Abstract
♦ Background: Peritoneal dialysis (PD) is a treatment for selected acute kidney injury patients (AKI), but little is known about its metabolic implications. The aim of the present study was to evaluate the metabolic implications of glucose absorption, sodium removal, protein loss into the dialysate, and catabolism in AKI patients undergoing high-volume PD and to identify risk factors associated with those metabolic effects.
♦ Methods: A prospective cohort study over 18 consecutive months evaluated 208 sessions of high-volume PD performed in 31 AKI patients. One session of high-volume PD lasted 24 hours. Repeated-measures analysis was performed, and correlations were calculated using the Spearman test for continuous variables and generalized linear models for categorical variables.
♦ Results: Glucose absorption remained at approximately 35.3% ± 10.5% per session. Protein loss measured 4.2 ± 6.1 g daily, with higher values initially, which declined significantly after 2 sessions. Nitrogen balance (NB) was initially negative, but stabilized at approximately zero after 3 sessions. Glucose uptake was positively correlated with the Acute Tubular Necrosis Individual Severity Score [ATNISS (r = 0.21, p = 0.0036)], C-reactive protein (r = 0.26, p = 0.0167), protein loss (r = 0.36, p < 0.0001), and sodium removal (r = 0.24, p = 0.002). Protein loss was positively correlated with sodium removal (r = 0.22, p = 0.0085) and gastrointestinal disease (p = 0.0004). Sodium removal was positively correlated with serum sodium (r = 0.21, p = 0.0064), ATNISS (r = 0.15, p = 0.0411), urea nitrogen appearance [UNA (r = 0.24, p = 0.0019)], and fluid overload as an indication for dialysis (p < 0.0001). Urea nitrogen appearance was positively correlated with the indication for dialysis (electrolyte disturbances: p = 0.0287) and negatively correlated with nephrotoxic AKI (p < 0.0001). Nitrogen balance was negatively correlated with UNA (r = -0.389, p < 0.0001) and ischemic AKI (p = 0.0047).
♦ Conclusions: High-volume PD did not increase hypercatabolism in AKI patients, and protein loss and glucose uptake remained constant during treatment. Those parameters were influenced by the clinical condition of the patients, including the cause of AKI, inflammation, and comorbidities—factors that should be known before the prescription of dialysis and nutrition, thus avoiding metabolic complications such as hyperglycemia, hypernatremia, and worsening catabolism.
Keywords: Acute kidney injury, metabolic implications
During the 1970s and 1980s, peritoneal dialysis (PD) was widely accepted as the standard treatment for acute kidney injury (AKI) (1). However, advances in techniques for extracorporeal blood purification gradually reduced the use of PD, making it an underused modality. In developing countries, PD is still commonly used because of its low cost and minimal infrastructure requirements (2). In a recent study, Gabriel et al. (3) showed that high-volume PD administered continuously using a flexible catheter and cycler is an effective treatment in AKI because it ensures adequate fluid status and metabolic control without causing hemodynamic instability or disequilibrium syndrome (4).
However, the use of PD in AKI also has several limitations: it needs an intact peritoneal cavity, and it is less effective than extracorporeal blood purification techniques in emergency situations such as severe fluid overload and severe hyperkalemia (4). Metabolic, infectious, and mechanical disorders are also limitations. Among the metabolic complications of PD are hyperglycemia, hypernatremia, protein loss into the dialysate, and hypercatabolism. Hyperglycemia is caused by the use of high glucose dialysate. Hypernatremia is caused by the short time that the dialysate dwells in the peritoneal cavity because of the rapid changes used in high-volume PD. Protein loss into the dialysate can reach 48 g daily, worsening the nutrition status of patients already depleted by AKI (3-5). Severe hypercatabolism is controversial and is attributed to the inability of PD to provide an adequate dose of dialysis for patients with AKI.
Few studies have assessed the metabolic implications of PD in patients with AKI. Evaluation of those implications is relatively simple, requires no additional costs, and can provide information about the severity of the disease. It can also guide selection of therapeutic, dialytic, and nutrition measures to prevent metabolic complications.
The objectives of the present study were to evaluate the metabolic implications of glucose uptake, sodium removal, protein loss into dialysate, and catabolism in patients with AKI undergoing high-volume PD and to identify the risk factors associated with those disorders.
Methods
Study Population
Our prospective cohort study evaluated 208 high-volume PD sessions performed in 31 patients with AKI admitted to the Clinical Hospital of Botucatu School of Medicine during 18 consecutive months. The study was approved by the medical ethics committee for local research, and informed consent was obtained from all participants or their legal representatives.
Inclusion criteria were age more than 18 years; a clinical diagnosis of AKI caused by ischemic, nephrotoxic, or mixed acute tubular necrosis; a requirement for dialysis; and treatment with high-volume PD for at least 1 session. Exclusion criteria were a pre-renal or post-renal cause of AKI, severe hemodynamic instability (systolic blood pressure below 80 mmHg or use of norepinephrine at a dose exceeding 1 μg/kg/min), absolute contraindications for PD use, early mechanical complications related to PD (occurring within 24 hours), pregnancy, severe chronic renal disease (baseline serum creatinine > 4 mg/dL), and kidney transplant.
Study Protocol
The treatment modality used in the patients with AKI was continuous high-volume PD, which is designed to achieve higher small-solute clearances. Dialysis was performed using a flexible catheter, an automated cycler, and a high volume of dialysis fluid as described in previous studies (2-5). Each session of high-volume PD lasted 24 hours, and sessions were repeated daily, 7 times per week. The total dialysate volume per session ranged from 36 L to 44 L. Peritoneal access was established by the nephrology team through a percutaneous Tenckhoff catheter. The dialysate (135 mEq/L Na; 3.5 mEq/L Ca; 1.5 mEq/L K; 40 mEq/L lactate; and 1.5%, 2.5%, or 4.25% glucose) was Dianeal (Baxter, São Paulo, Brazil), and exchanges were performed using a HomeChoice cycler (Baxter). The prescribed Kt/V was 0.50 per session (3).
We collected clinical and laboratory data on sex, age, main diagnosis, date of admission, AKI cause, presence or absence of sepsis, need for dialysis, specific prognostic score for AKI [Acute Tubular Necrosis Individual Severity Score (ATNISS)], start date of dialysis, and comorbidities (diabetes, previous chronic kidney disease, hypertension).
At the beginning and after each high-volume PD session, samples of blood, urine, and dialysate were collected and analyzed for urea, creatinine, serum sodium, and glucose. Plasma albumin, total protein, total leukocytes, C-reactive protein (CRP), hemoglobin, and hematocrit were measured every 3 days.
All dialysate was collected, and 3 mL of each 24-hour effluent collection was obtained for measurement of urea nitrogen, sodium, glucose, and total protein. Every 3 days, a cell count and a dialysate culture were evaluated for potential peritonitis. Peritonitis was diagnosed if we found cloudy effluent and a white blood cell count exceeding 100/mm3, with at least 50% polymorphonuclear cells, or if the effluent was culture-positive.
A daily urine sample was also collected for analysis of urinary urea. The following calculations were performed daily: delivered Kt/V, glucose absorption from the dialysate, protein loss into the dialysate, dialytic sodium removal, and degree of patient catabolism.
The protocol was interrupted in the case of partial recovery of renal function (urine output > 1000 mL in 24 hours and a progressive decline in creatinine and urea levels to below 4 mg/dL and 100 mg/dL respectively), a change in the dialysis method, more than 30 days of follow-up, or patient death.
Statistical Analysis
The statistical analysis was conducted using SAS for Windows (version 9.1.3: SAS Institute, Cary, NC, USA). Variables with a normal distribution are presented as mean ± standard deviation, and those with a non-normal distribution, as medians with interquartile range.
For analysis of continuous variables, the Student t-test was used for data with a parametric distribution, and the Kruskal-Wallis test, for non-normal data. For the analysis of categorical variables, a chi-square or Fisher exact test was used.
For continuous variables, correlations between metabolic parameters and clinical and laboratory findings were determined using the Spearman test. For categorical variables, correlations were determined using a repeated-measures generalized linear model, assuming a normal distribution, identity link function, gamma distribution, and log link function.
In all tests, differences were considered significant at 5%.
Calculations
The ATNISS score (6) was determined as follows:
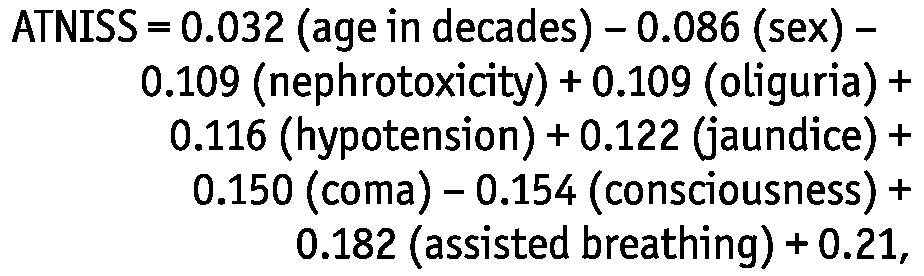 |
where sex is either 1 (for men) or 0 (for women); oliguria is defined as urine output less than 400 mL/24 h; hypotension is defined as systolic blood pressure less than 100 mmHg for more than 10 hours, regardless of vasoactive drugs; and jaundice is defined as total bilirubin less than 2 mg/dL.
In the prescribed Kt/V (7,8), K is the prescribed dialysate volume in 24 hours (in milliliters) multiplied by 0.60 (because the dialysate-to-plasma ratio of creatinine is considered to equal 0.6 for a dwell time between 30 and 60 minutes), t is 1 (for 1 day), and V is the distribution volume of urea (in milliliters). After the K value was calculated, it was divided by 2 L to yield the number of dialysis solution exchanges in 24 hours.
Delivered Kt/V (9) was determined using the formula
 |
Quantification of protein loss into effluent was determined using the formula
 |
Percentage absorption of glucose from dialysate was determined using the formula
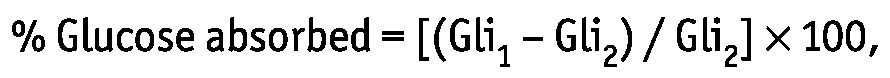 |
where Gli1 is the glucose contained in 24-hour effluent in grams, and Gli2 is the glucose contained in the dialysate infused over 24 hours in grams, calculated as
 |
where “dialysate infused” refers to available solutions for PD containing 1.5%, 2.5%, or 4.25% dextrose. Thus, the concentrations of glucose were 1.36%, 2.27%, or 3.86%.
Removal of sodium (rem Na in milliequivalents) was determined using the formula:
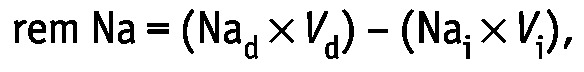 |
where Nad is the sodium concentration in effluent (in milliequivalents per liter), Vd is the effluent volume in liters, Nai is the sodium concentration in the infused dialysate (in milliequivalents per liter), and Vi is the infused dialysate volume in liters.
To estimate the degree of catabolism, the Druml (10) formula for urea nitrogen appearance (UNA) was used. Patients were classified according to their excess of urea as having low catabolism (loss exceeding nitrogen intake by 5 g), moderate catabolism (loss exceeding nitrogen intake by 5 - 10 g), or severe catabolism (loss exceeding nitrogen intake by >10 g). The nitrogen balance (NB) was determined by subtracting total nitrogen loss from dietary nitrogen intake.
Nitrogen intake was calculated by converting the quantity of dietary protein to grams of nitrogen by dividing the protein quantity in grams by 6.25 (that is, 6.25 g of protein generates 1 g of nitrogen).
Nitrogen excretion included non-urea and urea nitrogen losses. Non-urea nitrogen losses (which do not vary substantially with diet) were estimated using the Maroni formula (11):
 |
where ND is dietary nitrogen in grams, NUN is non-urea nitrogen in grams, and UNA is given in grams.
The formula used to determine NUN was (11)
 |
The Druml (10) formula was used to determine UNA in grams:
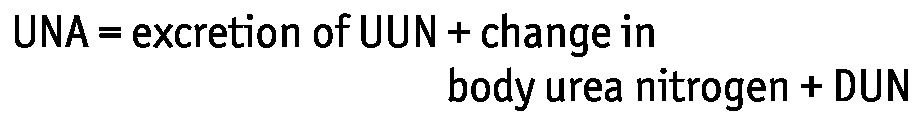 |
where UUN is urinary urea nitrogen in grams per day, and DUN is dialysate urea nitrogen in grams per liter. Expanded, this equation becomes
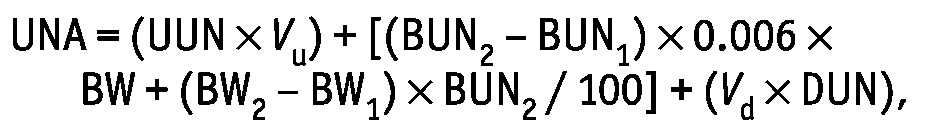 |
where Vu is urine volume in liters, BUN1 and BUN2 are the blood urea nitrogen readings in milligrams per deciliter on days 1 and 2, BW1 and BW2 are body weight in kilograms on days 1 and 2, and Vd is dialysate volume in liters.
Results
The mean age of the 31 study patients was 67.3 ± 12.7 years, 77.4% were men, and 51.6% were hospitalized in the intensive care unit. Ischemia was the main cause of AKI (83.9%), and uremia or azotemia was the main indication for dialysis (86.6%). The ATNISS was 0.56 ± 0.3. The average number of sessions per patient was 6.7, the average delivered Kt/V was 0.38 ± 0.1, and the weekly delivered Kt/V was 2.71 ± 0.6. Mortality was 61%. Table 1 shows the clinical, laboratory, and dialysis characteristics of the study patients.
TABLE 1.
Clinical, Laboratory, and Dialysis Characteristics of Patients with Acute Kidney Injury Undergoing High-Volume Peritoneal Dialysis
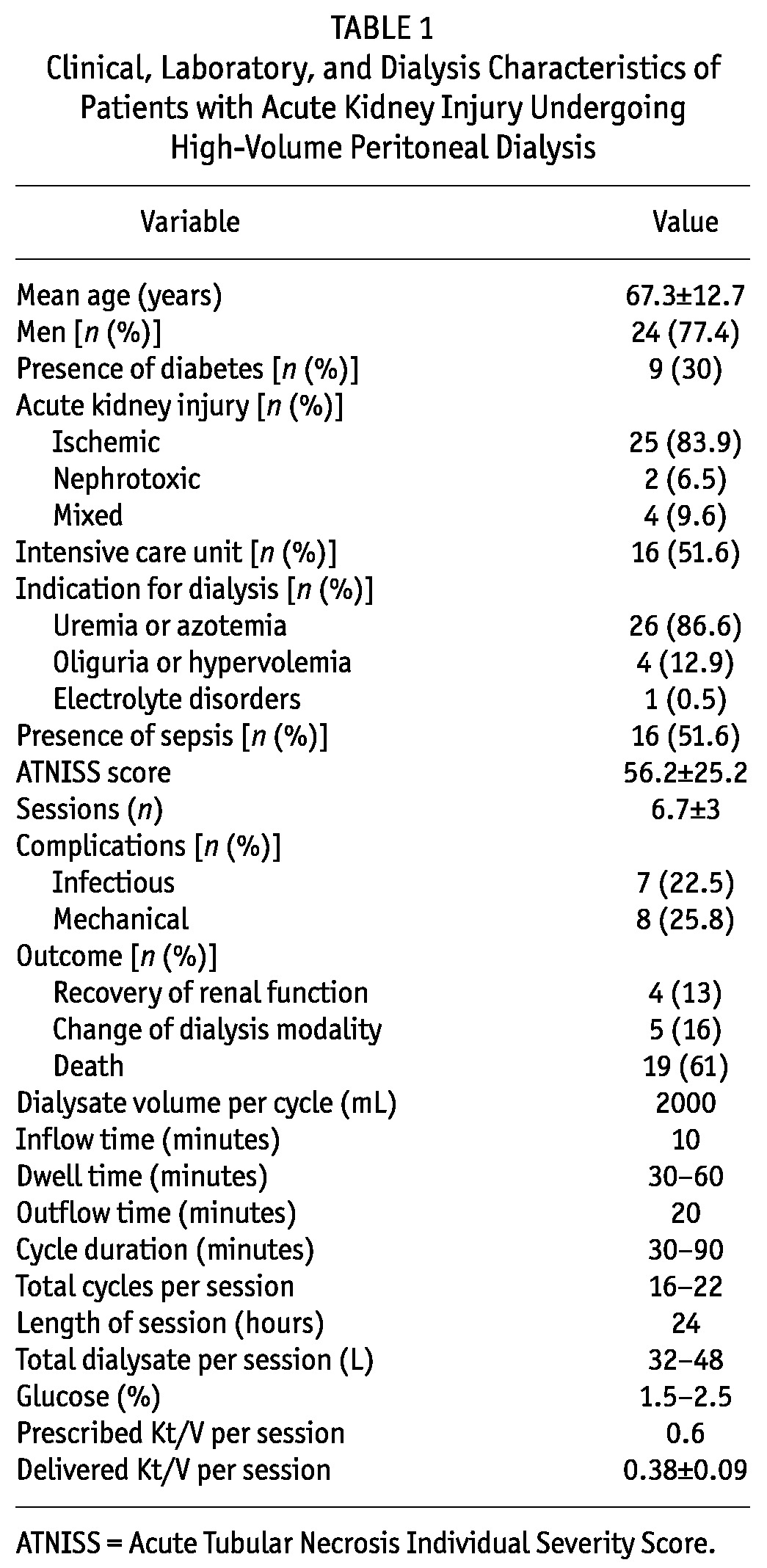
Table 2 shows metabolic and fluid control in the patients. After the second session, serum creatinine stabilized at approximately 2.9 mg/dL. After the third session, urea, pH, and bicarbonate remained stable (137 ± 32 mg/dL, 7.3 ± 0.07, and 20 ± 2.7 mEq/L respectively). Ultrafiltrate remained stable after the second session [1 L (range: 0.1 - 2.4 L)]. Serum sodium was significantly higher before PD and stabilized at approximately 140 mEq/L after PD. No significant difference was found in the concentration of dextrose used per session (2.0 - 2.2 mg/dL). Total protein, albumin, and glucose showed no statistically significant changes during treatment. The sodium concentration in effluent remained stable (127.5 - 131.8 mEq/L) and was lower than the concentration in serum (p = 0.02).
TABLE 2.
Metabolic Control and Parameters of Dialysis in Patients with Acute Kidney Injury Undergoing High-Volume Peritoneal Dialysis (PD)
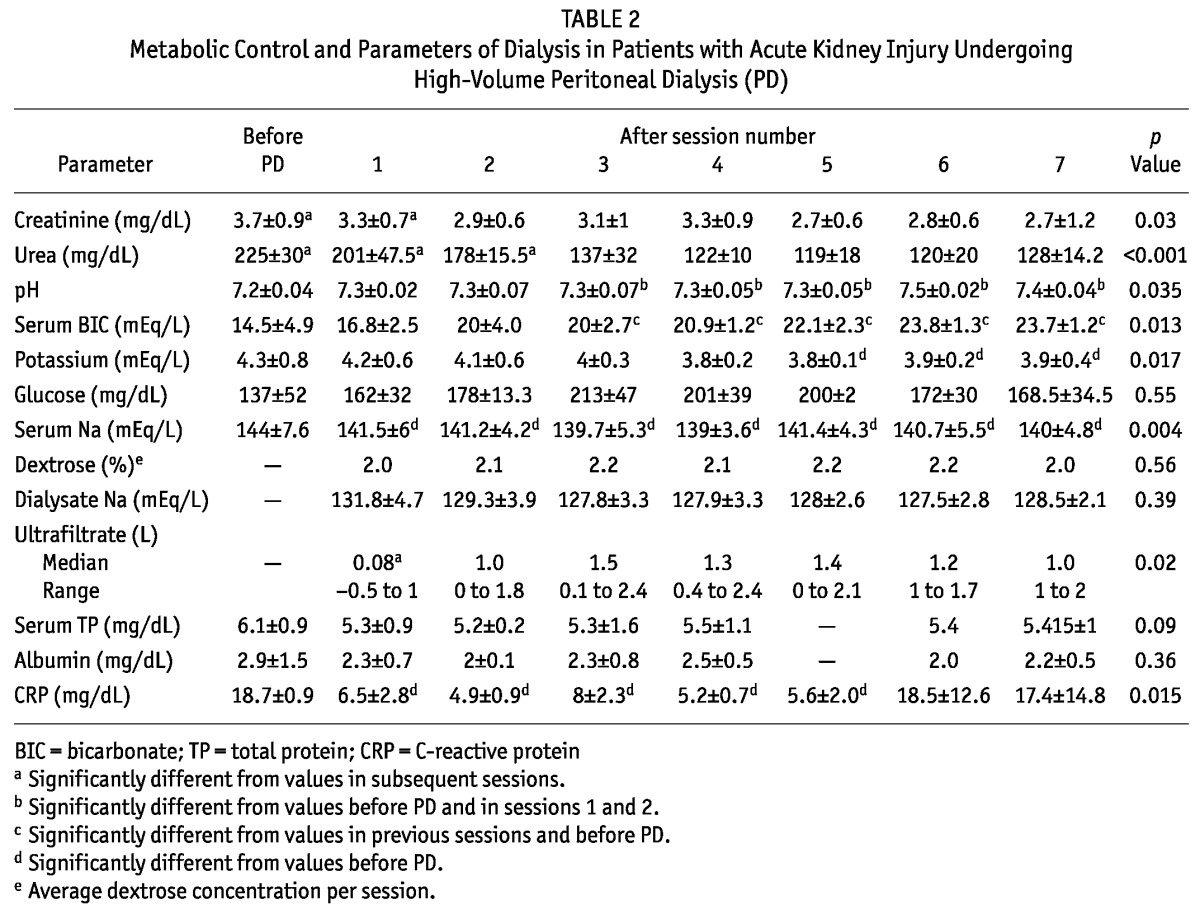
Table 3 shows the metabolic implications of high-volume PD in the patients with AKI. Glucose absorption was constant during treatment, at approximately 35.3% ± 10.5%. Average daily protein loss was 4.2 ± 6.1g, being significantly higher during the first session, declining after the second session, and then remaining stable at approximately 3 g. The NB was initially negative, but after 3 sessions, it was maintained near zero. The UNA remained constant during each session, but sodium removal varied greatly throughout the treatment period.
TABLE 3.
Metabolic Implications of High-Volume Peritoneal Dialysis in Patients with Acute Kidney Injury
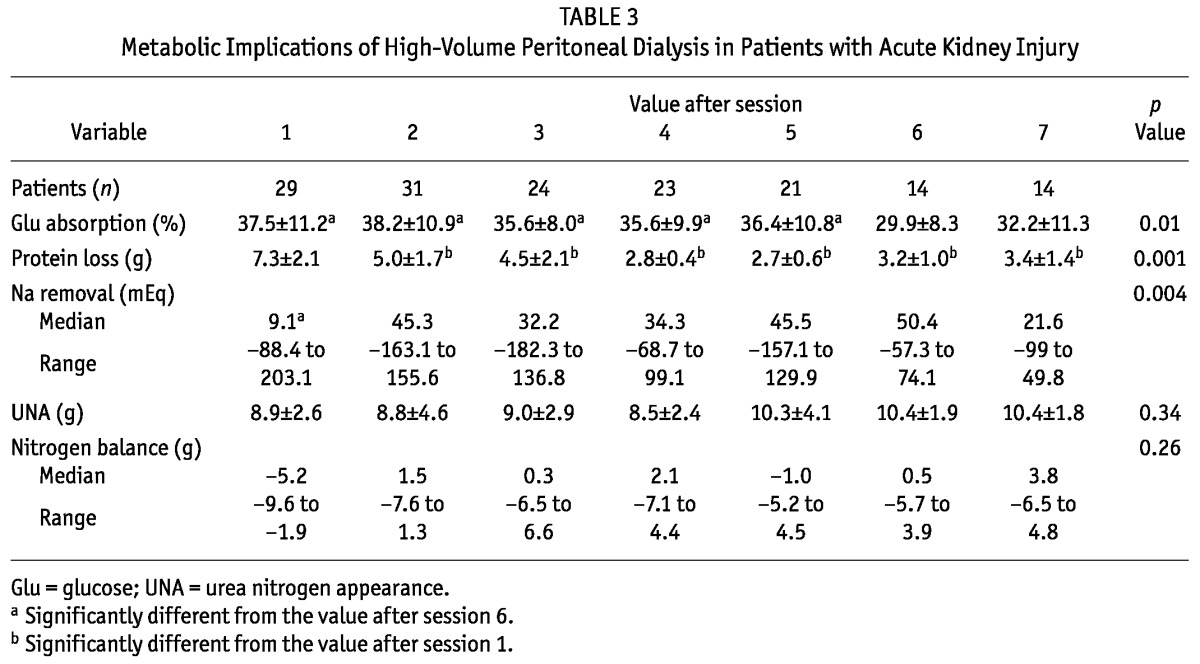
Table 4 presents the correlations between metabolic, clinical, and laboratory parameters. Glucose absorption was higher in patients with higher ATNISS scores, higher CRP levels, higher protein loss, and higher sodium removal (p values of 0.0036, 0.0167, <0.0001, and 0.002 respectively). Patients who absorbed more glucose did not experience increased blood glucose. Glucose absorption (37.6% ± 9.6% vs 36% ± 11.3%) and blood glucose levels (181.5 ± 24.5 mg/dL vs 162 ± 42 mg/dL) were not significantly different between patients with and without diabetes. Of the 31 patients, 22 (71%) received intravenous insulin, and 12 (38.7%) received intraperitoneal insulin after the start of high-volume PD.
TABLE 4.
Spearman Correlations for Metabolic, Clinical, and Laboratory Parameters in Patients with Acute Kidney Injury Treated with High-Volume Peritoneal Dialysis
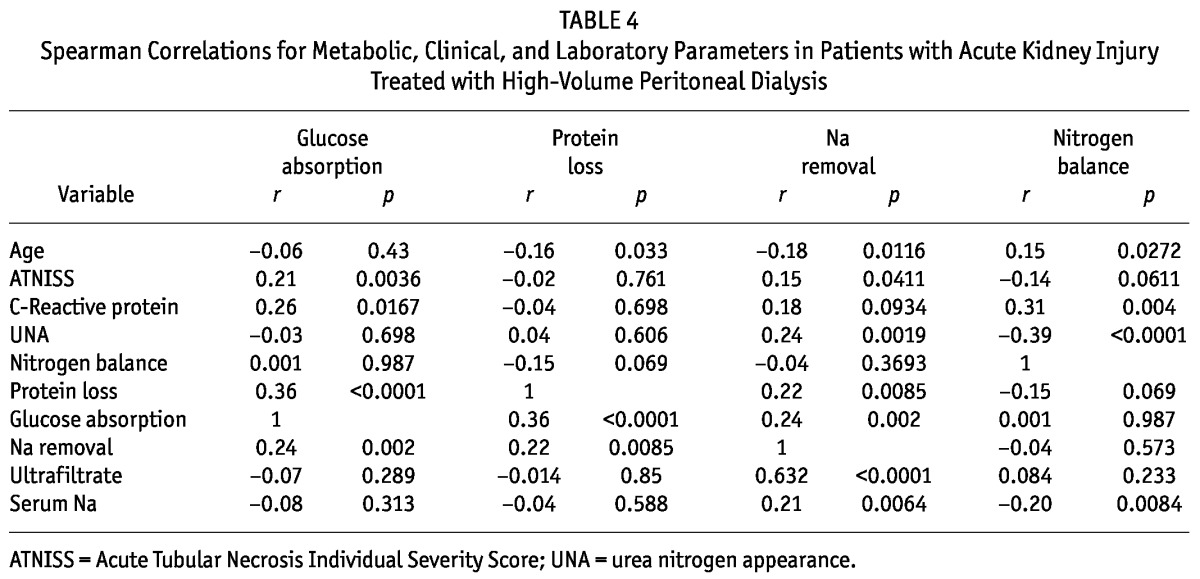
Increased protein losses were observed in younger patients and in those with higher sodium removal (p = 0.033 and 0.0085 respectively). Mean plasma albumin was 2.3 ± 0.86 mg/dL, with no significant change during high-volume PD and no correlation with protein loss. Sodium removal was positively correlated with serum sodium, ATNISS index, UNA, and ultrafiltrate, (p values of 0.0064, 0.0411, 0.0019, and <0.0001 respectively) and negatively correlated with age (p = 0.0116). Nitrogen balance was negatively correlated with UNA (p < 0.0001).
Table 5 shows the clinical parameters associated with metabolic effects. Glucose absorption was higher when the indication for dialysis was an electrolyte disorder than when the indication was uremia (41% ± 12.7% vs 36.4% ± 11.7%, p < 0.0001). Sodium removal was lower in nephrotoxic AKI [-83.9 mEq (range: -189.2 to 19.63 mEq)] than in ischemic AKI [12.1 mEq (range: -147.3 to 136.8 mEq)] or mixed acute tubular necrosis [19.64 mEq (range: -124.1 to 82.7 mEq), p < 0.0001]. Sodium removal was increased in patients in whom dialysis was indicated by fluid overload [54.6 mEq (range: -44.6 to 268.2 mEq)] compared with patients in whom the indication was an electrolyte disturbance [46.5 mEq (range: 9 - 84 mEq)] or uremia [-3.2 mEq (range: -50 to 99.1 mEq), p < 0.0001].
TABLE 5.
Correlationsa Between Clinical and Metabolic Parameters in Patients with Acute Kidney Injury Treated with High Volume Peritoneal Dialysis
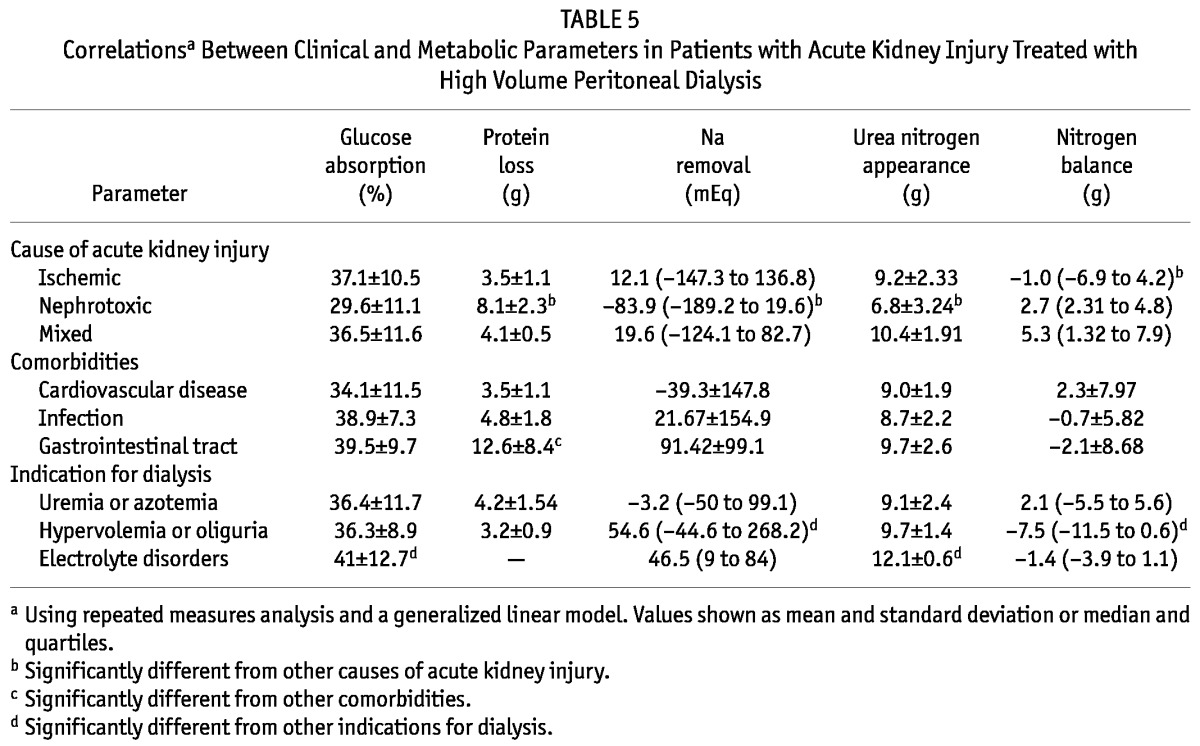
Higher UNA was observed in patients in whom dialysis was indicated by an electrolyte disturbance (12.1 ± 0.6 g) than in those in whom the indication was fluid overload (9.7 ± 1.4 g) or uremia (9.1 ± 2.4 g, p = 0.028). Lower UNA values were observed in patients with nephrotoxic AKI (6.8 ± 3.2 g) than in patients with mixed AKI (10.4 ± 1.9 g) or ischemic AKI (9.2 ± 2.3 g, p < 0.0001). Values for NB were lower in patients with ischemic AKI [-1.0 g (range: -6.9 to 4.2 g)] than in patients with nephrotoxic AKI [2.7 g (range: 2.31 - 4.8 g)] or mixed AKI [5.3 g (range: 1.32 - 7.9 g), p < 0.0001]. Values for NB were also lower (more negative) in patients who required dialysis because of fluid overload [-7.5 g (range: -11.5 to 0.64 g)] than in those with an electrolyte disorder [-1.4 g (range: -3.9 to 1.1 g)] or uremia [2.1 g (range: -5.5 to 5.6 g), p < 0.0001].
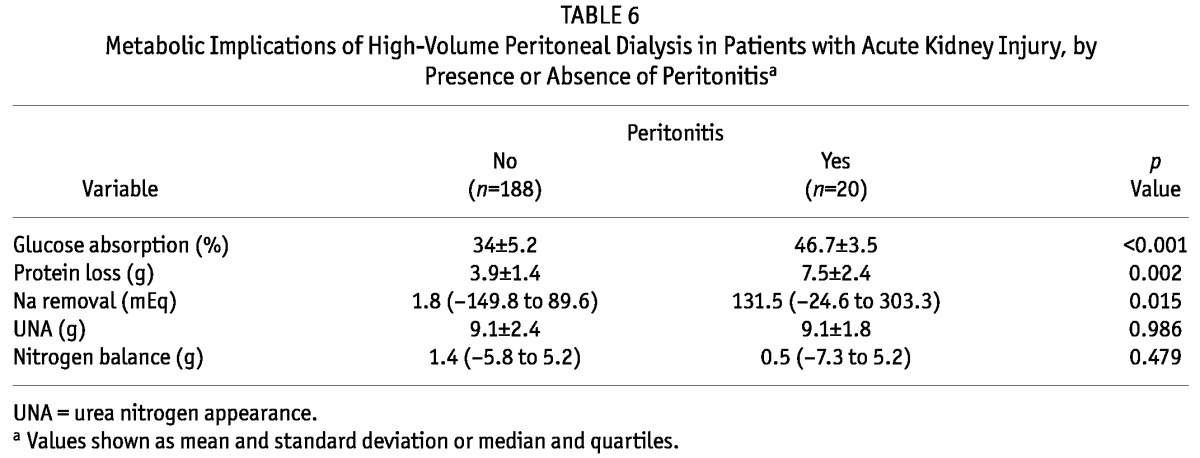
Table 6 shows an evaluation of the metabolic implications of high-volume PD during peritonitis episodes. In patients without and with peritonitis, glucose absorption (34% ± 5.2% vs 46.7% ± 3.5%, p < 0.001), protein loss into dialysate (3.9 ± 1.4 g vs 7.5 ± 2.4 g, p = 0.002), and sodium removal [1.8 mEq (range: -149.8 to -89.6 mEq) vs 131.5 mEq (range -24.6 to 303.3 mEq), p = 0.015] were higher in the presence of infection.
TABLE 6.
Metabolic Implications of High-Volume Peritoneal Dialysis in Patients with Acute Kidney Injury, by Presence or Absence of Peritonitisa
Discussion
Our prospective study aimed to describe the metabolic implications—in terms of glucose uptake, sodium removal, protein loss into dialysate, and catabolism—in patients with AKI undergoing high-volume PD and to identify factors associated with changes in those metabolic variables. Few studies have been published on PD in AKI, and metabolic implications were not evaluated in most (12,13).
In a classic article, Boen (12) discussed the kinetics of PD (using the intermittent method) and changes in the plasma concentrations of several solutes during dialysis. Diffusion curves and peritoneal clearances were determined and compared. Urea diffuses most rapidly and shows the highest peritoneal clearance, followed by potassium, inorganic phosphate, creatinine, uric acid, calcium, and magnesium. The peritoneal clearances of all substances studied showed a rise when the rate of dialysis was increased, until a maximum value was reached at volume of 3.5 L per hour, and after 30 minutes in the peritoneal cavity, 52 - 12 g of glucose was absorbed per liter when the initial concentration in the irrigation fluid was 2% - 3.5%.
Our study showed that glucose absorption from the dialysate was constant during therapy and remained at approximately 35.3% ± 10.5%, below the value proposed by Podel et al. (14), who estimated that glucose absorption in patients with AKI and end-stage renal disease treated by PD would be approximately 40% - 50%. We also observed no significant difference in glucose uptake between patients with and without diabetes. No previous studies have measured glucose absorption from dialysate in patients with AKI undergoing PD. In patients treated with chronic PD, glucose absorption ranges from 60% to 80%, according to Gahl and Hain (15).
Although we observed no significant difference in glycemic control during dialysis, those data must be analyzed critically, because recent studies showed an association between glycemic control and mortality in critically ill patients (16,17). In the present study, after initiation of therapy, serum glucose levels were maintained between 163 mg/dL and 213 mg/dL. According to Basi et al. (18), hyperglycemia is an independent predictor of death in patients with AKI, even after adjustment for age, sex, race, disease severity score for cortisol (a hormone marker of stress), AKI severity, and nutrition status. Although the optimal target for glycemic control is not yet known, the NICE-SUGAR (Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) study (19) showed lower mortality when serum glucose levels were below 180 mg/dL, suggesting that glucose levels in critically ill patients should lie between 110 mg/dL and 180 mg/dL. Thus, in those patients, serum glucose should be monitored frequently (approximately every 6 hours), and intravenous and intraperitoneal insulin should be considered (4). In the present study, 71% of the patients received intravenous insulin, and 38.7% received intraperitoneal insulin after the start of high-volume PD.
In the present study, glucose uptake was influenced by inflammation and disease severity. Uptake was higher in patients with higher CRP levels, in patients with peritonitis, and in patients with a higher ATNISS score. Determining glucose uptake in critically ill patients is important to avoid hyperglycemia caused by hyperalimentation. Inadequate nutrition in this population has distinct effects on immune-inflammatory pathways, is associated with increased morbidity, and might affect survival. Overfeeding can cause metabolic disorders and dysfunction in organs such as lungs and liver (20).
In addition to hyperglycemia, a possible metabolic complication of PD in patients with AKI is protein loss into the dialysate, which may worsen the nutrition status of already compromised patients, causing impaired prognosis (3,4,21). In the present study, daily protein loss was 4.23 ± 2.44 g, lower than reported elsewhere. Previous studies showed that daily protein loss into dialysate may reach 48 g in patients with AKI treated with intermittent PD (12,22-24). In India, Chitalia et al. (13) performed a prospective, randomized study that evaluated 2 modes of automated PD—continuous and tidal—in patients with AKI. The authors concluded that, at the same volume, tidal PD resulted in greater protein loss (10.5 ± 1.5 g vs 6.6 ± 1.2 g, p < 0.001). In another study, Gabriel et al. (3) reported an average daily protein loss of 21.7 g (range: 9.1 - 29.8 g) during treatment with high-volume PD; that loss was aggravated by peritonitis or ascites. In our study, we also observed a greater daily loss of protein in the presence of peritonitis (7.5 ± 2.4 g vs 3.9 ± 1.4 g).
Protein loss was greater in patients with higher glucose uptake and increased sodium removal, consistent with a more permeable peritoneum. Recent studies in end-stage renal disease patients receiving PD support the hypothesis that the peritoneal transport of macromolecules is elevated in high-transport patients, which might be caused by inflammatory mediators (25). In that study, protein loss was not correlated with CRP, but it was negatively correlated with age and positively correlated with the presence of gastrointestinal tract comorbidity, which can be attributed to the inclusion of liver patients with ascites in that group.
As observed in earlier studies (3,5), protein loss into the dialysate does not seem to influence serum albumin, nor is it correlated with a higher degree of catabolism assessed by NB. In our study, glucose absorption and protein loss were both lower than previously reported in the literature, probably as a result of short dwells, which is characteristic of the many cycles and rapid exchanges in high-volume PD. Similar results were observed in earlier studies that compared glucose absorption and protein loss in chronic and acute patients treated using different dwell times (13,26-28).
No other studies have evaluated sodium balance in patients with AKI treated by high-volume PD. Physiologically, the concentration of sodium in dialysate declines during the initial phase of a dwell using hypertonic solution; a gradual rise follows. The minimum value is usually reached after 1 or 2 hours. It is likely that this so-called sieving of sodium is caused by transcellular water transport through the ultrasmall pores (aquaporin-1). However, other mechanisms such as temporal binding of sodium in the interstitial tissue cannot be excluded with certainty. Water transport rates are high during the initial phase of a hypertonic exchange. The decline in dialysate sodium is therefore a dilutional phenomenon, implying that during short dwells with hypertonic dialysate, much more water than sodium is removed from the extracellular volume. The removal of water can lead to hypernatremia (29,30).
The hindrance in the removal of sodium compared with the removal of water is not clinically important during continuous ambulatory PD because the increment in the concentration gradient is counteracted by increased diffusion of sodium. However, during short dwells, as are often applied in automated PD, much more water than sodium can be removed from the extracellular volume (29,30). In that context, it is likely that the concentration of sodium in the dialysate does not have time to equilibrate with the plasma concentration during high-volume PD.
An increase in the glucose concentration is proposed for sodium removal, but the removal of water can still greatly exceed the removal of sodium. Lowering the sodium concentration in the dialysate is another way to increase the diffusion of sodium; however, a low-sodium dialysate is typically limited to just 1 exchange daily, because more frequent use could lead to hyponatremia. Results of long-term studies are not available.
The sodium concentration in dialysate for short-exchange treatments has been studied in patients receiving intermittent PD. A short-term study using 7% glucose-based dialysis solution for 30-minute dwells showed that a dialysate sodium concentration of 110 mmol/L was required to obtain isonatric fluid contraction of the extracellular compartment (30). A study of 3- to 5-day ultrafiltration PD in edematous patients using 2-hour exchanges with either 7% glucose and sodium at a concentration of 120 mmol/L or 4.5% glucose and sodium at a concentration of 130 mmol/L revealed that both combinations could prevent hypernatremia (31).
A short-term study in children with AKI treated with automated PD demonstrated that hypernatremia develops in the patients treated with solutions containing 3.86% glucose and sodium at a concentration of 132 mmol/L. The hypernatremia improved in patients switched to a dialysate containing 3.86% glucose and sodium at 128 mmol/L (32). It can therefore be concluded that the sodium concentration should be lower in dialysis solutions used for automated PD (125 - 130 mmol/L) than in solutions used for continuous ambulatory PD.
In the present study, we did not observe hypernatremia. Sodium removal was variable, being higher in patients with higher serum sodium, showing that high-volume PD does not cause hypernatremia and can correct that condition if necessary. Sodium removal was also positively correlated with ultrafiltrate, ATNISS, and UNA, and inversely correlated with age (sodium removal was higher in younger patients). Sodium removal was also higher in patients with higher catabolism and a worse prognosis. Increased sodium removal was also observed in patients in whom dialysis was indicated because of an electrolyte disorder. Nephrotoxic AKI was negatively correlated with sodium removal.
Our patients were treated with high-volume PD (prescribed Kt/V: 0.5), although no consensus has been reached on the optimal dose of renal replacement therapy for patients with AKI. Data on the effect of dialysis dose on AKI are limited, and the capacity of PD to achieve adequate renal replacement therapy doses in hypercatabolic AKI has been a subject of controversy (4). Some authors showed inadequate metabolic control using PD, but they used a rigid peritoneal catheter, performed exchanges manually, and used short dwells, leading to inadequate solute clearance and low dialysis efficiency (33,34). However, other studies reported positive outcomes with PD in hypercatabolic AKI (3,35). High-volume, tidal, and continuous-flux PD allow for higher doses if desired (4,36).
A recent trial by our group showed no difference in survival for patients with AKI who received a weekly Kt/V of 3 (delivered Kt/V: 0.43 per session) or of 4.2 (delivered Kt/V: 0.6 per session). More studies are needed to determine the ideal delivered PD dose in patients with AKI (37).
Some authors found that hypercatabolic patients treated with PD became more hypercatabolic, but those studies used inadequate parameters to measure protein catabolism (33, 38). The “gold standard” for assessing the degree of protein anabolism and catabolism is NB, but few studies have used it to assess the catabolic state in patients with AKI (34). In our study, high-volume PD did lead to greater catabolism. Levels of UNA remained at approximately 10.2 g per day during dialysis, and NB remained neutral or positive from the 3rd dialysis session onward. In clinical conditions such as AKI, the need for dialysis is related to the extent of catabolism. In our study, NB was lower with ischemic AKI than with other types of AKI; it was also lower in patients who required dialysis for fluid overload rather than for other indications.
Conclusions
Despite some limitations, such as the small number of patients and the lack of multivariate analysis, our study is the first to describe the metabolic implications of high-volume PD in patients with AKI and to identify the main factors associated with those effects. Furthermore, our study confirmed that protein loss and glucose absorption remain constant during therapy and that high-volume PD using dialysate with an increased glucose concentration does not lead to hypernatremia or hypercatabolism in patients with AKI. Those parameters are influenced by clinical conditions, such as the cause of AKI and the presence of inflammation and comorbidities, factors that should be taken into account to avoid metabolic complications such as hyperalimentation, hyperglycemia, and hypercatabolism when dialysis and nutrition are prescribed.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
Our study was partially supported by a grant from the Brazilian funding agency Conselho Nacional de Desenvolvimento Científico e Tecnológico.
References
- 1. Teschner M, Heidland A, Klassen A, Sebekova K, Bahner U. Georg Ganter—a pioneer of peritoneal dialysis and his tragic academic demise at the hand of the Nazi regime. J Nephrol 2004; 17:457–60 [PubMed] [Google Scholar]
- 2. Ponce D, Balbi AL. Peritoneal dialysis in acute kidney injury: a viable alternative. Perit Dial Int 2011; 31:387–9 [DOI] [PubMed] [Google Scholar]
- 3. Gabriel DP, Nascimento GV, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis for acute renal failure. Perit Dial Int 2007; 27:277–82 [PubMed] [Google Scholar]
- 4. Burdmann EA, Chakravarthi R. Peritoneal dialysis in acute kidney injury: lessons learned and applied. Semin Dial 2011; 24:149–56 [DOI] [PubMed] [Google Scholar]
- 5. Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int Suppl 2008; (108):S87–93 [DOI] [PubMed] [Google Scholar]
- 6. Liaño F, Gallego A, Pascual J, García-Martín F, Teruel JL, Marcén R, et al. Prognosis of acute tubular necrosis: an extended prospectively contrasted study. Nephron 1993; 63:21–31 [DOI] [PubMed] [Google Scholar]
- 7. Korbet SM. Acute peritoneal dialysis prescription. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001: 333–42 [Google Scholar]
- 8. Nolph KD, Sorkin MI. Peritoneal dialysis in acute renal failure. In: Brenner BM, Lazarus MJ, eds. Acute Renal Failure. 2nd ed. New York, NY: Churchill Livingstone; 1988: 809–34 [Google Scholar]
- 9. Daugirdas JT, Van Stone JC. Physiologic principles and urea kinetic modeling. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001: 15–45 [Google Scholar]
- 10. Druml W. Nutritional support in acute renal failure. In: Mitch WE, Klahr S, eds. Handbook of Nutrition and the Kidney. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998: 213–36 [Google Scholar]
- 11. Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 1985; 27:58–65 [DOI] [PubMed] [Google Scholar]
- 12. Boen ST. Peritoneal dialysis: a clinical study of factors governing its effectiveness. 1959. Kidney Int Suppl 2008; (108):S5–17 [DOI] [PubMed] [Google Scholar]
- 13. Chitalia VC, Almeida AF, Rai H, Bapat M, Chitalia KV, Acharya VN, et al. Is peritoneal dialysis adequate for hypercatabolic acute renal failure in developing countries? Kidney Int 2002; 61:747–57 [DOI] [PubMed] [Google Scholar]
- 14. Podel J, Hodelin-Wetzel R, Saha DC, Burns G. Glucose absorption in acute peritoneal dialysis. J Ren Nutr 2000; 10:93–7 [DOI] [PubMed] [Google Scholar]
- 15. Gahl GM, Hain H. Nutrition and metabolism in continuous ambulatory peritoneal dialysis. Contrib Nephrol 1990; 84:36–44 [DOI] [PubMed] [Google Scholar]
- 16. Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Curr Opin Clin Nutr Metab Care 2010; 13:198–204 [DOI] [PubMed] [Google Scholar]
- 17. Bochicchio GV, Joshi M, Bochicchio KM, Pyle A, Johnson SB, Meyer W, et al. Early hyperglycemic control is important in critically injured trauma patients. J Trauma 2007; 63:1353–8 [DOI] [PubMed] [Google Scholar]
- 18. Basi S, Pupim LB, Simmons EM, Sezer MT, Shyr Y, Freedman S, et al. Insulin resistance in critically ill patients with acute renal failure. Am J Physiol Renal Physiol 2005; 289:F259–64 [DOI] [PubMed] [Google Scholar]
- 19. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. on behalf of the NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–97 [DOI] [PubMed] [Google Scholar]
- 20. De Waele E, Spapen H, Honoré PM, Mattens S, Rose T, Huyghens L. Bedside calculation of energy expenditure does not guarantee adequate caloric prescription in long-term mechanically ventilated critically ill patients: a quality control study. ScientificWorldJournal 2012; 2012:909564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiaccadori E, Cremaschi E, Regolisti G. Nutritional assessment and delivery in renal replacement therapy patients. Semin Dial 2011; 24:169–75 [DOI] [PubMed] [Google Scholar]
- 22. Blumenkrantz MJ, Gahl GM, Kopple JD, Kamdar AV, Jones MR, Kessel M, et al. Protein losses during peritoneal dialysis. Kidney Int 1981; 19:593–602 [DOI] [PubMed] [Google Scholar]
- 23. Cameron JS, Ogg C, Trounce JR. Peritoneal dialysis in hypercatabolic acute renal failure. Lancet 1967; 1:1188–91 [DOI] [PubMed] [Google Scholar]
- 24. Posen GA, Luiscello J. Continuous equilibration peritoneal dialysis in the treatment of acute renal failure. Perit Dial Bull 1980; 1:6–8 [Google Scholar]
- 25. Balafa O, Halbesma N, Struijk DG, Dekker FW, Krediet RT. Peritoneal albumin and protein losses do not predict outcome in peritoneal dialysis patients. Clin J Am Soc Nephrol 2011; 6:561–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tjiong HL, Zijlstra FJ, Rietveld T, Wattimena JL, Huijmans JGM, Swart GR, et al. Peritoneal protein losses and cytokine generation in automated peritoneal dialysis with combined amino acids and glucose solutions. Mediators Inflamm 2007; 2007:97272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perez RA, Blake PG, McMurray S, Mupas L, Oreopoulos DG. What is the optimal frequency of cycling in automated peritoneal dialysis? Perit Dial Int 2000; 20:548–56 [PubMed] [Google Scholar]
- 28. Westra WM, Kopple JD, Krediet RT, Appell M, Mehrotra R. Dietary protein requirements and dialysate protein losses in chronic peritoneal dialysis patients. Perit Dial Int 2007; 27:192–5 [PubMed] [Google Scholar]
- 29. Struijk DG, Krediet RT. Sodium balance in automated peritoneal dialysis. Perit Dial Int 2000; 20(Suppl 2):S101–5 [PubMed] [Google Scholar]
- 30. Ahearn DJ, Nolph KD. Controlled sodium removal with peritoneal dialysis. Trans Am Soc Artif Intern Organs 1972; 18:423-8,440 [DOI] [PubMed] [Google Scholar]
- 31. Raja RM, Kramer MS, Rosenbaum JL, Manchanda R, Lazaro N. Evaluation of hypertonic peritoneal dialysis solutions with low sodium. Nephron 1973; 11:342–53 [DOI] [PubMed] [Google Scholar]
- 32. Vande Walle J, Raes A, Castillo D, Lutz-Dettinger N, Dejaegher A. Advantages of HCO3 solution with low sodium concentration over standard lactate solutions for acute peritoneal dialysis. Adv Perit Dial 1997; 13:179–82 [PubMed] [Google Scholar]
- 33. Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med 2002; 347:895–902 [DOI] [PubMed] [Google Scholar]
- 34. Wooley JA, Btaiche IF, Good KL. Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nutr Clin Pract 2005; 20:176–91 [DOI] [PubMed] [Google Scholar]
- 35. Amerling R, Merouani A. Continuous-flow peritoneal dialysis as acute therapy. In: Ronco C, Bellomo R, Kellum JA, eds. Critical Care Nephrology. 2nd ed. Philadelphia, PA: Saunders/Elsevier; 2009:1509–15 [Google Scholar]
- 36. Ponce D, Balbi AL, Amerling R. Advances in peritoneal dialysis in acute kidney injury. Blood Purif 2012; 34:107–16 [DOI] [PubMed] [Google Scholar]
- 37. Ponce D, Brito GA, Abrão JG, Balb AL. Different prescribed doses of high-volume peritoneal dialysis and outcome of patients with acute kidney injury. Adv Perit Dial 2011; 27:118–24 [PubMed] [Google Scholar]
- 38. Mehta RL, Letteri JM. Current status of renal replacement therapy for acute renal failure. A survey of US nephrologists. The National Kidney Foundation Council on Dialysis. Am. J. Nephrol. 1999;19(3):377–82 [DOI] [PubMed] [Google Scholar]


