Editor:
Peritonitis is a frequent complication of peritoneal dialysis (PD) and the most common cause of PD failure. The mainstay of treatment is antimicrobial therapy. Guidelines advocate intraperitoneal (IP) administration of antibiotics, because the IP route is considered to be superior to intravenous (IV) administration (1). With the current rise in multiresistant gram-negative bacteria, the carbapenem antibiotic meropenem is more frequently used. However, no reports on the pharmacokinetics of meropenem when administered IP have been published.
In this case, we report on the pharmacokinetics of IP compared with IV meropenem as treatment for PD peritonitis. The patient was a 72-year-old man on continuous ambulatory PD (four 2-L exchanges daily) because of end-stage diabetic nephropathy (creatinine clearance 11 mL/min and urine output 1 L/24 h).
In 2011, the patient was admitted for severe PD peritonitis. He was initially treated with vancomycin and gentamicin IP. No clinical improvement occurred, and the effluent white cell count remained high.
Gram-negative Enterobacter cloacae was isolated from the effluent. The antibiogram indicated that the organism was resistant to amoxicillin-clavulanate potassium, cefuroxime, cefoxitin, trimethoprim, gentamicin, and tobramycin. Although the organism was sensitive to the third-generation cephalosporins in vitro, those agents are discouraged because of the risk of selecting for resistance. We therefore started meropenem, to which the organism was sensitive, at a dose of 1 g IV once daily. The patient recovered, with no clinical signs of peritonitis.
Because a 3-week antibiotic course is advised (1), IP administration is desirable to facilitate outpatient treatment. The patient provided informed consent to undergo additional blood samples to examine the pharmacokinetics of IV compared with IP meropenem administration. Serial blood (plasma and serum) samples were drawn at 1, 4, 12, and 24 hours after both IV (day 11 of admission) and IP (day 14) administration. On day 13, the IV administration had already been changed to IP (1 g meropenem IP daily given in the night dwell). The blood samples were immediately processed and stored at -80°C. They were then analyzed by the high-performance liquid chromatography method at the University Medical Center-Groningen, Groningen, Netherlands.
Figure 1 shows the serial meropenem concentrations during the 24 hours of IV and IP administration. The concentrations of meropenem found in serum were similar to those found in plasma, and therefore only plasma concentrations are shown.
Figure 1 —
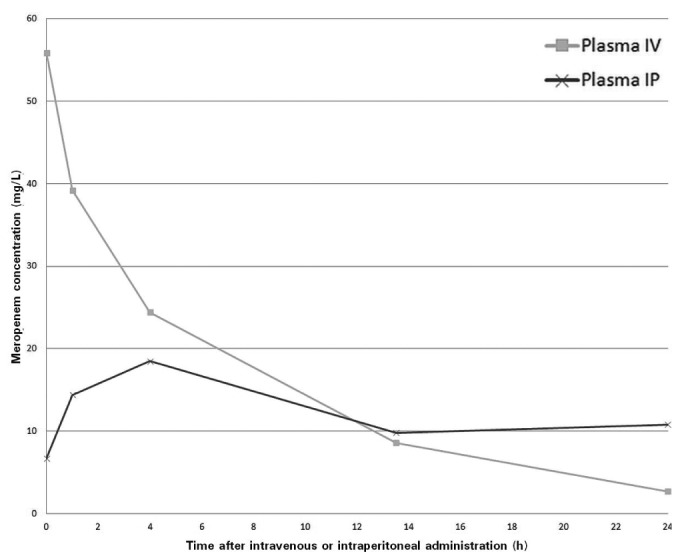
Meropenem plasma concentration after intravenous (IV) and intraperitoneal (IP) administration.
With respect to pharmacokinetics, the highest concentration of meropenem was found soon after a single dose IV. Because of the limited number of measurements, we cannot determine the exact time to the highest concentration after IP administration. However, the curve indicates that the concentration after IP administration was highest after about 4 hours and reached a plateau phase after approximately 14 hours. The area under the curve during 24 hours (AUC24h) for IP and IV administration are largely similar at 325 mg·h/L and 376 mg·h/L respectively. The bioavailability of IP meropenem is therefore estimated at 86%. With regard to pharmacodynamics, the percentage of free time above the minimum inhibitory concentration (fT > MIC) for bacteriostasis [>2 mg/L for Enterobacter cloacae (2)] is close to 100% for both IV and IP administration.
The present report indicates that IP meropenem is feasible for the treatment of PD peritonitis. Further, it provides the first insight into the pharmacokinetics and pharmacodynamics of IP meropenem.
The use of meropenem IP in humans has been reported once before by Van Ende et al. (3). They used a similar dose of 1 g/24 h to treat a wound infection in a PD patient. They concluded that IP administration of meropenem is safe and well tolerated. However, no serial measurements of drug concentration were performed. Although meropenem has a good tolerability profile, the risk of under- or overdosing and of side effects may be increased in patients on renal replacement therapy (RRT) (4).
In our patient, the AUC24h and the fT > MIC for meropenem were good during IP administration and comparable with IV administration. During peritonitis at least, the bioavailability of IP meropenem is reasonably high, indicating that an IP dose of 1 g/24 h was effective for the treatment of PD peritonitis in this patient, although a lower dose might have been possible. It remains unclear whether the risk of accumulation is increased, in particular because the AUC24h found in our patient is higher than that found in patients with normal kidney function (AUC24h of 150 - 200 mg·h/L with a bolus of 1 g IV 3 times daily) (2). Further, the measured concentration 24 hours after the first IP administration may not reflect steady-state levels after a longer period of IP administration. Indeed, earlier studies found large inter-patient variation in elimination of the drug, depending on the type of RRT (1,4). Monitoring of meropenem concentrations may therefore be indicated to limit the risk of under- and overdosing in patients on RRT. Alternatively, future pharmacokinetic research might be directed toward the design of a meropenem dose schedule for peritonitis.
Conclusions
Intraperitoneal meropenem seems to be feasible for the treatment of PD peritonitis. To limit the risk of under- and overdosing, routine measurement of blood concentrations may be indicated when meropenem is used for a longer period in patients on RRT.
Disclosures
All authors declare that they have no financial conflicts of interest. MvH has received speaker or consultancy fees from Bayer, Boehringer Ingelheim, and Bio-Rad, but not in relation to the present research or on the subject of antibiotics in general.
Acknowledgments
We thank the Department of Clinical Pharmacy and Toxicology, University Medical Center-Groningen, for analysis of the meropenem concentrations.
References
- 1. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]
- 2. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Meropenem: Rationale for the EUCAST Clinical Breakpoints, Version 1.5. Växjö, Sweden: EUCAST; 2009. [Available online at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Meropenem_EUCAST_Rationale_Document_1.5_090601.pdf; accessed 10 June 2012] [Google Scholar]
- 3. Van Ende C, Tintillier M, Cuvelier C, Migali G, Pochet JM. Intraperitoneal meropenem administration: a possible alternative to the intravenous route. Perit Dial Int 2010; 30:250–1 [DOI] [PubMed] [Google Scholar]
- 4. Thalhammer F, Hörl WH. Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin Pharmacokinet 2000; 39:271–9 [DOI] [PubMed] [Google Scholar]


