Abstract
♦ Background: Although several studies have demonstrated the economic advantages of peritoneal dialysis (PD) over hemodialysis (HD), few reports in the literature have compared the costs of HD and PD access. The aim of the present study was to compare the resources required to establish and maintain the dialysis access in patients who initiated HD with a tunneled cuffed catheter (TCC) or an arteriovenous fistula (AVF) and in patients who initiated PD.
♦ Methods: We retrospectively analyzed the 152 chronic kidney disease patients who consecutively initiated dialysis treatment at our institution in 2008 (HD-AVF, n = 65; HD-CVC, n = 45; PD, n = 42). Detailed clinical and demographic information and data on access type were collected for all patients. A comprehensive measure of total dialysis access costs, including surgery, radiology, hospitalization for access complications, physician costs, and transportation costs was obtained at year 1 using an intention-to-treat approach. All resources used were valued using 2010 prices, and costs are reported in 2010 euros.
♦ Results: Compared with the HD-AVF and HD-TCC modalities, PD was associated with a significantly lower risk of access-related interventions (adjusted rate ratios: 1.572 and 1.433 respectively; 95% confidence intervals: 1.253 to 1.891 and 1.069 to 1.797). The mean dialysis access-related costs per patient-year at risk were €1171.6 [median: €608.8; interquartile range (IQR): €563.1 - €936.7] for PD, €1555.2 (median: €783.9; IQR: €371.4 - €1571.7) for HD-AVF, and €4208.2 (median: €1252.4; IQR: €947.9 - €2983.5) for HD-TCC (p < 0.001). In multivariate analysis, total dialysis access costs were significantly higher for the HD-TCC modality than for either PD or HD-AVF (β = -0.53; 95% CI: -1.03 to -0.02; and β = -0.50; 95% CI: -0.96 to -0.04).
♦ Conclusions: Compared with patients initiating HD, those initiating PD required fewer resources to establish and maintain a dialysis access during the first year of treatment.
Keywords: Cost analysis, health economics, hemodialysis, dialysis access, vascular access, peritoneal catheter
End-stage renal disease (ESRD) patients who choose hemodialysis (HD) require a vascular access, and those who choose peritoneal dialysis (PD) require a peritoneal catheter before initiation of renal replacement therapy (RRT). The type of vascular access used in HD patients is recognized to have a significant influence on patient survival. Compared with use of a native arterio-venous fistula (AVF), use of a tunneled cuffed catheter (TCC) is associated with a substantially greater risk of sepsis, hospitalization, and mortality (1-8). By contrast, PD catheter complications have declined in recent years, with low rates of bacteremia and sepsis (9-22). Recently, Perl et al. (9) observed that, compared with patients starting PD or starting HD with a functioning AVF, patients starting HD with a TCC had a higher risk of death during the first year. However, that finding didn’t necessarily demonstrate causality between use of a HD catheter and patient death.
Several studies have reported that HD is more expensive than PD, mainly because of costs related to dialysis staff, patient transportation, and overhead (23-30). However, vascular access care accounts for a significant proportion of the health care costs in both incident and prevalent HD patients (31-33). Nonetheless, to our knowledge, few reports have compared the costs of PD and HD access (32). The aim of the present study was to compare the resources required to establish and maintain dialysis access in patients initiating HD with a TCC or with an AVF and in those initiating PD.
Methods
Study Design
Our retrospective cost analysis included local chronic kidney disease patients (age 18 years and older at the start of RRT) who consecutively initiated HD between 1 January 2008 and 1 July 2008, or PD between 1 January 2008 and 1 July 2009 at our hospital.
The study was approved by the Ethics Committee for Health and the Local Institutional Review Board of São João Hospital Centre, EPE, Porto, Portugal.
Patient Cohort
The incidence of ESRD—that is, patients who start any RRT modality for the first time—is higher in Portugal than in other European countries (34). An incidence rate of 217 HD patients and 18 PD patients per million population were registered by the Portuguese Society of Nephrology in 2010. Patients were recruited from the nephrology department of São João Hospital Centre, which is a tertiary-care university hospital responsible for nephrologic medical support to ESRD patients starting RRT in the northwest region of Portugal. Patients were enrolled if they had a diagnosis of end-stage chronic kidney disease according to a nephrologist and if they had received outpatient chronic dialysis treatment. Patients who had previously undergone RRT (HD, PD, or transplantation) and those who restarted during the study period or who transferred to another district immediately after RRT start were excluded. The program provided free choice to patients who were eligible for both therapies, but some patients in the HD group had no choice because of contraindications for PD. Treatment modality was assigned at the time of the first attempt at dialysis access placement, on an intention-to-treat basis. Patients were considered PD patients if they had chosen PD and if an attempt was made to place a PD catheter. Otherwise, the patients were considered HD patients. The HD group was subdivided into patients who underwent AVF creation or TCC placement as a first vascular access. Patients were followed for 1 year from the date of dialysis initiation, or until death or switch from their RRT modality. Because of the relatively lower number of patients who initiated PD between 1 January and 1 July 2008, compared with those who initiated HD, the recruitment period for incident PD patients was extended to July 2009.
A total of 191 chronic kidney disease patients started RRT during the study period (133 HD, 58 PD). Among those 191 patients, 23 HD patients were excluded because of previous RRT (n = 13) or loss to follow-up after transfer to another district (n = 10), and 16 PD patients were excluded because of previous RRT (HD, n = 11; transplantation, n = 5). The remaining 152 patients were included in the final analysis. Of the 110 incident HD patients, 65 underwent AVF creation, and 45 underwent TCC placement. Three cohorts of incident dialysis patients were therefore established: HD-AVF (n = 65), HD-TCC (n = 45), and PD (n = 42).
Data Collection
Clinical information was collected from hospital and dialysis unit records as appropriate. The presence of comorbidity at the enrolment date was assessed by a physician undertaking a complete review of the patient’s records. Information was collected for the 19 variables that constitute the Charlson comorbidity index (35), which has been validated for use in patients with ESRD. Information on all dialysis access surgeries, radiologic imaging studies, and dialysis catheter interventions was collected from our hospital database. Because an access was created before dialysis initiation in some patients, all attempts at dialysis access placement were recorded and included in the final analysis. The clinical records from all hospitalizations for all patients were reviewed by a physician. Information on hospital admissions for which the primary reason for admission was access-related care— as defined by the discharge diagnosis (coded according to the International Classification of Diseases, Ninth Revision)—was captured for all patients.
Procedures
Access Surgery: Peritoneal dialysis-related procedures (PD catheter insertion, replacement, repositioning, or removal; omentectomy; lysis of adhesions; correction of peritoneal leaks and abdominal hernias) were performed by a dedicated group of general surgeons and nephrologists, in the operating room, under general anesthesia. Fistula-related procedures (fistula creation, revision, and ligation) were performed by vascular surgeons in a specialized room, under local anesthesia. Preoperative ultrasonography screening of vessels and peripheral venograms for access planning were not routinely performed.
Diagnostic Imaging: Diagnostic imaging studies included fistulograms, access-directed thrombolysis, and access-related angioplasties—that is, radiology procedures performed as part of access-related care. These procedures were performed by a dedicated interventional nephrologist in the angiographic suite, under local anesthesia (36).
TCC-Related Interventions: Central venous catheter-related interventions included insertion, exchange, and removal. These procedures were performed by nephrologists at the bedside, under local anesthesia. Catheter dysfunction, defined as the complete inability to withdraw blood or the inability to withdraw blood at a sufficient rate to sustain dialysis (blood flow less than 300 mL/min), was routinely managed by dialysis nurses with local instillation of tissue plasminogen activator.
Cost Analysis
Our study was performed from the public administration perspective, including direct medical and nonmedical costs. Annual dialysis access costs were evaluated using a mixed costing method. All resources used were valued using 2010 prices, and costs are reported in 2010 euros.
The resources required to care for a patient’s dialysis access were divided into the categories of access surgery, diagnostic imaging, TCC-related interventions, hospitalization, and patient transportation. Access surgery, diagnostic imaging, and TCC-related intervention costs were obtained using a micro-costing approach:
The professional fee per intervention was determined from the average fee charged by physicians per year.
Technical costs per intervention—including supplies, pharmacy and radiology costs, and additional overhead expenses—were obtained for all procedures.
The “total expense” represents the sum of the technical and overhead costs and the professional fees (37). Cost data for dialysis access-related hospitalizations were extracted from the Ministry of Health and Welfare Ordinance Legislation—Diário da República (1st series, No. 147, 31 July 2009, No. 839, and 2nd series, No. 81, 5 April 2000, clause No. 7376/2000). Costs of patient transport for dialysis access care were included in the analysis (€0.47/1 km).
Outcomes
The primary outcome was the costs related to dialysis access at 1 year from the time of first dialysis. The secondary outcome was the dialysis access-related intervention rate per patient-year.
Statistical Analysis
Data are presented as percentages and means ± standard deviation. Costs are given as means with 95% confidence intervals (CIs). Categorical variables were compared using the Fisher exact test. The Kruskal-Wallis test was used to analyze differences between continuous variables. Rates were calculated for each of the patients by dividing the number of events or procedures by the duration of follow-up in years. Between study groups, the mean intervention rates per patient were compared using Poisson regression. Because costs were not normally distributed, they were log-transformed before statistical testing. Multivariate linear regression was used to assess the impact of various comorbid factors on the dialysis access-related costs. Covariates were included if the baseline difference between the three groups was less than 0.10 in the univariate comparison. To address the impact on costs of variations in duration of follow-up resulting from early death, the year 1 cost of patient care by access type and dialysis modality was calculated by direct extrapolation from the truncated costing period for patients who died during year 1. This approach permitted the cost per patient-year at risk to be reported. All tests were two-sided, and differences were considered significant at p < 0.05. All statistical analyses were performed using the SPSS software application (version 19: SPSS, Chicago, IL, USA).
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of the study population. Compared with the PD patients, the HD-TCC and HD-AVF patients were more likely to be older and to have a higher frequency of diabetes mellitus, coronary artery disease, congestive heart failure, and cerebrovascular disease. Time from referral to dialysis initiation was significantly lower in the HD-TCC patients than in the HD-AVF and PD patients.
TABLE 1.
Baseline Characteristics of Enrolled Patients by Dialysis Modality and Vascular Access Type
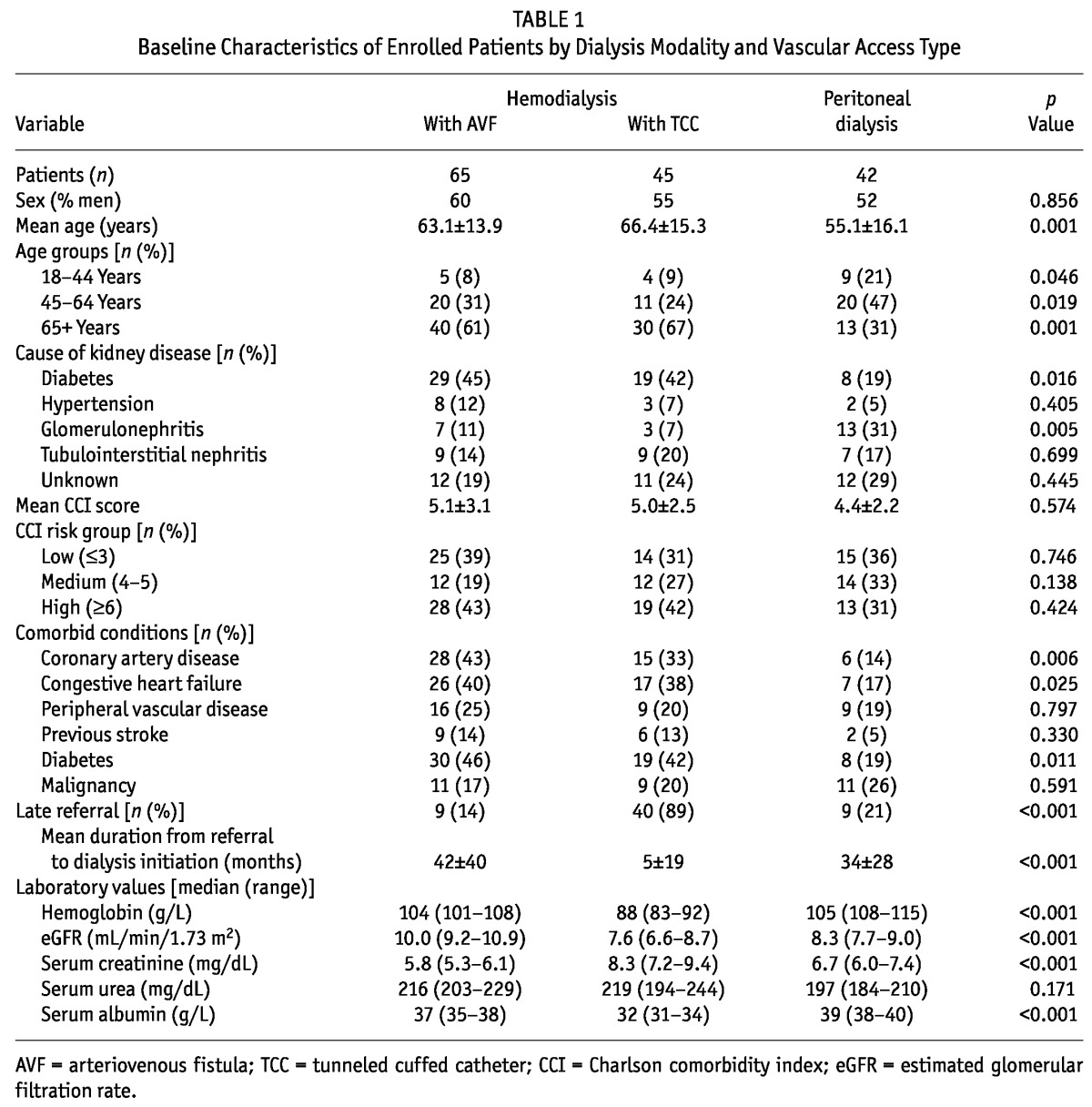
The mean distances between the homes of the HD-AVF, HD-TCC, and PD patients and our hospital center were 42.1 ± 33.9 km, 53.0 ± 33.8 km, and 30.3 ± 23.4 km respectively (p = 0.004).
Resource Use
We were able to assess costs for the full 12-month observation period in 131 of the 152 study patients. For the remaining 21 patients (16 of whom died, 2 of whom received a renal graft, and 3 of whom permanently switched from PD to HD), only the corresponding portion of the 12-month period was costed.
Description of Procedures
Table 2 presents the frequencies and types of invasive procedures performed during the interventions. In the PD group, 76% and 24% of the procedures were related to PD and HD catheters respectively. Eight PD patients used at least 1 HD catheter. The reasons for HD catheter use in the PD group were catheter malfunction (n = 2), peritonitis (n = 2), catheter “break-in” period (n = 2), abdominal leak (n = 1), and requirement for continuous renal replacement therapy (n = 1). In the HD-AVF group, 75% and 25% of the procedures were related to the AVF and the TCC accesses respectively. Eleven patients required at least 1 TCC insertion during dialysis because of AVF failure. In the HD-TCC group, 30% and 70% of the procedures were related to the AVF and TCC accesses respectively. During dialysis, 34 patients underwent at least 1 AVF creation attempt. The primary failure rates (including failed attempts) were 2% for the PD group (1 of 44), 23% for the HD-AVF group (17 of 75), and 9% for HD-CVC group (6 of 67).
TABLE 2.
Invasive Access Interventions by Dialysis Modality and Vascular Access Type
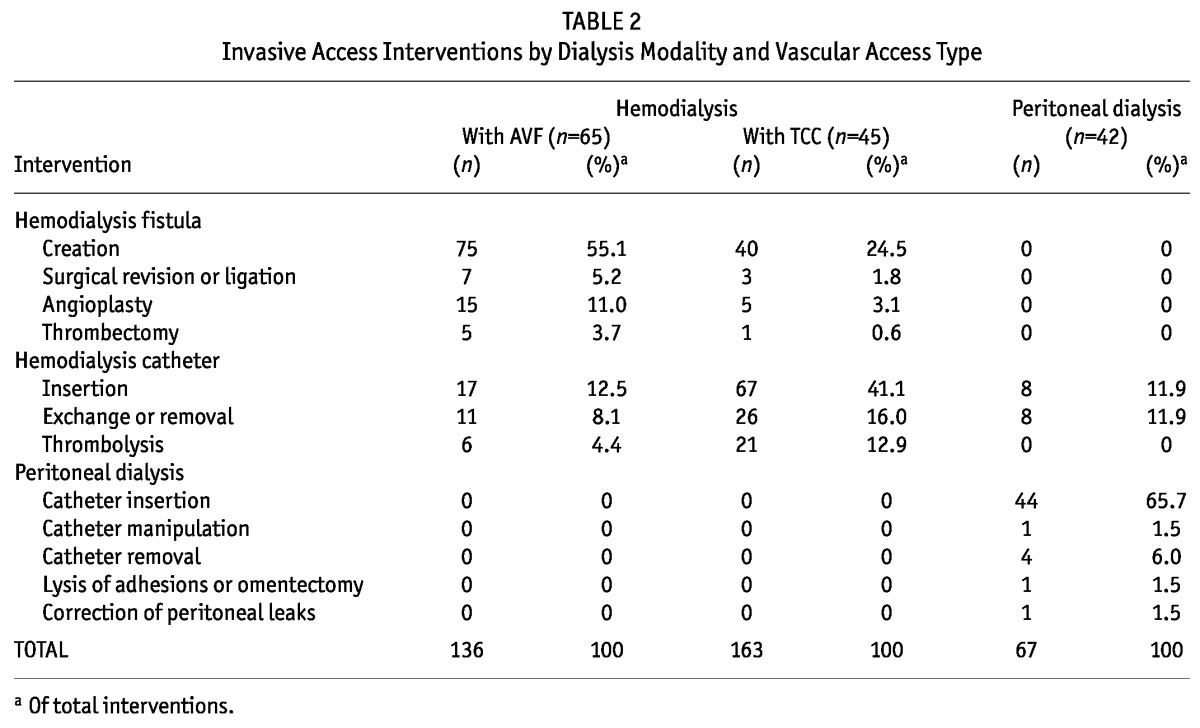
Table 3 lists the mean numbers of interventions in the study population. The mean numbers of access surgeries and diagnostic imaging studies were higher for the HD-AVF group than for the HD-TCC and PD groups (p =0.083 and p < 0.001 respectively). In contrast, the mean numbers of TCC-related interventions and hospitalizations were significantly higher for the HD-TCC group than for either the HD-AVF or the PD group (p < 0.001 and p =0.025 respectively). The main causes of dialysis access-related hospital admissions were peritonitis (n = 4, 67%) for PD patients, access surgery (n = 3, 75%) for HD-AVF patients, and catheter-related bacteremia (n = 13, 81%) for HD-TCC patients. The mean number of bacteremic episodes for HD-TCC patients was 0.58 ± 1.18 per patient-year at risk.
TABLE 3.
Dialysis Access-Related Interventionsa of Enrolled Patients, by Dialysis Modality and Vascular Access Type, per Patient-Year at Risk
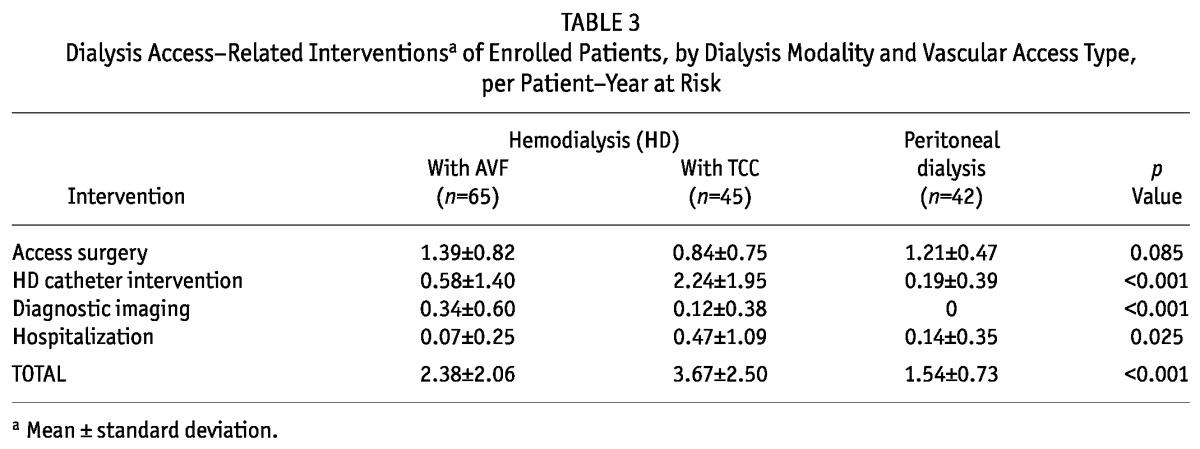
Overall, rates for dialysis access-related interventions were significantly lower in the PD group than in either the HD-AVF or the HD-TCC group (p < 0.001, Table 3). In multivariate analysis, the PD modality was associated with a significantly lower risk of access-related interventions than were the HD-AVF and HD-TCC modalities (adjusted rate ratios: 1.572 and 1.433 respectively; 95% CIs: 1.253 to 1.891 and 1.069 to 1.797). None of the covariates in the models were associated with the risk or rate of intervention.
Cost Analysis
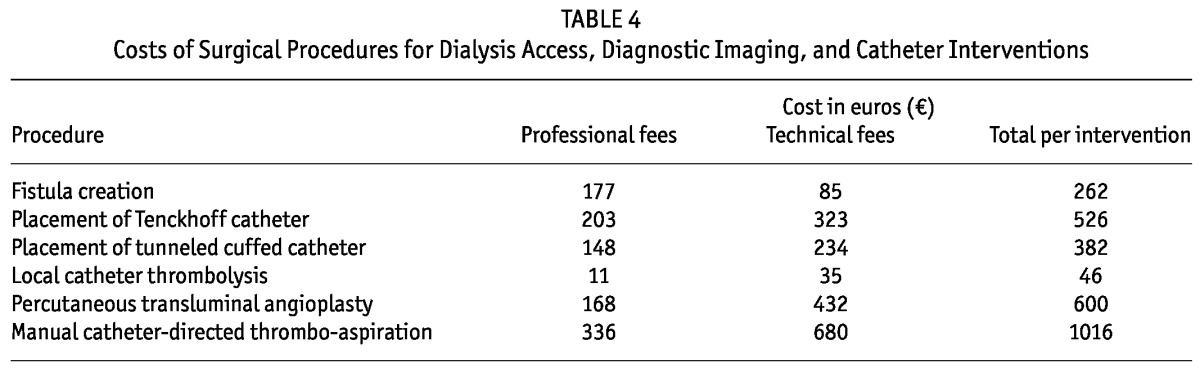
Table 4 sets out the itemized dialysis access-related costs.
TABLE 4.
Costs of Surgical Procedures for Dialysis Access, Diagnostic Imaging, and Catheter Interventions
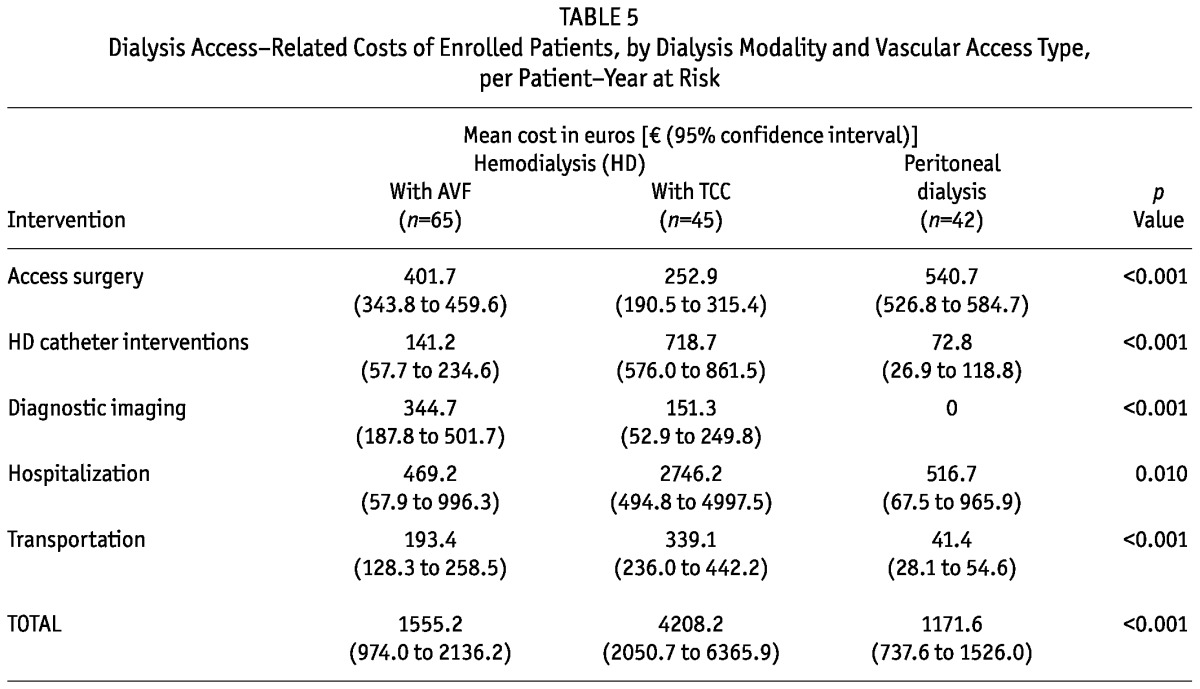
The mean cost of access surgery per patient-year was higher for PD patients than for either the HD-AVF or the HD-TCC patients (p < 0.001, Table 5). On the other hand, the costs of diagnostic imaging procedures were higher for the HD-AVF patients (p < 0.001, Table 5), and the costs of hospitalization related to TCC interventions and of patient transportation were higher for the HD-TCC patients (p =0.010 and p < 0.001 respectively; Table 5). Overall, the mean dialysis access-related costs per patient-year at risk were €1171.6 [median: €608.8; interquartile range (IQR): 563.1 - 936.7] for the PD patients, €1555.2 (median: €783.9; IQR: 371.4 - 1571.7) for the HD-AVF patients, and €4208.2 (median: €1252.4; IQR: 947.9 - 2983.5) for the HD-TCC patients (p < 0.001, Table 5). In multivariate analysis, total access-related costs were significantly higher for the HD-TCC modality than for either the PD or the HD-AVF modality (β = -0.53; 95% CI: -1.03 to -0.02; and β = -0.50; 95% CI: -0.96 to -0.04).
TABLE 5.
Dialysis Access-Related Costs of Enrolled Patients, by Dialysis Modality and Vascular Access Type, per Patient-Year at Risk
Discussion
The present study demonstrates that dialysis access-related intervention rates were significantly lower for patients initiating PD than for those initiating HD. Peritoneal dialysis patients had the lowest numbers of access surgeries and catheter-related interventions. In contrast, HD-AVF patients underwent a higher number of access surgeries and diagnostic imaging procedures, and HD-TCC patients underwent a higher number of catheter-related interventions and hospitalizations (mainly because of catheter-related bacteremia). Our results accord with those of Oliver et al. (38) who recently reported that, compared with patients who chose HD, those who chose PD had a lower risk of invasive access interventions. In addition, we further demonstrated that the risks of catheter-related interventions and hospitalizations were significantly lower with the PD modality than with the HD-TCC modality, emphasizing the fact that patients who choose PD do not face an increased risk of catheter-related adverse events (10,39-41).
Our cost analysis showed that the costs related to dialysis access were lower for the PD modality. Even after considering the additional technical and overhead costs associated with PD catheter placement (operating room, general anesthesia, and surgical team) and the costs associated with primary nonfunction of all access types, patients who initiated PD incurred the lowest costs, and those who initiated HD-TCC, the highest costs during the first year of dialysis. In this regard, Lee et al. (32) reported that costs related to catheter placement and diagnostic imaging procedures accounted for the higher expenditure observed among prevalent HD patients with permanent catheters than among HD-AVF and PD patients. On the other hand, Manns et al. (31) observed that the largest cost component in patients dialyzed exclusively with a HD catheter (rather than an AVF) was hospitalization for access-related complications. In the present study, we observed that, in PD and HD-AVF patients, about 50% of dialysis access costs were related to access surgery, HD catheter interventions, and diagnostic imaging studies; in the HD-TCC group, about 75% of dialysis access costs were related to vascular access-related hospitalizations and patient transportation. In this regard, we observed that HD-TCC patients incurred the highest number of transportation runs (with the highest mean distances) between their homes and our hospital center. Total access-related costs were not statistically significantly different between the PD modality and the HD-AVF modality. Nevertheless, we observed that the costs for invasive interventions related to the dialysis access (mainly diagnostic imaging studies and catheter-related procedures) were higher in the HD-AVF modality. In this regard, Oliver et al. (38) also reported that, compared with PD patients, HD-AVF patients incurred a higher risk of invasive interventions.
The cost factor plays a leading role in health care economics. Because it is not easy to extrapolate costs from one country to another, studies that evaluate local realities are needed to guide appropriate economic decisions about the dialytic management of ESRD patients. Within the Portuguese National Health System, RRT is free of charge for the patient. In 2008, concerned with budget constraints and the exponential annual rise in dialysis costs, the Portuguese health authorities changed the reimbursement system for both HD and PD treatment to a per capita system that includes equipment costs, staff, patient follow-up and checkups, consumables, reverse-osmosis water, regular laboratory tests, radiology, and all medications for the treatment of anemia, bone-mineral disease, nutrition, cardiovascular complications, and in-dialysis intravenous antibiotics. The reimbursement per patient-week was set by law at €547.94 [Ministry of Health and Welfare Ordinance Legislation—Diário da República (2nd series, No. 35, 19 February 2008, clause No. 4325/2008)] for the HD and PD modalities alike. This package did not include vascular and PD access-related procedures, hospitalizations, or patient transportation. Our results, based on patients treated with contemporary dialysis modalities in Portugal, suggest that when a health care reimbursement system is the same for HD and PD, as occurs in Portugal, dialysis access-related costs may account for an approximate 4%, 5%, and 15% increase in annual dialysis treatment expenses for the PD, HD-AVF, and HD-TCC modalities respectively. Our findings accord with those of Manns et al. (31), who reported that HD vascular access costs may account for approximately 10% of the health care cost for incident HD patients, with patients selected for arteriovenous graft or catheter placement incurring the highest costs.
The present study may have important implications for policymakers. For health care systems that are promoting PD as a strategy to lower consumption of health care resources, our study suggests that the resources required to establish and maintain a dialysis access in the first year of treatment are lower for patients who chose PD.
As with all retrospective studies, selection bias may have occurred, in particular influenced by patient treatment preferences and time of referral to the nephrologist. In addition, the time at risk after the first access attempt was different between study groups. Further, the small sample size, short-term follow-up, and single-center nature of the study may limit its reproducibility. Also, the PD patients were treated at a single academic nephrology center, and the HD patients were treated at separate peripheral renal centers (although this situation reflects the distribution of patients between modalities in our country). The costs of certain health care procedures vary between countries. However, the relative resources required for an intervention and the determinants of the costs of vascular access are likely to be similar between centers. Finally, the extrapolation of data may inflate costs in the groups containing sicker patients.
Conclusions
Our study suggests that, compared with patients who initiate HD, those who initiate PD require fewer resources to establish and maintain a dialysis access during the first year of treatment. In addition, our findings emphasize that PD is a cost-effective option for incident dialysis patients.
Disclosures
The authors have no equity interest or financial agreements with any company or commercial entity related to the content of the article and they have not received salary or support from any company related to the article.
Acknowledgments
This study was supported by PEst-OE/SAU/UI0725/2011 from FCT/COMPETE/FEDER. The authors thank Professor João Frazão for assistance with manuscript preparation, Ms. Doreen Raine for editing the English, and the clinical directors of the HD clinics who kindly agreed to collaborate in the study: A. Baldaia Moreira MD, A. Caldeira Gomes MD, A. Castro Henriques MD, Antunes de Azevedo MD, Eva Xavier MD, João C. Fernandes MD, João M. Frazão MD PhD, Jorge P. Baldaia MD, José M. Madureira MD, José Pinheiro MD, Odete Pereira MD, Sofia Pedroso MD, Susana Sampaio MD, and Vasco Miranda MD.
References
- 1. Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J. on behalf of the CHOICE Study. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol 2005; 16:1449–55 [DOI] [PubMed] [Google Scholar]
- 2. Rehman R, Schmidt RJ, Moss AH. Ethical and legal obligation to avoid long-term tunneled catheter access. Clin J Am Soc Nephrol 2009; 4:456–60 [DOI] [PubMed] [Google Scholar]
- 3. Moist LM, Trpeski L, Na Y, Lok CE. Increased hemodialysis catheter use in Canada and associated mortality risk: data from the Canadian Organ Replacement Registry 2001 - 2004. Clin J Am Soc Nephrol 2008; 3:1726–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrero JJ, de Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 2011; 6:1722–30 [DOI] [PubMed] [Google Scholar]
- 5. de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009; 302:1782–9 [DOI] [PubMed] [Google Scholar]
- 6. Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis 2005; 46:501–8 [DOI] [PubMed] [Google Scholar]
- 7. Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis 2006; 47:469–77 [DOI] [PubMed] [Google Scholar]
- 8. Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM. Associates of mortality and hospitalization in hemodialysis: potentially actionable laboratory variables and vascular access. Am J Kidney Dis 2009; 53:79–90 [DOI] [PubMed] [Google Scholar]
- 9. Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 2011; 22:1113–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aslam N, Bernardini J, Fried L, Burr R, Piraino B. Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol 2006; 1:1226–33 [DOI] [PubMed] [Google Scholar]
- 11. Rodrigues A. How to persuade peritoneal dialysis-skeptical hemodialysis fans. Contrib Nephrol 2009; 163:237–42 [DOI] [PubMed] [Google Scholar]
- 12. Dell’Aquila R, Chiaramonte S, Rodighiero MP, Spanó E, Di Loreto P, Kohn CO, et al. Rational choice of peritoneal dialysis catheter. Perit Dial Int 2007; 27(Suppl 2):S119–25 [PubMed] [Google Scholar]
- 13. Gokal R, Alexander S, Ash S, Chen TW, Danielson A, Holmes C, et al. Peritoneal catheters and exit-site practices toward optimum peritoneal access: 1998 update. Perit Dial Int 1998; 18:11–33 [PubMed] [Google Scholar]
- 14. Digenis GE, Abraham G, Savin E, Blake P, Dombros N, Sombolos K, et al. Peritonitis-related deaths in continuous ambulatory peritoneal dialysis (CAPD) patients. Perit Dial Int 1990; 10:45–7 [PubMed] [Google Scholar]
- 15. Wilkie M, Wild J. Peritoneal dialysis access—results from a UK survey. Perit Dial Int 2009; 29:355–7 [PubMed] [Google Scholar]
- 16. Moossavi S, Raasch E, Russell G, Moossavi S, Mauck V, Beekman D, et al. Comparison of dialysis access outcomes in peritoneal dialysis and hemodialysis patients at an academic medical center (Abstract). Perit Dial Int 2007; 27(Suppl 3):S24 [Google Scholar]
- 17. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20:155–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 2010; 21:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heaf JG, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 2002; 17:112–17 [DOI] [PubMed] [Google Scholar]
- 20. Korevaar JC, Feith GW, Dekker FW, van Manen JG, Boeschoten EW, Bossuyt PM, et al. on behalf of the NECOSAD Study Group. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003; 64:2222–8 [DOI] [PubMed] [Google Scholar]
- 21. Hiramatsu M. on behalf of the Japanese Society for Elderly Patients on Peritoneal Dialysis. How to improve survival in geriatric peritoneal dialysis patients. Perit Dial Int 2007; 27(Suppl 2):S185–9 [PubMed] [Google Scholar]
- 22. Huang CC, Cheng KF, Wu HD. Survival analysis: comparing peritoneal dialysis and hemodialysis in Taiwan. Perit Dial Int 2008; 28(Suppl 3):S15–20 [PubMed] [Google Scholar]
- 23. Klarenbach S, Manns B. Economic evaluation of dialysis therapies. Semin Nephrol 2009; 29:524–32 [DOI] [PubMed] [Google Scholar]
- 24. Salonen T, Reina T, Oksa H, Sintonen H, Pasternack A. Cost analysis of renal replacement therapies in Finland. Am J Kidney Dis 2003; 42:1228–38 [DOI] [PubMed] [Google Scholar]
- 25. Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. The cost of renal dialysis in a UK setting—a multicentre study. Nephrol Dial Transplant 2008; 23:1982–9 [DOI] [PubMed] [Google Scholar]
- 26. Sennfalt K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis—a cost-utility analysis. Perit Dial Int 2002; 22:39–47 [PubMed] [Google Scholar]
- 27. Pacheco A, Saffie A, Torres R, Tortella C, Llanos C, Vargas D, et al. Cost/utility study of peritoneal dialysis and hemodialysis in Chile. Perit Dial Int 2007; 27:359–63 [PubMed] [Google Scholar]
- 28. Villa G, Fernández-Ortiz L, Cuervo J, Rebollo P, Selgas R, González T, et al. Cost-effectiveness analysis of the Spanish renal replacement therapy program. Perit Dial Int 2012; 32:192–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Villa G, Rodríguez-Carmona A, Fernández-Ortiz L, Cuervo J, Rebollo P, Otero A, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant 2011; 26:3709–14 [DOI] [PubMed] [Google Scholar]
- 30. Haller M, Gutjahr G, Kramar R, Harnoncourt F, Oberbauer R. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant 2011; 26:2988–95 [DOI] [PubMed] [Google Scholar]
- 31. Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, et al. Establishment and maintenance of vascular access in incident hemodialysis patients: a prospective cost analysis. J Am Soc Nephrol 2005; 16:201–9 [DOI] [PubMed] [Google Scholar]
- 32. Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis 2002; 40:611–22 [DOI] [PubMed] [Google Scholar]
- 33. Allon M, Dinwiddie L, Lacson E, Jr, Latos DL, Lok CE, Steinman T, et al. Medicare reimbursement policies and hemodialysis vascular access outcomes: a need for change. J Am Soc Nephrol 2011; 22:426–30 [DOI] [PubMed] [Google Scholar]
- 34. ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2009. Amsterdam, Netherlands: Academic Medical Center, Department of Medical Informatics; 2011. [Available online at: http://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2009.pdf; accessed 19 January 2013] [Google Scholar]
- 35. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83 [DOI] [PubMed] [Google Scholar]
- 36. Coentrão L, Bizarro P, Ribeiro C, Neto R, Pestana M. Percutaneous treatment of thrombosed arteriovenous fistulas: clinical and economic implications. Clin J Am Soc Nephrol 2010; 5:2245–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bizarro P, Coentrão L, Ribeiro C, Neto R, Pestana M. Endovascular treatment of thrombosed dialysis fistulae: a cumulative cost analysis. Catheter Cardiovasc Interv 2011; 77:1065–70 [DOI] [PubMed] [Google Scholar]
- 38. Oliver MJ, Verrelli M, Zacharias JM, Blake PG, Garg AX, Johnson JF, et al. Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 2012; 27:810–16 [DOI] [PubMed] [Google Scholar]
- 39. Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant 2006; 21(Suppl 2):ii56–9 [DOI] [PubMed] [Google Scholar]
- 40. Povlsen JV. Unplanned start on assisted peritoneal dialysis. Contrib Nephrol 2009; 163:261–3 [DOI] [PubMed] [Google Scholar]
- 41. Povlsen JV, Ivarsen P. Assisted peritoneal dialysis: also for the late referred elderly patient. Perit Dial Int 2008; 28:461–7 [PubMed] [Google Scholar]


