Abstract
♦ Background: This study compared the lifetime costs for peritoneal dialysis (PD) and hemodialysis (HD) patients in Taiwan.
♦ Methods: Using the National Health Insurance (NHI) database of all end-stage renal disease patients on maintenance dialysis registered from July 1997 to December 2005, we matched eligible PD patients with eligible HD patients on age, sex, and diabetes status. The matched patients were followed until 31 December 2006. Patients were excluded if they were less than 18 years of age, had been diagnosed with cancer before dialysis, or had been dialyzed at centers or clinics other than hospitals. Outcomes—including life expectancy, total lifetime costs, and costs per life-year paid by the NHI—were estimated and compared.
♦ Results: The 3136 pairs of matched PD and HD patients had a mean age of 53.2 ± 15.4 years. The total lifetime cost for PD patients (US$139 360 ± US$8 336) was significantly lower than that for HD patients (US$185 235 ± US$9 623, p < 0.001). Except for patients with diabetes (who had a short life expectancy), the total lifetime cost was significantly lower for PD patients than for HD patients regardless of sex and age (p < 0.01).
♦ Conclusion: In Taiwan, the total lifetime costs paid by the NHI were lower for PD than for HD patients.
Keywords: Hemodialysis, lifetime cost
The prevalence and incidence of end-stage renal disease (ESRD) have not only increased worldwide (1), but have also imposed a great financial burden on many countries (2,3). How to provide cost-effective treatment for ESRD patients has therefore become a priority for governments, insurance carriers, and health care providers.
In Taiwan, dialysis costs for all patients with ESRD have been fully reimbursed by the National Health Insurance (NHI) since 1995. The NHI is the only funding source for dialysis providers. It covers the costs of almost all medications and materials needed for dialysis; it does not pay physician or transportation expenses for the patients. Because Taiwan has the highest ESRD prevalence among all countries, with 68 940 patients undergoing dialysis in 2010 (1,4), and because the survival time of dialysis patients in Taiwan is relatively long, with a lower cardiovascular mortality rate than is seen in Western countries (5), ESRD is an enormous financial burden on the country (6).
Hemodialysis (HD) is more popular than peritoneal dialysis (PD) in Taiwan, probably for three reasons. First, older patients in Taiwan tend to depend on medical staff rather than on themselves (especially when facing a devastating disease), and hence, they avoid PD, a more self-dependent treatment. Second, many patients are afraid of developing peritonitis, which has been one of the major causes of death among PD patients in Taiwan (7). Third, because the NHI payments are higher for HD than for PD, HD is chosen as the first-line modality of renal replacement therapy by many nephrologists not practicing in hospitals.
In addition to practicing ESRD prevention by prohibiting the use of herbs that contain aristolochic acid (8), the Taiwanese government has been trying to promote PD because of underutilization (less than 10%) and a lower monthly cost than that for HD (9,10). However, because of ethics concerns and feasibility, the lifetime costs for PD and HD have never been fairly compared using a randomized controlled trial. Thus, in the present study, we used a matching method to compare lifetime survival and direct medical costs paid by the NHI for PD and HD in Taiwan and to provide clinically relevant evidence-based data for policymakers to make the best use of limited resources for ESRD patient care. Dialysis patients in Taiwan usually experience long survival, and so our study also quantified the lifetime medical costs of ESRD dialysis patients for the stakeholders who are concerned about the long-term sustainability of the NHI system.
Methods
Study Subjects
Using the database of ESRD patients registered in the NHI program from July 1997 to December 2005, we identified a study cohort. To secure the comparability of the populations to be examined, patients who were less than 18 years of age, who had been diagnosed with cancer before the initiation of dialysis, who had received maintenance dialysis for less than 3 months (90 days), or who had been dialyzed at centers or clinics other than hospitals were excluded. The study was approved by the Institutional Review Board of National Taiwan University Hospital (201107040RC).
Estimation of Life Expectancy
Using an intention-to-treat design, all eligible patients were followed until 31 December 2006. Patients who had stopped using NHI subsidies for more than 3 months were considered deceased.
Life expectancy estimates (long-term survival) of PD and HD patients calculated using the Monte Carlo method were extrapolated to 50 years (lifetime) from a logit-transformed curve of the survival ratio between PD and HD patients and their respective age- and sex-matched reference populations (referents) using a semiparametric method based on the assumption of a constant excess hazard model (11,12). This method has been successfully applied to study the life expectancy of various kinds of patients having a relatively long survival (13-15). The iSQoL software that was used to estimate life expectancy can be freely downloaded from http://www.stat.sinica.edu.tw/jshwang.
Estimation of Total Lifetime Cost and Cost Per Life-Year
“Total lifetime cost” refers to all outpatient and inpatient medical expenses paid by the NHI for a patient from the start of maintenance dialysis to death, regardless of any modality changes. In other words, “total lifetime cost” equals the sum of lifetime costs for outpatient and inpatient care. The total lifetime cost of dialysis treatment was estimated by summing, for an entire lifetime, the products of the mean survival probability for a dialysis patient in a time period t and the average cost during that period. The mean cost for a patient during a particular time period t1 was calculated by dividing the sum of the costs incurred by each living patient within t1 by the total number of living patients within t1. Cost per patient per year was first adjusted to 2010 monetary values and then summed for the lifetime, after adjustment using the survival probability for each year (that is, total lifetime cost /life expectancy).
Statistical Analysis
Comparisons of demographic characteristics and diabetes status for all eligible PD and HD patients were performed using either the independent t-test or the chi-square test, as appropriate. Each eligible PD patient was then randomly matched with an eligible HD patient on age, sex, and diabetes status. Comparisons of demographic characteristics and diabetes status between the matched PD and HD patients were performed using the same statistical methods.
Life expectancies, total lifetime costs, and costs per life-year for PD and HD patients, assuming an annual 3% discount rate, were then compared using the z-test both before and after matching. Comparisons of the foregoing parameters, stratified by age, sex, and diabetes status, were also performed between the matched PD and HD patients. A series of one-way sensitivity analyses that assumed a 5% discount rate or that included the health care expenses of 1 - 3 months before dialysis start were then evaluated. All analyses were performed using the SAS software application (version 9.1: SAS Institute, Cary, NC, USA), and a two-sided p of 0.05 was set as the cut-off value for statistical significance.
Results
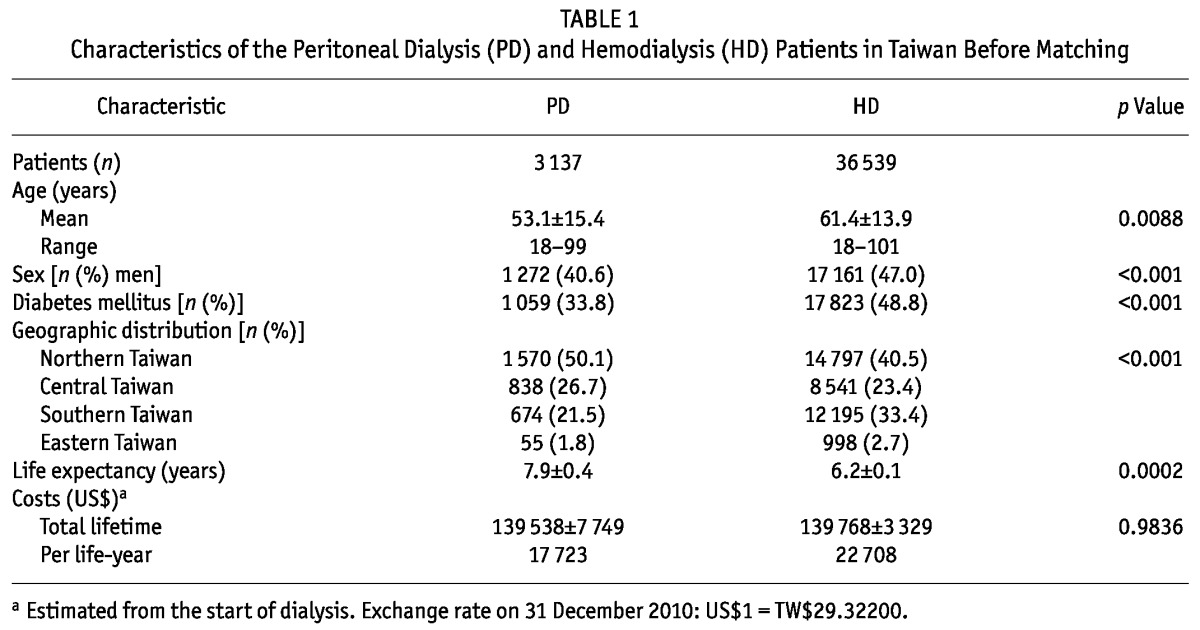
The cohort included 3137 PD patients and 36 539 HD patients (Table 1). Most of the patients, especially those who chose PD, lived in the northern part of Taiwan. Patients on HD, about half of whom were men and had diabetes mellitus, were older and thus had a shorter life expectancy. Before matching, the total lifetime cost was similar for PD patients and for HD patients.
TABLE 1.
Characteristics of the Peritoneal Dialysis (PD) and Hemodialysis (HD) Patients in Taiwan Before Matching
There were 3136 pairs of matched PD and HD patients [Table 2(A)]. Their mean age was 53.2 ± 15.4 years (range: 18 - 99 years). Life expectancies of these PD and HD patients after the start of maintenance dialysis were 7.9 ± 0.5 years and 8.7 ± 0.4 years respectively (p = 0.128). Figure 1 shows similar probabilities of survival across time for these PD and HD patients matched on age, sex, and diabetes status. After matching, the total lifetime cost for a PD patient (US$139 360 ± US$8 336) was noted to be significantly lower than that for a HD patient (US$185 235 ± US$9 623, p < 0.001).
TABLE 2.
Characteristics of the Matched Peritoneal Dialysis (PD) and Hemodialysis (HD) Patients in Taiwan
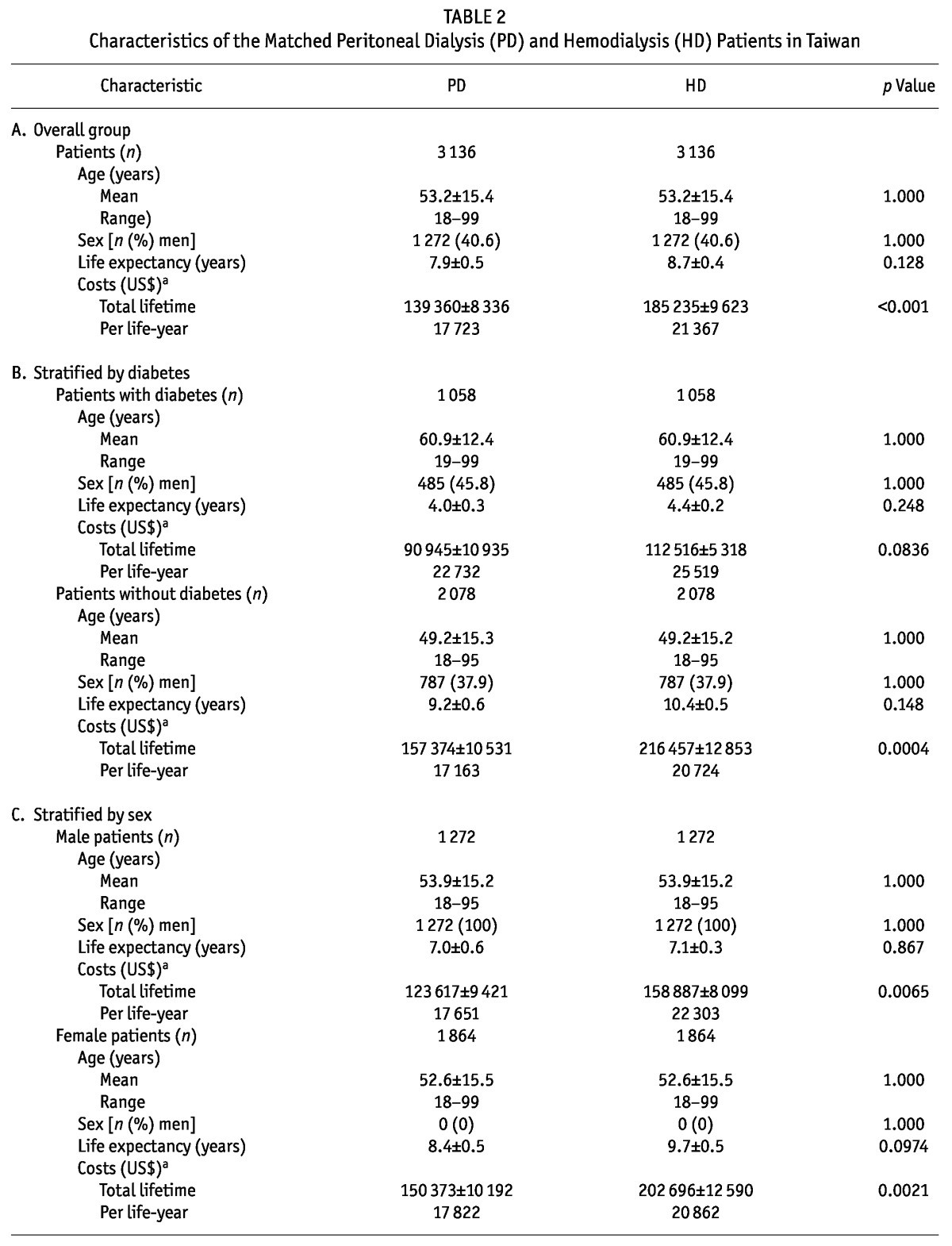
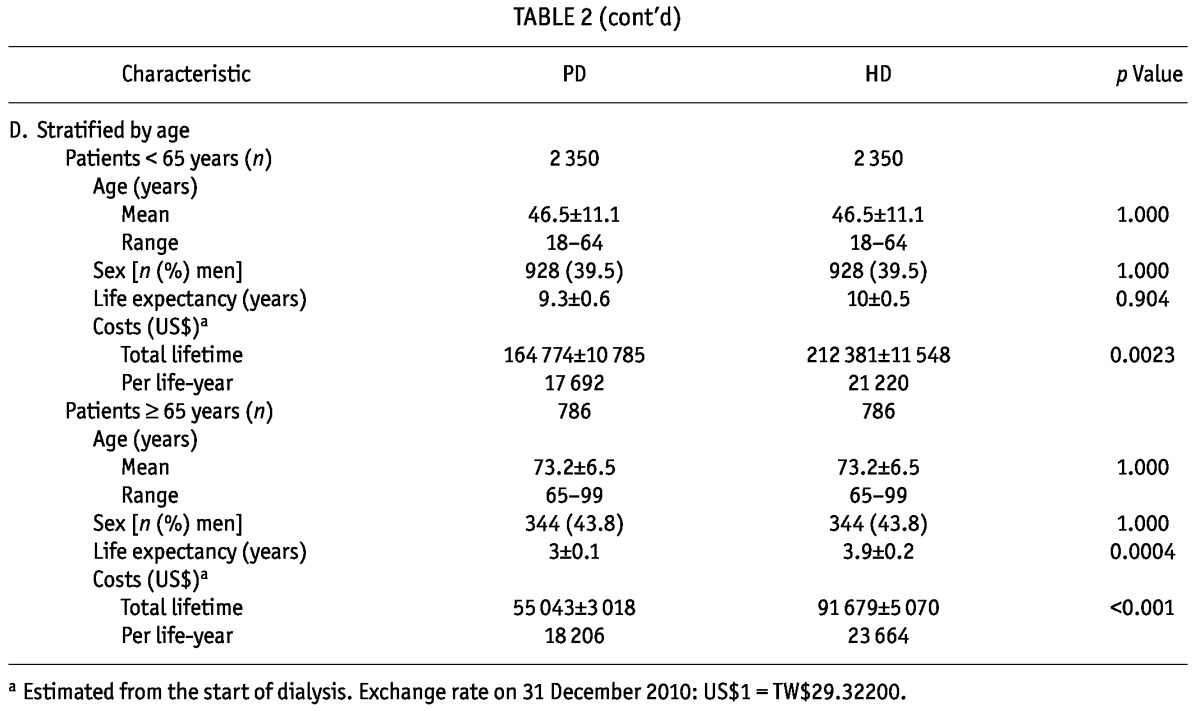
Figure 1 —
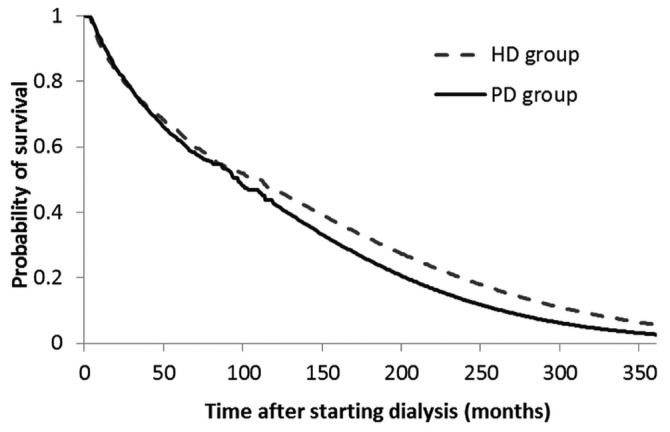
Comparison of the probability of survival across time for 3136 peritoneal dialysis (PD) and hemodialysis (HD) patients in Taiwan, matched for age, sex, and diabetes status.
When the matched patients were separated into groups by diabetes status [Table 2(B)], diabetic patients, whether receiving PD or HD, were found, compared with nondiabetic patients, to be older (mean age: 60.9 ± 12.4 years vs 49.2 ± 15.3 years) and to have a shorter life expectancy (about 4 years vs about 9 - 10 years). In the group with diabetes, PD and HD patients had similar total lifetime costs and costs per life-year (p = 0.0836); in the group without diabetes, PD patients (compared with HD patients) had a significantly lower total lifetime cost and cost per life-year (p =0.0004). Sensitivity analyses assuming annual discount rates of either 3% or 5% showed the same trend (data not shown).
Figure 2 shows the average monthly health care expense adjusted by survival probability and stratified by dialysis type (PD or HD) and diabetes status, plotted across time after starting dialysis. The area under the curve represents the sum of lifetime costs. Based on the intention-to-treat analysis, the curves representing the survival-adjusted monthly costs for diabetic PD and HD patients cross after 7 - 8 years of follow-up. If the analysis is limited to patients treated with one modality only, that phenomenon disappears.
Figure 2 —
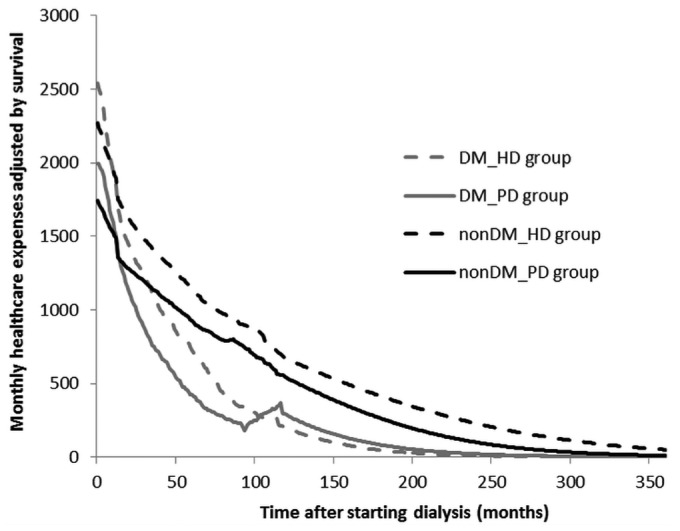
Average monthly health care expenses adjusted by survival probability, stratified by dialysis type and diabetes status, and plotted across time since dialysis start. DM = patients with diabetes mellitus; HD = hemodialysis; PD = peritoneal dialysis; nonDM = patients without diabetes mellitus.
When the matched patients were stratified by sex [Table 2(C)], life expectancy did not seem to be significantly different. However, the total lifetime cost for men on PD was significantly lower than that for men on HD (US$123 617 vs US$158 887, p = 0.0065). Similarly, the total lifetime cost for women on PD was significantly lower than that for women on HD (US$150 373 vs US$202 696, p = 0.0021).
The matched patients were also stratified by age into those less than 65 years of age and those 65 years of age or older [Table 2(D)]. The results showed that the lifetime cost for a PD patient was significantly lower than that for a HD patient in both groups: US$164 774 versus US$212 381 for those less than 65 years of age (p = 0.0023), and US$55 043 versus US$91 679 for those 65 years of age and older (p < 0.001). Comparisons of the cost per life-year for PD and HD patients in the various age groups yielded similar results.
Discussion
Table 1 shows the characteristics of ESRD patients in Taiwan. It can be seen that a larger proportion of PD patients live in Northern Taiwan. The fact that older patients in Taiwan are more dependent on medical staff and are thus more likely to avoid PD (which is a more self-dependent treatment) probably explains why the PD population is younger. As might be expected, the life-expectancy of a PD patient was longer than that of a HD patient. Because, compared with men, women generally have a smaller body size and need fewer daily exchanges to achieve adequate dialysis, the PD population included more women. In contrast, fewer patients with diabetes mellitus were found in the PD population, because glucose-containing PD fluids might affect glycemic control in diabetic patients (16). To be able to fairly compare PD and HD patients in Taiwan, we matched them on age, sex, and diabetes status (Table 2).
One conclusion from this nationwide hospital-based study is that the lifetime cost and the cost per life-year are, in general, both lower for PD patients than for HD patients in Taiwan [Table 2(A)]. A series of one-way sensitivity analyses that assumed a 5% discount rate or that included the health care expenses for 1 - 3 months before dialysis start showed the same trend. In other words, the differences in lifetime cost between PD and HD do not seem to be affected by changes in the discount rate or inclusion of costs from the immediate pre-dialysis phase. The following additional arguments support this finding:
Copayment was waived for all dialysis patients under the NHI scheme.
All patients diagnosed with cancer before dialysis were excluded to prevent any confounding from a comorbidity of this kind.
Every patient receiving PD was matched with a patient receiving HD based on age, sex, and diabetes status— risk factors that may potentially influence survival (17) and health care expenses (18).
Berger et al. (19) also showed that, compared with HD patients, PD patients in America had significantly lower total health care costs over 12 months of treatment. Similarly, in Sweden, the cost per quality-adjusted life year was lower for PD than for HD among all analyzed age groups after 5 years of follow-up (20).
Although most previous studies have focused mainly on the annual cost or cost per life-year (19-21), we analyzed lifetime cost for PD and HD (Tables 1 and 2) to address the long-term sustainability of our NHI system. According to Table 1, the lifetime cost was about the same for PD and HD before matching on various risk factors. However, the results after matching, as summarized in Table 2, consistently reveal a lower lifetime cost for PD, except for patients having comorbid diabetes. This analysis indicates that the long-term financial burden of the NHI can be lowered if PD is chosen as initial treatment for ESRD.
The total lifetime cost of PD and HD for patients with diabetes was, respectively, only about 42% and 48% of the cost for patients without diabetes because of the much shorter life expectancy for diabetic patients [Table 2(B)]. In fact, the cost per life-year of ESRD care was higher for patients with diabetes than for non diabetic patients. Because diabetes mellitus, the most common cause of ESRD (22,23), has been increasing worldwide, the issue of whether patients with diabetes should receive PD or HD deserves special attention. If, as demonstrated in our study, the life expectancy of matched PD and HD patients is the same, then choosing PD first for diabetic patients appears to be more cost-effective. Still, “prevention is better than cure” is a well-known axiom, and there are methods to retard the ESRD incidence among diabetic patients—for example, optimal control of blood glucose (23,24), hypertension (24), and hyperlipidemia (25), and early referral of chronic kidney disease patients to nephrology care (25,26), among others. Yet, how to promote PD initiation remains a great challenge in Taiwan and in other countries that have higher HD penetration.
Life expectancy was not different for men on PD and HD or for women on PD and HD [Table 2(C)]. Given that the total lifetime cost for men or women on HD was significantly higher than the cost for men or women on PD, our suggestion for choosing PD first in ESRD patients without contraindications seems reasonable.
In patients 65 years of age and older, life expectancy was slightly but significantly greater for those on HD than for those on PD. The reason may be that older patients generally have more comorbid conditions and that undergoing HD enables them to receive more intensive care from the dialysis staff. Given that the lifetime cost was significantly lower for PD patients than for HD patients, initiating home visits (27,28) for elderly patients might be a better way to reduce the NHI financial burden without compromising the quality of care during implementation of a “PD First” policy.
Our study has two noteworthy limitations. First, patients who had stopped using NHI subsidies for at least 3 months were considered deceased, meaning that patients who had gone abroad or emigrated were also censored. However, the effect was probably negligible, given that ESRD patients in Taiwan are eligible for dialysis free of charge, and few patients undergoing dialysis would choose to emigrate. Second, neither the lifetime expenses not paid by the NHI (for example, monthly transportation fees and costs for caregivers employed) nor the work status of the patients [the employment rate in PD patients is generally higher (29)] was included in the study. Transportation expenditures incurred by a family member or members accompanying a patient during HD may also directly or indirectly affect the cost of care. If such costs were to be carefully evaluated, the difference in lifetime cost for PD and HD patients might be even greater.
Conclusions
Our study found that both the total lifetime cost and the cost per life-year paid by the NHI were lower for PD patients than for HD patients in Taiwan. At present, more than 65 000 people are receiving long-term dialysis in Taiwan, among whom more than 90% are treated with HD (4). To make more cost-effective use of NHI resources, we recommend a policy of sequential dialysis care with PD first and then HD as required for incident ESRD patients (30-32). Strategies to control diabetes should also be encouraged and implemented.
Disclosures
None of the authors has any financial conflict of interest associated with the contents or publication of this manuscript. The first author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The authors thank the Ta-Tung Kidney Foundation, the Mrs. Hsiu-Chin Lee Kidney Research Fund, and the National Health Research Institutes of Taiwan (intramural project EO-101-EO-PP04) for their support of the present study. We also thank Miss Cecilia C.W. Kao for her proofreading efforts.
References
- 1. United States, Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, US Renal Data System (USRDS). 2011 Atlas of CKD & ESRD [Web resource]. Minneapolis, MN: USRDS; 2011. [Available at: http://www.usrds.org/atlas11.aspx; accessed 7 March 2013] [Google Scholar]
- 2. Kontodimopoulos N, Niakas D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy 2008; 86:85–96 [DOI] [PubMed] [Google Scholar]
- 3. Ranasinghe P, Perera YS, Makarim MF, Wijesinghe A, Wanigasuriya K. The costs in provision of haemodialysis in a developing country: a multi-centered study. BMC Nephrol 2011; 12:42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taiwan Society of Nephrology (TSN). History and Mission (Web page). Taipei, Taiwan: TSN; n.d. [Available at: http://www.tsn.org.tw/englishVersion/History.aspx; accessed 7 March 2013] [Google Scholar]
- 5. Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462,293 adults in Taiwan. Lancet 2008; 371:2173–82 [DOI] [PubMed] [Google Scholar]
- 6. Yang WC, Hwang SJ. on behalf of the Taiwan Society of Nephrology. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transplant 2008; 23:3977–82 [DOI] [PubMed] [Google Scholar]
- 7. Shiao CC, Kao TW, Hung KY, Chen YC, Wu MS, Chu TS, et al. Seven-year follow-up of peritoneal dialysis patients in Taiwan. Perit Dial Int 2009; 29:450–7 [PubMed] [Google Scholar]
- 8. Lai MN, Lai JN, Chen PC, Hsieh SC, Hu FC, Wang JD. Risks of kidney failure associated with consumption of herbal products containing mu tong or fangchi: a population-based case-control study. Am J Kidney Dis 2010; 55:507–18 [DOI] [PubMed] [Google Scholar]
- 9. Government of Taiwan, Department of Health. Bureau of National Health Insurance [Web page]. Taipai, Taiwan: Department of Health; 2006. [Available online at: http://www.nhi.gov.tw/english/index.aspx; accessed 7 March 2013] [Google Scholar]
- 10. Just PM, Riella MC, Tschosik EA, Noe LL, Bhattacharyya SK, de Charro F. Economic evaluations of dialysis treatment modalities. Health Policy 2008; 86:163–80 [DOI] [PubMed] [Google Scholar]
- 11. Hwang JS, Tsauo JY, Wang JD. Estimation of expected quality adjusted survival by cross-sectional survey. Stat Med 1996; 15:93–102 [DOI] [PubMed] [Google Scholar]
- 12. Hwang JS, Wang JD. Monte Carlo estimation of extrapolation of quality-adjusted survival for follow-up studies. Stat Med 1999; 18:1627–40 [DOI] [PubMed] [Google Scholar]
- 13. Fang CT, Chang YY, Hsu HM, Twu SJ, Chen KT, Lin CC, et al. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM 2007; 100:97–105 [DOI] [PubMed] [Google Scholar]
- 14. Kao TW, Huang JW, Hung KY, Chang YY, Chen PC, Yen CJ, et al. Life expectancy, expected years of life lost and survival of hemodialysis and peritoneal dialysis patients. J Nephrol 2010; 23:677–82 [PubMed] [Google Scholar]
- 15. Hung MC, Yan YH, Fan PS, Lin MS, Chen CR, Kuo LC, et al. Estimation of quality-adjusted life expectancy in patients under prolonged mechanical ventilation. Value Health 2011; 14:347–53 [DOI] [PubMed] [Google Scholar]
- 16. Huang CC. Treatment targets for diabetic patients on peritoneal dialysis: any evidence? Perit Dial Int 2007; 27(Suppl 2):S176–9 [PubMed] [Google Scholar]
- 17. Villar E, Remontet L, Labeeuw M, Ecochard R. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol 2007; 18:2125–34 [DOI] [PubMed] [Google Scholar]
- 18. Yang WC, Hwang SJ, Chiang SS, Chen HF, Tsai ST. The impact of diabetes on economic costs in dialysis patients: experiences in Taiwan. Diabetes Res Clin Pract 2001; 54(Suppl 1):S47–54 [DOI] [PubMed] [Google Scholar]
- 19. Berger A, Edelsberg J, Inglese GW, Bhattacharyya SK, Oster G. Cost comparison of peritoneal dialysis versus hemodialysis in end-stage renal disease. Am J Manag Care 2009; 15:509–18 [PubMed] [Google Scholar]
- 20. Sennfält K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis—a cost-utility analysis. Perit Dial Int 2002; 22:39–47 [PubMed] [Google Scholar]
- 21. Pacheco A, Saffie A, Torres R, Tortella C, Llanos C, Vargas D, et al. Cost/utility study of peritoneal dialysis and hemodialysis in Chile. Perit Dial Int 2007; 27:359–63 [PubMed] [Google Scholar]
- 22. Gupta A, Gupta P, Biyani M. Targeted therapies in diabetic nephropathy: an update. J Nephrol 2011; 24:686–95 [DOI] [PubMed] [Google Scholar]
- 23. Fioretto P, Bruseghin M, Berto I, Gallina P, Manzato E, Mussap M. Renal protection in diabetes: role of glycemic control. J Am Soc Nephrol 2006; 17(Suppl 2):S86–9 [DOI] [PubMed] [Google Scholar]
- 24. Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD). Nephrol Dial Transplant 2012; 27:1847–54 [DOI] [PubMed] [Google Scholar]
- 25. Slade H, Williams SM, Manning PJ, Walker RJ. High-risk diabetic nephropathy patients: the outcome of evidence-based clinical practice in an outpatient clinic. Diabetes Res Clin Pract 2011; 92:356–60 [DOI] [PubMed] [Google Scholar]
- 26. Chan MR, Dall AT, Fletcher KE, Lu N, Trivedi H. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med 2007; 120:1063–70 [DOI] [PubMed] [Google Scholar]
- 27. Warmington V. A home visit program for CAPD. Perit Dial Int 1996; 16(Suppl 1):S475–8 [PubMed] [Google Scholar]
- 28. Nayak KS, Sinoj KA, Subhramanyam SV, Mary B, Rao NVV. Our experience of home visits in city and rural areas. Perit Dial Int 2007; 27(Suppl 2):S27–31 [PubMed] [Google Scholar]
- 29. Helanterä I, Haapio M, Koskinen P, Grönhagen-Riska C, Finne P. Employment of patients receiving maintenance dialysis and after kidney transplant: a cross-sectional study from Finland. Am J Kidney Dis 2012; 59:700–6 [DOI] [PubMed] [Google Scholar]
- 30. Thodis ED, Oreopoulos DG. Home dialysis first: a new paradigm for new ESRD patients. J Nephrol 2011; 24:398–404 [DOI] [PubMed] [Google Scholar]
- 31. Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health 2007; 10:61–72 [DOI] [PubMed] [Google Scholar]
- 32. Villa G, Rodríguez-Carmona A, Fernández-Ortiz L, Cuervo J, Rebollo P, Otero A, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant 2011; 26:3709–14 [DOI] [PubMed] [Google Scholar]


