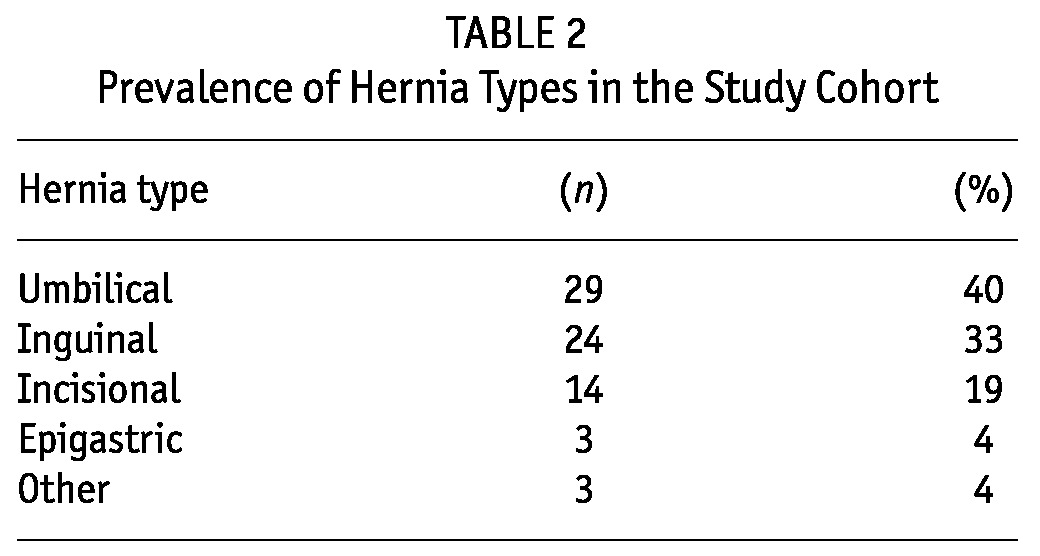
Before they started PD, patients were asked about a history of hernia, and an abdominal examination was performed. Once on PD, development of a hernia was, in almost all instances, self-reported by the patients. Most hernias (n = 52, 71%) were diagnosed and managed after the successful initiation of PD. Diagnosis and repair of a hernia occurred before dialysis initiation in 10 cases (14%), and another 11 hernias diagnosed before initiation (15%) were repaired at the time of PD catheter insertion. Overall, umbilical hernias were the most common hernia type encountered (40%), followed by inguinal and incisional hernias (Table 2). Of the 14 incisional hernias in this series, most (79%) were related to the PD catheter; 3 were related to the renal transplantation incision.
TABLE 2.
Prevalence of Hernia Types in the Study Cohort
In the control group, 39 patients (62%) were on CAPD; the remaining 38% were on APD. By contrast, the PD modality in the hernia group varied depending on whether the patient was or was not on PD at time of diagnosis (Figure 2). Among patients already on PD, 63% were on CAPD, and 37% were on APD. Among patients who underwent hernia repair at the time of catheter insertion, 64% were started on APD. The overall mean dialysate fill volume at the time of hernia diagnosis was 1901 ± 314 mL, with no significant difference between the hernia group and the control group (1953 ± 623 mL vs 1858±308 mL respectively, p = 0.26)
Figure 2 —
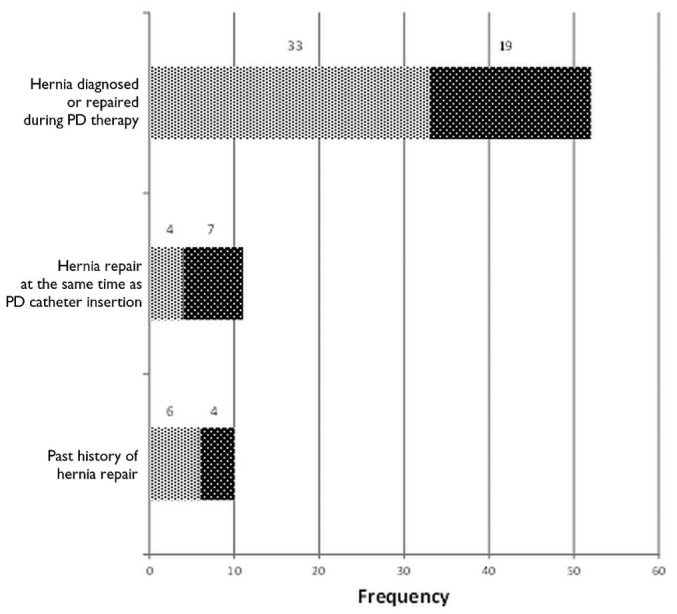
Peritoneal dialysis (PD) modality—CAPD (light gray bars) and APD (dark gray bars)—used by patients at the time of hernia diagnosis (73 hernias).
Of the 51 hernias that were surgically repaired, nearly all were handled electively (49 of 51, 96%); the remaining 2 hernias required emergency surgery. Before 2005, surgery was performed by general surgical teams; since 2005, surgery has been performed by the renal transplant surgical team. For hernias detected after PD start, the mean time from diagnosis to hernia repair was 6 months. Surgery is usually performed within 2 - 3 months of referral (range: 3 days - 28 months); the occasional delays typically reflect patient preference. In our experience, patients with small hernias often hesitate about surgical referral or prefer to have surgery close to specific dates.
In all cases of surgical repair, tension-free hernioplasty was performed. In 53% of cases, there was no need for early postoperative dialysis; intermittent PD was performed in 6 cases, low-volume APD in 3, and temporary hemodialysis in 7 (14%). The decision about the dialysis modality to use depended on various clinical factors, such as whether the patient was trained on APD, whether a bed was available for intermittent PD, and how urgent the need for dialysis was. Only 1 postoperative complication was recorded, a self-limited hematoma. Within 6 months of hernia repair, 4 episodes of peritonitis occurred, for an incidence rate of 1 episode in 44.5 months. Seven hernias (10%) recurred, with an average time to recurrence of 12 months (range: 7 - 32 months).
We observed no significant differences in serum albumin between the groups at baseline (mean: 29.1 ± 7 g/L in the hernia group vs 30.5 ± 5.4 g/L in the control group; p = 0.19). At 6 months, RRF had declined in both groups (to 5.60 ± 4.65 mL/min from 6.41 ± 4.75 mL/min in the hernia group, and to 4.48 ± 4.41 mL/min from 5.03 ± 4.18 mL/min in the control group), a difference that did not achieve statistical significance (p = 0.12 and p = 0.10 respectively).
At 1, 2, and 3 years, PD technique survival was, respectively, 67%, 47%, and 34% in the hernia cohort and 76%, 47%, and 36% in control subjects, censoring for death and transplantation (Figure 3). There was therefore no statistically significant difference in long-term technique survival between the groups. At 1, 2, 3, and 5 years, cumulative patient survival was, respectively, 93%, 90%, 82%, and 59% in the hernia group and 92%, 88%, 84%, and 64% in the control group (Figure 4), with no significant difference between groups. Those rates are representative of overall PD patient survival at our center during the study period: 95% at 1 year, 91% at 2 years, and 88% at 3 years.
Figure 3 —
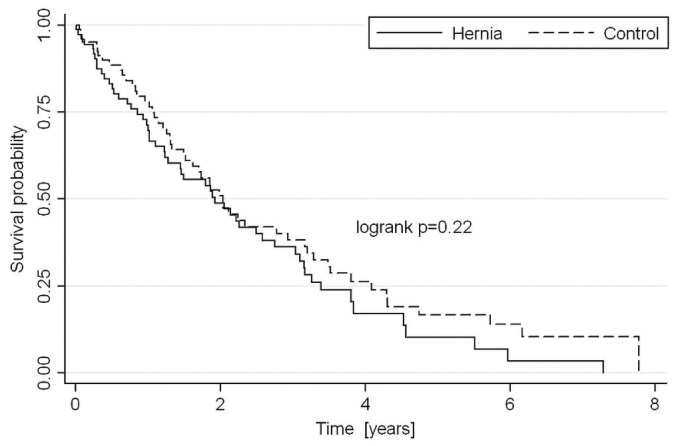
Censored peritoneal dialysis (PD) technique survival in the hernia and control groups, censored for death, transplantation, transfer to another center, or loss to follow-up and adjusted for PD vintage.
Figure 4 —
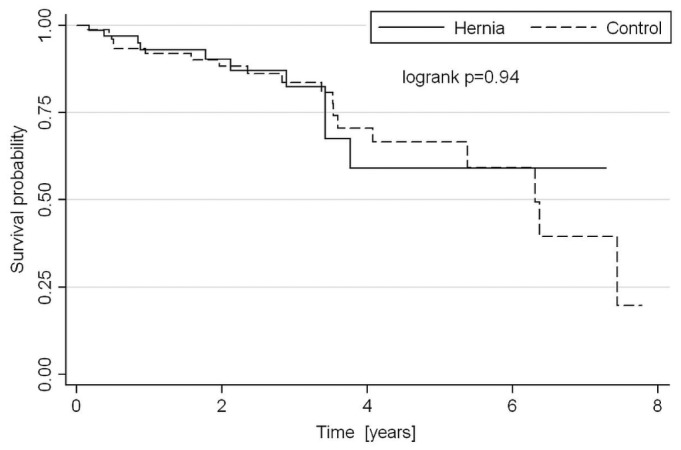
Cumulative patient survival in the study cohort after censoring for death, transplantation, change in dialysis modality, transfer to another center, dialysis withdrawal, and loss to follow-up.
Discussion
The present study constitutes the one of the largest published series describing the management and clinical impact of hernias in patients on PD. We have demonstrated for the first time that, with appropriate management, this pathology does not significantly affect long-term PD technique survival or change in RRF.
The overall prevalence of hernias in our study was 20%, which is comparable to prevalences in earlier series (1,2,4,6,13,14) and which reflects an overall incidence in our PD population of 0.08 incidents per year at risk, in keeping with other reported rates of 0.04 - 1.11 hernias per year at risk (1,2,15). Hernias already present in patients about to start PD have been reported in 5% - 12% of cases (2,6,16). By contrast, we found that 29% of the hernias in our study cohort occurred before PD start—although that finding might reflect the inclusion of patients with a prior history of hernia rather than diagnoses during PD assessment as in the series by Garcia-Urena et al. (2).
Elevations in intra-abdominal pressure from large volumes of dialysate might explain the higher incidence of hernias in PD cohorts than in a general population, but that hypothesis remains controversial: some studies showed no association (1,5,17), others observed higher rates of hernia formation with greater pressures (18). Notably, we observed no significant difference in fill volume between the hernia and control cohorts in our study.
Upright posture also increases intra-abdominal pressure, and APD is often favored over CAPD to allow for equivalent fill volumes. In established PD patients, we found a higher percentage of hernias occurring among patients on CAPD than among those on APD (63% vs 47%), although PD modality per se has not been shown to be an independent risk factor for hernia formation (1). Hernias were also more common in patients who had undergone transplantation. In those patients, the hernia was rarely associated with the transplant incision, and so those hernias might be related to the use of steroids and subsequent changes in abdominal musculature.
In our study, umbilical hernias were the most common subtype, followed by inguinal hernias. That finding is consistent with results in previous reports (2,8), but contrasts with data for the general UK population, in whom inguinal hernias are the most common. However, more modern cohorts show a rising incidence of umbilical hernias (19). Surgical treatment was well tolerated in our study, and the 10% recurrence rate is comparable to rates in older series (6-10,20). Only 7% of our patients undergoing surgery had to convert permanently to hemodialysis, a proportion similar to those reported by other authors (2,10).
The PD technique survival of 47% at 2 years in our series is similar to that reported by the UK Renal Registry: specifically, 45% technique failure rate over a mean observation period of 430 days (21). The hernia and control groups experienced similar levels of comorbidity, although polycystic kidney disease was more common in the hernia group.
It might be expected that the pain from symptomatic hernias, concerns about hernia enlargement with ongoing PD, the pain and emotional upheaval of abdominal surgery, and the potential for a temporary change of dialysis modality would predispose the hernia group to worse PD technique survival. Interestingly, we found no such association in our study, which adds to the reassuring body of literature about the management of this condition while maintaining patients on their chosen dialysis modality. That finding has added importance at a time of increased awareness about the barriers to starting and maintaining PD (22,23), a dialysis therapy that would be the treatment of choice for approximately half the incident dialysis population (24). However, because of the higher risk of transmural translocation of enteric bacteria, we certainly advocate that a period of a few days elapse between surgery and resumption of PD when there is evidence of compromised bowel (because of strangulation or ischemia).
The reduction in RRF that we noted in both study groups accords with the known changes in that parameter over time. Whether changes in RRF become more marked over the long term is unclear, although acute renal injury relating to surgery would be expected to manifest as a significant change within the 6-month timeframe used in this study.
Conclusions
Our data support timely management of abdominal wall hernias in patients on PD. Recurrence rates are low, and there is no negative impact of that approach on PD technique survival, RRF, or patient survival.
Disclosures
EB has received speaker fees and research funding from Baxter Healthcare. The remaining authors have no financial conflicts of interest to declare.
Acknowledgments
SB was supported by a grant from the Spanish Society of Nephrology “Ayuda Fundación Senefro.” The authors also thank all the PD nurses for their assistance.
References
- 1. del Peso G, Bajo MA, Costero O, Hevia C, Gil F, Diaz C, et al. Risk factors for abdominal wall complications in peritoneal dialysis patients. Perit Dial Int 2003; 23:249–54 [PubMed] [Google Scholar]
- 2. Garcia-Urena MA, Rodriguez CR, Vega Ruiz V, Carnero Hernandez FJ, Fernandez-Ruiz E, Vazquez Gallego JM, et al. Prevalence and management of hernias in peritoneal dialysis patients. Perit Dial Int 2006; 26:198–202 [PubMed] [Google Scholar]
- 3. Suh H, Wadhwa NK, Cabralda T, Sokunbi D, Pinard B. Abdominal wall hernias in ESRD patients receiving peritoneal dialysis. Adv Perit Dial 1994; 10:85–8 [PubMed] [Google Scholar]
- 4. O’Connor JP, Rigby RJ, Hardie IR, Wall DR, Strong RW, Woodruff PW, et al. Abdominal hernias complicating continuous ambulatory peritoneal dialysis. Am J Nephrol 1986; 6:271–4 [DOI] [PubMed] [Google Scholar]
- 5. Dejardin A, Robert A, Goffin E. Intraperitoneal pressure in PD patients: relationship to intraperitoneal volume, body size and PD-related complications. Nephrol Dial Transplant 2007; 22:1437–44 [DOI] [PubMed] [Google Scholar]
- 6. Afthentopoulos IE, Panduranga Rao S, Mathews R, Oreopoulos DG. Hernia development in CAPD patients and the effect of 2.5 L dialysate volume in selected patients. Clin Nephrol 1998; 49:251–7 [PubMed] [Google Scholar]
- 7. Wetherington GM, Leapman SB, Robison RJ, Filo RS. Abdominal wall and inguinal hernias in continuous ambulatory peritoneal dialysis patients. Am J Surg 1985; 150:357–60 [DOI] [PubMed] [Google Scholar]
- 8. Shah H, Chu M, Bargman JM. Perioperative management of peritoneal dialysis patients undergoing hernia surgery without the use of interim hemodialysis. Perit Dial Int 2006; 26:684–7 [PubMed] [Google Scholar]
- 9. Guzman-Valdivia G, Zaga I. Abdominal wall hernia repair in patients with chronic renal failure and a dialysis catheter. Hernia 2001; 5:9–11 [DOI] [PubMed] [Google Scholar]
- 10. Martinez-Mier G, Garcia-Almazan E, Reyes-Devesa HE, Garcia-Garcia V, Cano-Gutierrez S, Mora y Fermín R, et al. Abdominal wall hernias in end-stage renal disease patients on peritoneal dialysis. Perit Dial Int 2008; 28:391–6 [PubMed] [Google Scholar]
- 11. van den Wall Bake AW, Kooman JP, Lange JM, Smit W. Adequacy of peritoneal dialysis and the importance of preserving residual renal function. Nephrol Dial Transplant 2006; 21(Suppl 2):ii34–7 [DOI] [PubMed] [Google Scholar]
- 12. Lubowitz H, Slatopolsky E, Shankel S, Rieselbach RE, Bricker NS. Glomerular filtration rate. Determination in patients with chronic renal disease. JAMA 1967; 199:252–6 [DOI] [PubMed] [Google Scholar]
- 13. Cherney DZ, Siccion Z, Chu M, Bargman JM. Natural history and outcome of incarcerated abdominal hernias in peritoneal dialysis patients. Adv Perit Dial 2004; 20:86–9 [PubMed] [Google Scholar]
- 14. Tokgoz B, Dogukan A, Guven M, Unluhizarci K, Oymak O, Utas C. Relationship between different body size indicators and hernia development in CAPD patients. Clin Nephrol 2003; 60:183–6 [DOI] [PubMed] [Google Scholar]
- 15. Hussain SI, Bernardini J, Piraino B. The risk of hernia with large exchange volumes. Adv Perit Dial 1998; 14:105–7 [PubMed] [Google Scholar]
- 16. Nicholson ML, Madden AM, Veitch PS, Donnelly PK. Combined abdominal hernia repair and continuous ambulatory peritoneal dialysis (CAPD) catheter insertion. Perit Dial Int 1989; 9:307–8 [PubMed] [Google Scholar]
- 17. Durand PY, Chanliau J, Gamberoni J, Hestin D, Kessler M. Routine measurement of hydrostatic intraperitoneal pressure. Adv Perit Dial 1992; 8:108–12 [PubMed] [Google Scholar]
- 18. Bargman JM. Complications of peritoneal dialysis related to increased intraabdominal pressure. Kidney Int Suppl 1993; 40:S75–80 [PubMed] [Google Scholar]
- 19. Dabbas N, Adams K, Pearson K, Royle G. Frequency of abdominal wall hernias: is classical teaching out of date? JRSM Short Rep 2011; 2:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gianetta E, Civalleri D, Serventi A, Floris F, Mariani F, Aloisi F, et al. Anterior tension-free repair under local anesthesia of abdominal wall hernias in continuous ambulatory peritoneal dialysis patients. Hernia 2004; 8:354–7 [DOI] [PubMed] [Google Scholar]
- 21. Tangri N, Ansell D, Naimark D. Predicting technique survival in peritoneal dialysis patients: comparing artificial neural networks and logistic regression. Nephrol Dial Transplant 2008; 23:2972–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown EA. How to address barriers to peritoneal dialysis in the elderly. Perit Dial Int 2011; 31(Suppl 2):S83–5 [DOI] [PubMed] [Google Scholar]
- 23. Oliver MJ, Garg AX, Blake PG, Johnson JF, Verrelli M, Zacharias JM, et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant 2010; 25:2737–44 [DOI] [PubMed] [Google Scholar]
- 24. Little J, Irwin A, Marshall T, Rayner H, Smith S. Predicting a patient’s choice of dialysis modality: experience in a United Kingdom renal department. Am J Kidney Dis 2001; 37:981–6 [DOI] [PubMed] [Google Scholar]


