Abstract
Smooth muscle neoplasms of the vulva can be mistaken for Bartholin duct cysts, which can lead to a delay in diagnosis. We present a case of vulvar leiomyoma and a case of leiomyosarcoma that clinically mimicked Bartholin duct cysts. Identification of leiomyosarcomas in this region is particularly important; due to the risk of recurrence, patients may need radiation and/or chemotherapy in addition to adequate surgical treatment and appropriate follow up. Prior series have shown that risk of recurrence is related to inadequate resection and not to the size or grade of tumor. It is critical that pathologists recognize smooth muscle tumors of the vulva and communicate to clinicians the importance of clear margins and wide local excision in cases of malignancy.
Leiomyomas, benign soft tissue tumors that arise from smooth muscle, account for approximately 3.8% of all benign soft tissue tumors (1). While they can develop anywhere in the body where smooth muscle is present, the most common site is the uterine myometrium (2). External genital leiomyomas arising within Bartholin glands are rare and usually mimic a Bartholin gland cyst. Leiomyomas are typically single mass lesions that are easily excised and contain histologically bland spindled cells that demonstrate estrogen and progesterone receptor positivity with immunohistochemical evaluation. Leiomyomas express smooth muscle markers by immunohistochemical stains, including desmin, smooth muscle actin, and muscle-specific actin.
Sarcomas account for only 1% to 2% of tumors arising in the vulva (3). Leiomyosarcomas are the most common type of sarcoma presenting in the vulva, and they are believed to arise from the smooth muscles within erectile tissue, blood vessels, rough ligaments, and erector-pili muscles (4). Most of the literature characterizing vulvar smooth muscle tumors comprises single case reports with reviews of the literature, and few large case series exist. The largest case series reported recurrence in 10 out of 25 vulvar and vaginal sarcomas, which required re-excision and radiation treatment. The overall 5-year survival rate was 70% (5).
CASE REPORTS
Patient 1. A 50-year-old postmenopausal woman presented with a large left labial mass in the area of the Bartholin glands. It had been slowly increasing in size over the past 5 years. She denied pain, bleeding, discharge, or pruritus. The mass was initially drained, as it was clinically believed to be a cystic lesion, but no fluid could be aspirated. The patient had lost a significant amount of weight over the previous 2 years, but had been on a rigorous diet and exercise program. Other significant medical history included coronary artery disease with coronary artery bypass grafting, hypertension, and diabetes mellitus. A 6 × 4 cm mobile, solid mass was present on the left labia majora free from underlying levator muscles. No evidence of erythema, bleeding, or discharge was noted.
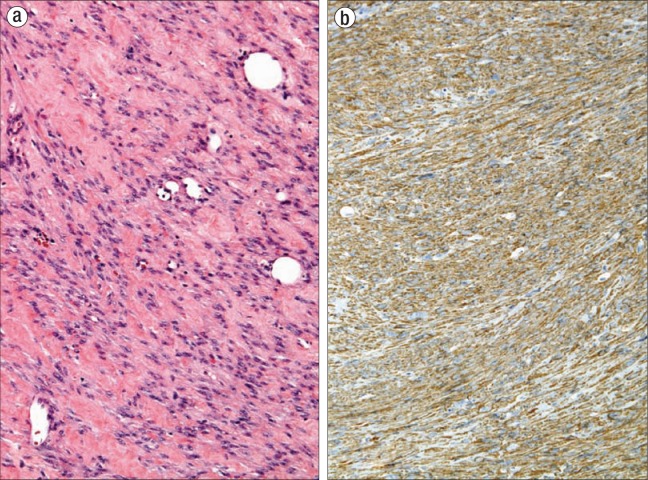
An elective excision was performed under local anesthesia. The mass was easily removed and was not adherent to the levator muscles, rectum, vagina, or pubic ramus. The mass was removed in fragments, the largest of which measured 6.5 × 5.5 × 2.5 cm; the remaining tissue fragments measured 3.0 × 2.0 × 1.5 cm in aggregate. The tissue fragments had tan-white smooth cut surfaces. On microscopy, all sections contained interweaving fascicles of bland spindled cells with well-circumscribed margins (Figure 1a). There was minimal to no cytologic atypia, less than 2 mitoses per 10 high-power fields (HPF), and no necrosis. Smooth muscle actin immunohistochemical stain highlighted the smooth muscle cells, confirming the diagnosis of benign leiomyoma (Figure 1b).
Figure 1.
The leiomyoma in patient 1 (a) displays interweaving fascicles of bland spindle cells with minimal to no cytologic atypia (hematoxylin and eosin, × 200) and (b) has a diffusely positive stain for smooth muscle actin by immunohistochemistry, classifying the tumor as a leiomyoma (immunohistochemical stain, × 200).
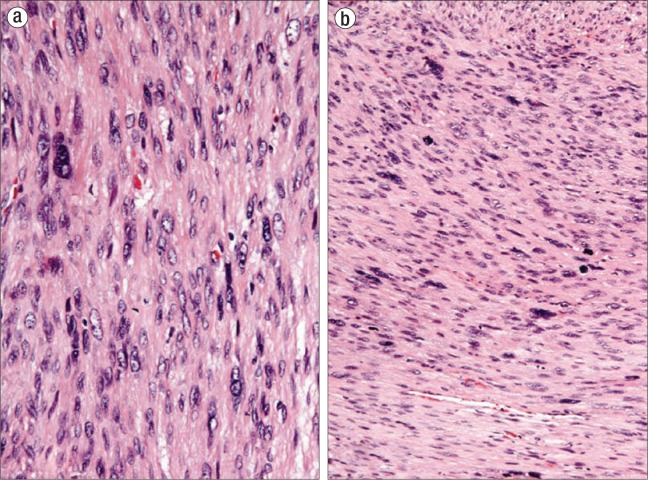
Patient 2. A 57-year-old postmenopausal woman had a 4 × 2 cm mass in the perineal region of the left labia that was tender and uncomfortable and had increased in size over the past 4 to 6 weeks. She denied pain, bleeding, discharge, or pruritus. The mass was clinically diagnosed as a Bartholin gland duct cyst and drainage was attempted, but no fluid could be aspirated. An elective excision was performed under general anesthesia. The mass was easily removed from the left perineal region. It measured 2.5 cm and was composed of tan-pink, soft tissue. On microscopy, sections showed interlacing fascicles of spindled cells with diffuse pleomorphism (Figure 2a), increased mitotic activity (up to 16 mitoses per 10 HPF with atypical forms) (Figure 2b), and a focally infiltrative border. The combination of these three features was sufficient for the diagnosis of leiomyosarcoma. Immunohistochemical stains for smooth muscle actin and desmin highlighted the smooth muscle cells, confirming the diagnosis of leiomyosarcoma. The neoplasm was present at the surgical margin, and additional therapy was recommended. The clinician was contacted to discuss the unexpected results and the significance of a positive surgical margin, which expedited the referral to a gynecologic oncologist for radical vulvectomy. Radical excision was performed and no residual disease was identified.
Figure 2.
The leiomyosarcoma in patient 2 displays (a) diffuse nuclear pleomorphism with hyperchromatic, enlarged, and atypical nuclei (hematoxylin and eosin, × 400) and (b) increased mitotic activity (up to 16 mitoses per 10 high-power fields with atypical forms) (hematoxylin and eosin, × 200).
DISCUSSION
We present two cases of vulvar smooth muscle tumors that clinically mimicked Bartholin gland cysts. These cases suggest that a biopsy should be performed, rather than a drainage procedure, if the mass appears firm or solid on palpation, is ulcerated instead of smooth, or presents in a slightly different location than the usual region of the Bartholin ducts (6). Histologic evaluation is necessary for diagnosis. While vulvar leiomyosarcomas are rare, they do occur in the same location as Bartholin duct cysts. Initial clinical misdiagnosis as a Bartholin duct cyst can result in a delay of diagnosis and worse prognosis (7).
Both leiomyomas and leiomyosarcomas are immunopositive for muscle markers, including desmin, smooth muscle actin, and muscle-specific actin, and they can be focally positive for S-100 and cytokeratin. The diagnosis of extrauterine leiomyosarcomas in the gynecologic tract requires the presence of at least three of the following characteristics: a diameter > 5 cm, infiltrative margins, > 5 mitotic figures per 10 HPF, and moderate to severe cytologic atypia. Lesions that have only one of these characteristics should be diagnosed as leiomyomas, and cases with two characteristics should be considered atypical leiomyomas (8).
Due to the low incidence of these tumors, there are no evidence-based diagnostic algorithms or published recommendations for treatment. However, prior reports have recommended surgical excision with the potential addition of radiation therapy. Decisions are made based upon the individual case presentation and pathology evaluation. Leiomyosarcomas are generally treated by complete excision with a goal of pathologic confirmation of negative margins. Conversations between pathologists and clinicians can provide guidance to ensure adequate surgical excisions are performed. Prior studies have shown that risk of recurrence is most closely related to inadequate resection of margins (9). The overall prognosis is best correlated to histologic grade (5). Close monitoring of the patient is advised, as these entities have almost a 50% recurrence rate (5).
The value of adjuvant chemotherapy is uncertain but has produced regression of metastases in vulvar sarcomas (9). Adjuvant chemotherapy and radiation therapy for completely resected low-grade mesenchymal tumors have not been shown to improve outcomes (3, 5). Small case series have shown benefit in treating high-grade sarcomas or recurrent low-grade sarcomas with postoperative radiation; however, it is very difficult to compare treatment regimens at different institutions as there are no standardized guidelines (5).
References
- 1.Misumi S, Irie T, Fukuda K, Tada S, Hosomura Y. A case of deep soft tissue leiomyoma: CT and MRI findings. Radiat Med. 2000;18(4):253–256. [PubMed] [Google Scholar]
- 2.Kumar V. The female genital tract. In: Kumar V, Abbas AK, Fausto N, Aster J, editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia: Saunders Elsevier; 2010. pp. 1005–1062. [Google Scholar]
- 3.DiSaia PJ, Pecorelli S. Gynecological sarcomas. Semin Surg Oncol. 1994;10(5):369–373. doi: 10.1002/ssu.2980100510. [DOI] [PubMed] [Google Scholar]
- 4.Lösch A, Joura EA, Stani J, Breitenecker G, Lahodny J. Leiomyosarcoma of the vulva. A case report. J Reprod Med. 2001;46(6):609–612. [PubMed] [Google Scholar]
- 5.Curtin JP, Saigo P, Slucher B, Venkatraman ES, Mychalczak B, Hoskins WJ. Soft-tissue sarcoma of the vagina and vulva: a clinicopathologic study. Obstet Gynecol. 1995;86(2):269–272. doi: 10.1016/0029-7844(95)00160-s. [DOI] [PubMed] [Google Scholar]
- 6.Dewdney S, Kennedy CM, Galask RP. Leiomyosarcoma of the vulva: a case report. J Reprod Med. 2005;50(8):630–632. [PubMed] [Google Scholar]
- 7.González-Bugatto F, Añón-Requena MJ, López-Guerrero MA, Báez-Perea JM, Bartha JL, Hervías-Vivancos B. Vulvar leiomyosarcoma in Bartholin's gland area: a case report and literature review. Arch Gynecol Obstet. 2009;279(2):171–174. doi: 10.1007/s00404-008-0652-1. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen GP, Rosenberg AE, Koerner FC, Young RH, Scully RE. Smooth-muscle tumors of the vulva. A clinicopathological study of 25 cases and review of the literature. Am J Surg Pathol. 1996;20(7):779–793. doi: 10.1097/00000478-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Aartsen EJ, Albus-Lutter CE. Vulvar sarcoma: clinical implications. Eur J Obstet Gynecol Reprod Biol. 1994;56(3):181–189. doi: 10.1016/0028-2243(94)90168-6. [DOI] [PubMed] [Google Scholar]