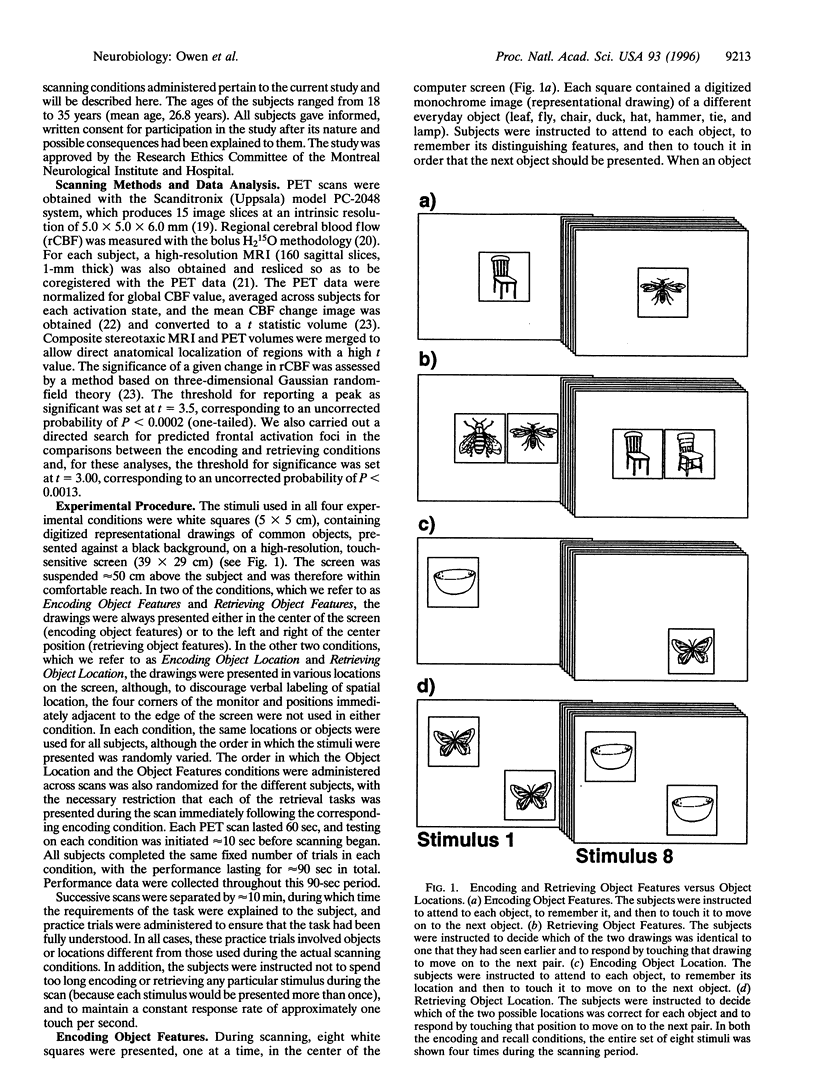
Abstract
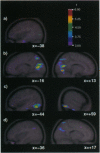
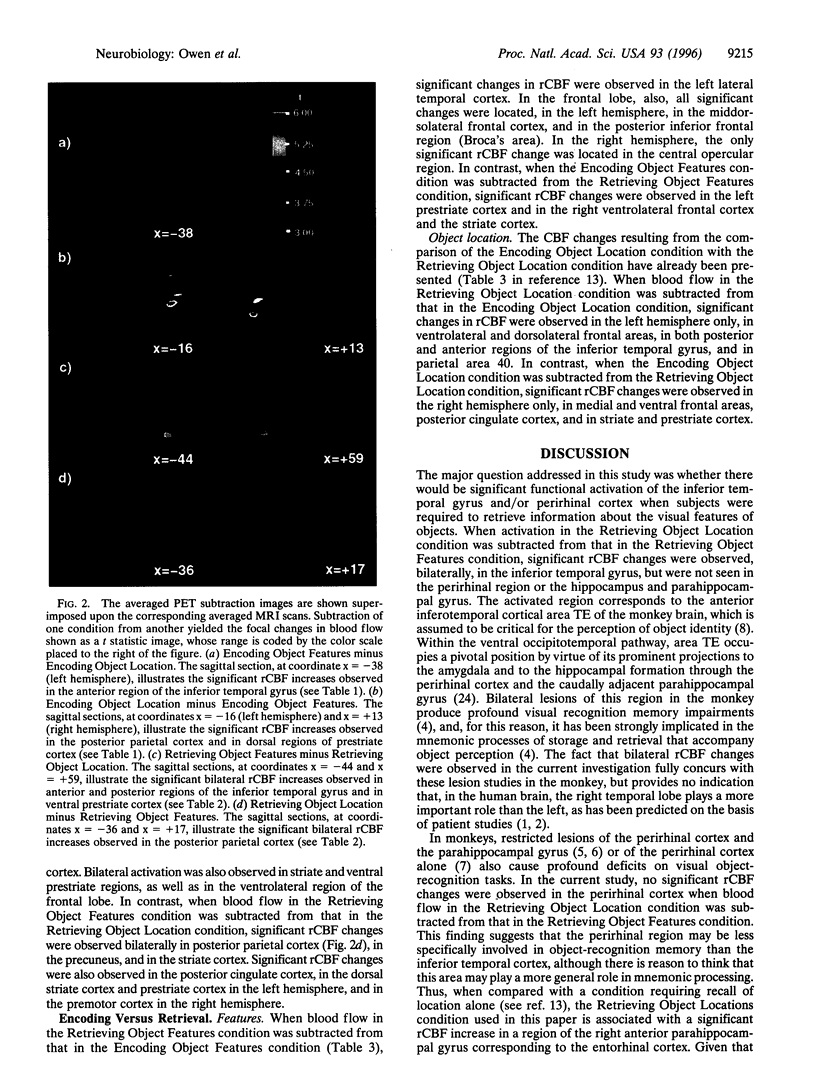
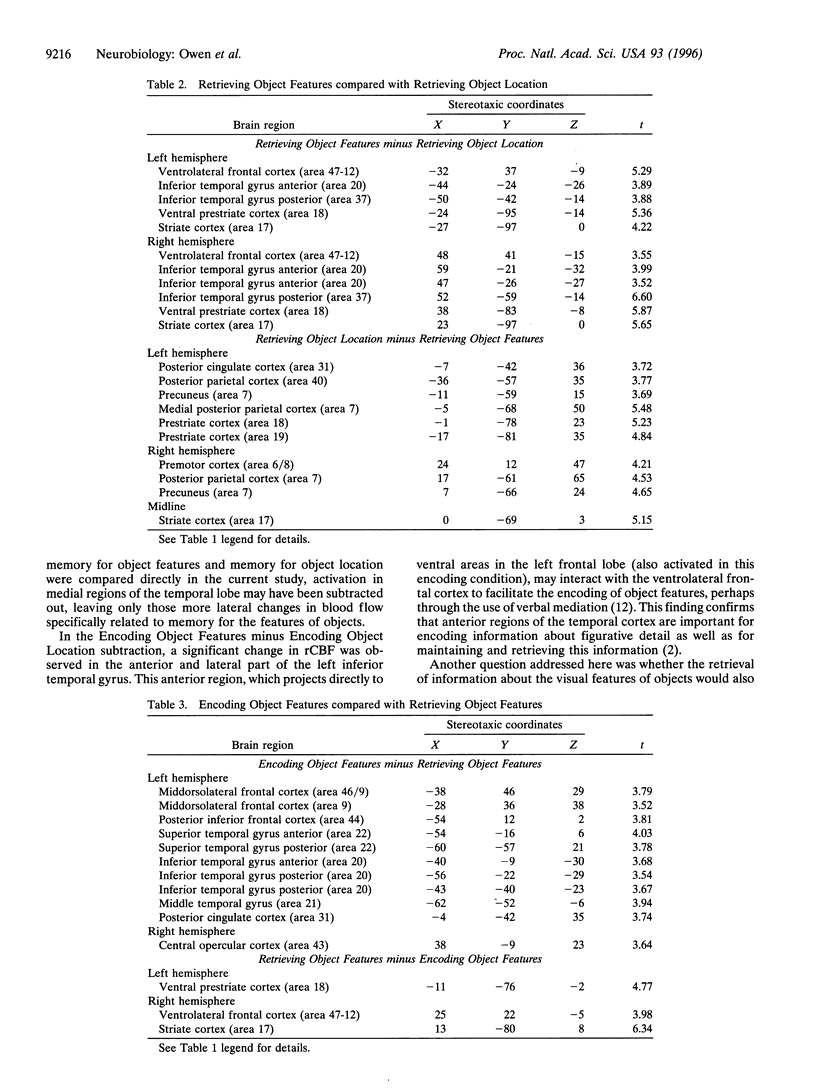
Regional cerebral blood flow was measured with positron-emission tomography during two encoding and two retrieval tasks that were designed to compare memory for object features with memory for object locations. Bilateral increases in regional cerebral blood flow were observed in both anterior and posterior regions of inferior temporal cortex and in ventral regions of prestriate cortex, when the condition that required retrieval of object locations was subtracted from the condition that required retrieval of object features. During encoding, these changes were less pronounced and were restricted to the left inferior temporal cortex and right ventral prestriate cortex. In contrast, both encoding and retrieval of object location were associated with bilateral changes in dorsal prestriate and posterior parietal cortex. Finally, the two encoding conditions activated left frontal lobe regions preferentially, whereas the two retrieval conditions activated right frontal lobe regions. These findings confirm that, in human subjects, memory for object features is mediated by a distributed system that includes ventral prestriate cortex and both anterior and posterior regions of the inferior temporal gyrus. In contrast, memory for the locations of objects appears to be mediated by an anatomically distinct system that includes more dorsal regions of prestriate cortex and posterior regions of the parietal lobe.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachevalier J., Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986 Jun;20(3):249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Evans A. C., Marrett S., Torrescorzo J., Ku S., Collins L. MRI-PET correlation in three dimensions using a volume-of-interest (VOI) atlas. J Cereb Blood Flow Metab. 1991 Mar;11(2):A69–A78. doi: 10.1038/jcbfm.1991.40. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Perlmutter J. S., Raichle M. E. A stereotactic method of anatomical localization for positron emission tomography. J Comput Assist Tomogr. 1985 Jan-Feb;9(1):141–153. doi: 10.1097/00004728-198501000-00025. [DOI] [PubMed] [Google Scholar]
- Haxby J. V., Horwitz B., Ungerleider L. G., Maisog J. M., Pietrini P., Grady C. L. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994 Nov;14(11 Pt 1):6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J. V., Ungerleider L. G., Horwitz B., Maisog J. M., Rapoport S. I., Grady C. L. Face encoding and recognition in the human brain. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S., Kapur S., Moscovitch M., Winocur G., Houle S. Dissociation of pathways for object and spatial vision: a PET study in humans. Neuroreport. 1995 Oct 2;6(14):1865–1868. doi: 10.1097/00001756-199510020-00011. [DOI] [PubMed] [Google Scholar]
- Martin A., Wiggs C. L., Ungerleider L. G., Haxby J. V. Neural correlates of category-specific knowledge. Nature. 1996 Feb 15;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Meunier M., Bachevalier J., Mishkin M., Murray E. A. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993 Dec;13(12):5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch C., Kapur S., Köhler S., Houle S. Distinct neural correlates of visual long-term memory for spatial location and object identity: a positron emission tomography study in humans. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3721–3725. doi: 10.1073/pnas.92.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Tulving E., Habib R., Nilsson L. G., Kapur S., Houle S., Cabeza R., McIntosh A. R. Functional brain maps of retrieval mode and recovery of episodic information. Neuroreport. 1995 Dec 29;7(1):249–252. [PubMed] [Google Scholar]
- Petrides M., Alivisatos B., Evans A. C. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci. 1995 Jan;15(1 Pt 1):359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott S., Milner B. Memory for different aspects of complex visual scenes after unilateral temporal- or frontal-lobe resection. Neuropsychologia. 1993 Jan;31(1):1–15. doi: 10.1016/0028-3932(93)90076-c. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Martin W. R., Herscovitch P., Mintun M. A., Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983 Sep;24(9):790–798. [PubMed] [Google Scholar]
- Shallice T., Fletcher P., Frith C. D., Grasby P., Frackowiak R. S., Dolan R. J. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994 Apr 14;368(6472):633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Suzuki W. A., Zola-Morgan S., Squire L. R., Amaral D. G. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci. 1993 Jun;13(6):2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E., Kapur S., Craik F. I., Moscovitch M., Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K. J., Evans A. C., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992 Nov;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G., Suzuki W. A. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989 Dec;9(12):4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]