Vascular smooth muscle cells (VSMCs) are key component cells of the vascular wall that are critical for contractility of the aorta1. They respond to biochemical and mechanical signals and play an important role in the regulation of blood pressure. Dysregulation of VSMCs is a hallmark of cardiovascular diseases (CVDs) such as restenosis, hypertension, aneurysms and atherosclerosis. Investigating the signaling pathways in both normal and dysregulated VSMCs is important for understanding how these CVDs develop and for the development of more effective therapies for these potentially life threatening disorders.
Aneurysms are dangerous vascular diseases characterized by aortic dilatation with thinning of the medial VSMC layer. Several mechanisms have been implicated in the development of human aortic aneurysms, including the role of transforming growth factor-β1 (TGF-β1), although there are some controversies in the field2-6. Evidence shows that VSMCs from thoracic aortic aneurysms (TAA) exhibit enhanced activity of SMAD2, a transcription factor that is part of the canonical TGF-β1 pathway and a key effector of the actions of TGF-β17. TGF-β1 signaling results in the phosphorylation and translocation of cytoplasmic SMAD2 into the nucleus and consequent transcriptional activation of its target genes8, 9. However, the activation of SMAD2 in TAA has previously been shown to be dissociated from the TGF-β1 pathway. Instead, the SMAD2 hyperactivity is suggested to be due to the constitutive overexpression of SMAD2 at the transcriptional level10. Furthermore, SMAD2 overexpression was associated with key chromatin histone modifications at the SMAD2 promoter suggesting an element of epigenetic control10.
In the nucleus of mammalian cells, chromosomal DNA is tightly packaged into ‘chromatin’, a higher order structure made up of arrays of subunits called nucleosomes. Each nucleosome consists of an octamer of histones wrapped around DNA. Chromatin modifying enzymes such as histone acetyltransferases (HATs) can be recruited to specific chromatin sites to modify histone tails. These so called ‘epigenetic’ modifications, including DNA methylation and histone post-translational modifications, can affect the dynamics of DNA-nucleosome interactions and gene expression11. For example, acetylation of histone tails has been shown to influence DNA-nucleosome contacts allowing for more accessibility of the DNA for proteins such as transcription factors and the transcriptional machinery12. Over thirty years ago, Weintraub et al suggested that post-translational modifications of chromatin could be heritable13 and more recent investigations by many groups have shown that chromatin states can be maintained from mother to daughter cells, though the exact mechanism remains to be elucidated14.
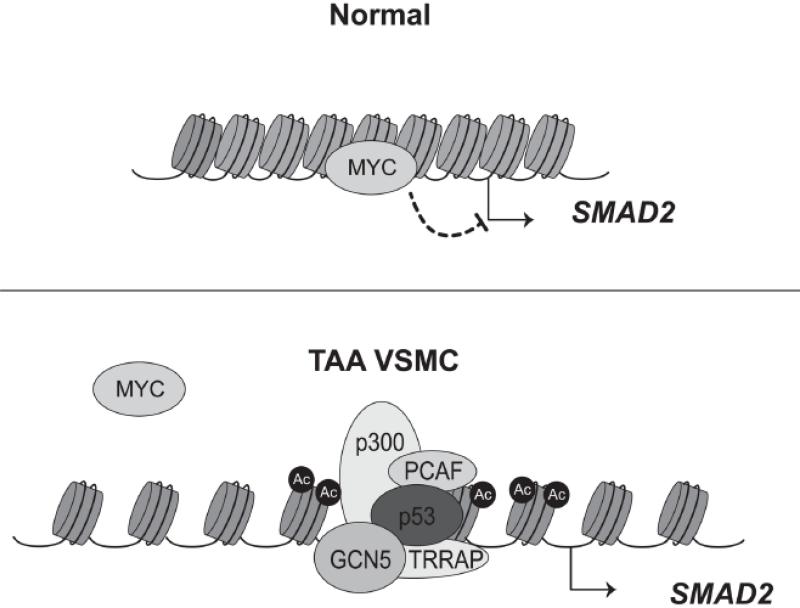
In this issue of Circulation Research, Gomez et al investigated the molecular mechanism associated with the constitutive transcription of SMAD2 in human TAA VSMCs15. They had previously observed that TAA VSMCs retain constitutive transcription of SMAD2 even after three passages in vitro suggesting a heritable, cell autonomous mechanism of cellular “memory”10. Interestingly, histones of the SMAD2 promoter in TAA VSMCs are hyperacetylated compared to controls10. The hyperacetylation of histones at the SMAD2 promoter is dependent on PCAF and p300, two HATs that are specifically recruited to the SMAD2 locus. With HAT inhibitors of p300 and PCAF, Gomez et al found that SMAD2 transcription is attenuated suggesting that the histone acetylation is an important step for transcription of SMAD2. Furthermore, they observed a decrease in Myc binding and an increase in p53 binding at the SMAD2 promoter of TAA VSMCs compared to controls. The results demonstrated a switch in recruitment between Myc and p53 at the SMAD2 promoter, with Myc acting as a transcriptional repressor and p53 functioning as a transcriptional activator. Overall, these observations lead to a model in which loss of Myc binding, increased p300/PCAF-dependent histone acetylation and p53 activation are necessary components for the sustained transcription of SMAD2 in TAA VSMCs (Figure 1).
Figure 1.
Vascular smooth muscle cells (VSMCs) from thoracic aortic aneurysm (TAA) switch from a Myc-dependent repression to a p53- and histone acetylation-dependent activation of the SMAD2 promoter. Top: In normal VSMCs, Myc binding prevents transcription of the SMAD2 locus and the nearby chromatin is quite compact. Bottom: In TAA VSMCs, Myc is displaced and p53 binding and histone acetylation of the promoter driven by p300 and PCAF, two histone acetyltransferases, leads to chromatin relaxation and activates transcription of SMAD2. Nucleosomes are schemactically represented as cyclinders wrapped with DNA.
The findings of Gomez et al are very interesting because they discover that in TAA VSMCs p53 binding and histone acetylation at the SMAD2 promoter drive constitutive SMAD2 transcription. Also, these data suggest that p53 binding and histone acetylation are key components to the heritability of the phenotype in the in vitro cultured TAA VSMCs that is described in their earlier work. Building upon their findings, further work to elucidate the molecular mechanism(s) that allows TAA VSMCs in culture to “remember” their previous states in vivo is an interesting avenue of research. Are p53 binding and histone acetylation maintained through mitosis or reestablished after mitosis is complete in daughter cells?
In recent years, there has been increasing evidence that transcription factors once thought to be completely excluded from mitotic chromosomes, can be at least partially bound to mitotic chromosomes leading to the phenomenon termed “mitotic bookmarking”16. For example, GATA1, a zinc finger protein that is important for erythroid development, has been shown to bind to specific sites on the mitotic chromosomes that allow for the rapid recruitment of its co-activators and reactivation of target genes following mitosis17. It has also been shown that MLL, a metazoan transcription factor which has H3K4 methyltransferase activity, also binds to mitotic chromosomes and allows for the fast reactivation of target genes18. It is not clear whether the methyltransferase activity of MLL plays a role in the fast reactivation since local H3K4 methylation does not depend on MLL. Together, these studies indicate that transcription factors, including chromatin modifiers, may contribute to persistent transcriptional changes that are inherited through mitosis. To date, there is no evidence to conclude whether or not p300, PCAF, and p53 bind to mitotic chromosomes. These studies, especially at the SMAD2 promoter in TAA VSMCs would certainly be worth exploring.
How TAA VSMCs initially establish histone acetylation or p53 binding at the SMAD2 promoter is another avenue of interest. Is there an intrinsic propensity such as genetic predisposition for these human aneurysmal VSMCs to constitutively transcribe SMAD2 or is the overexpression the result of an environmental signal that is maintained in the cells? Of note, Gomez et al observed that the global level of p300/PCAF-dependent histone acetylation is increased in TAA VSMCs. This suggests that there may be additional loci that are also dysregulated in addition to the promoter of SMAD2. Epigenome-wide analysis to determine differentially histone acetylated regions of the genome in TAA VSMCs compared to controls would be valuable to determine other dysregulated loci in addition to SMAD2. It may also lead to the identification of SMAD target genes that may contribute the phenotype of the aneurysms. Since, unlike genetic changes, the reversibility of epigenetic changes presents an additional window of opportunity for therapeutic intervention alone or in combination with traditional therapies. The observation that HAT inhibitors can reduce the expression of SMAD2 suggests that specific p300/PCAF inhibitors might be effective in the clinical setting for TAAs which are not always easy to diagnose and treat.
The study presented by Gomez et al also highlight the importance of chromatin dynamics in modulating VSMC gene expression which is associated with physiological and pathological states of the vessel wall and progression of vascular diseases such as aneurysms. It provides additional evidence of a role for epigenetic mechanisms to facilitate cellular “memory”. Interestingly, cellular “memory” is not limited to human TAA VSMCs. In another report, it was shown that, relative to those from non-diabetic mice, VSMCs isolated from the aorta of diabetic mice displayed increased migration, adhesion and inflammatory gene expression even after several passages in vitro, and this was associated with decreased expression and promoter occupancy of the repressive histone methyltranferase, Suv39h119. Clearly, both human and mouse VSMCs may “remember” their past disease states and for human VSMCs, forgetting to switch off SMAD2 can lead to aneurysmal diseases.
Acknowledgments
Sources of Funding:
This study was supported by grants to R. Natarajan from the National Institutes of Health (R01 HL106089, R01 HL087864, and R01 DK 065073) and the Juvenile Diabetes Research Foundation, and by NIH (NIDDK) T32 fellowship (T32 DK007571-24) to A. Leung.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 2.Dai J, Losy F, Guinault AM, Pages C, Anegon I, Desgranges P, Becquemin JP, Allaire E. Overexpression of transforming growth factor-beta1 stabilizes already-formed aortic aneurysms: a first approach to induction of functional healing by endovascular gene therapy. Circulation. 2005;112:1008–1015. doi: 10.1161/CIRCULATIONAHA.104.523357. [DOI] [PubMed] [Google Scholar]
- 3.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jondeau G, Michel JB, Boileau C. The translational science of Marfan syndrome. Heart. 2011;97:1206–1214. doi: 10.1136/hrt.2010.212100. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JA, Spinale FG, Ikonomidis JS. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. J Vasc Res. 2009;46:119–137. doi: 10.1159/000151766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, Jondeau G, Michel JB, Vranckx R. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol. 2009;218:131–142. doi: 10.1002/path.2516. [DOI] [PubMed] [Google Scholar]
- 8.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 10.Gomez D, Coyet A, Ollivier V, Jeunemaitre X, Jondeau G, Michel JB, Vranckx R. Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms. Cardiovasc Res. 2011;89:446–456. doi: 10.1093/cvr/cvq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol. 2013;20:259–266. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub H, Flint SJ, Leffak IM, Groudine M, Grainger RM. The generation and propagation of variegated chromosome structures. Cold Spring Harbor symposia on quantitative biology. 1978;42(Pt 1):401–407. doi: 10.1101/sqb.1978.042.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Moazed D. Mechanisms for the inheritance of chromatin states. Cell. 2011;146:510–518. doi: 10.1016/j.cell.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez D, Kessler K, Michel JB, Vranckx R. Modifications of Chromatin Dynamics Control the Smad2 Pathway Activation in Aneurysmal Smooth Muscle Cells. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301989. [DOI] [PubMed] [Google Scholar]
- 16.Kadauke S, Blobel GA. Mitotic bookmarking by transcription factors. Epigenetics & chromatin. 2013;6:6. doi: 10.1186/1756-8935-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadauke S, Udugama MI, Pawlicki JM, Achtman JC, Jain DP, Cheng Y, Hardison RC, Blobel GA. Tissue-specific mitotic bookmarking by hematopoietic transcription factor GATA1. Cell. 2012;150:725–737. doi: 10.1016/j.cell.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blobel GA, Kadauke S, Wang E, Lau AW, Zuber J, Chou MM, Vakoc CR. A reconfigured pattern of MLL occupancy within mitotic chromatin promotes rapid transcriptional reactivation following mitotic exit. Mol Cell. 2009;36:970–983. doi: 10.1016/j.molcel.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]