Abstract
Background
Hypotension is the most common problem with spinal anesthesia. This prospective study aimed to compare normotensive and hypertensive patients with respect to the hemodynamic effects of spinal anesthesia performed with hyperbaric bupivacaine.
Material/Methods
Sixty patients who were scheduled to undergo various elective operations under spinal anesthesia were included into the study. The patients were separated into 2 groups: hypertensive patients constituted Group H (n=30) and normotensive patients constituted Group N (n=30). After fluid loading, spinal anesthesia was performed with 3.5 ml 0.5% hyperbaric bupivacaine. Demographic characteristics and incidence of hypotension and bradycardia were compared. Systolic (SBP), diastolic (DBP), and mean blood pressures (MBP) and heart rate (HR) were also compared before and after spinal anesthesia.
Results
There was no significant difference between the groups with respect to demographic characteristics, maximal height of sensory block, incidences of hypotension and bradycardia, and the amount of fluids infused (p>0.05). In the hypertensive patient group, the SBP, DBP, and MBP values were significantly higher than in the normotensive patient group at all measurement times (p<0.05). Comparison within the groups did not reveal any significant differences in either group compared to the basal values (p>0.05). There were no significant differences in HR between or within groups (p>0.05).
Conclusions
There was no significant difference between normotensive and hypertensive patients in the incidences of hypotension caused by spinal anesthesia with 0.5% hyperbaric bupivacaine.
Keywords: spinal anesthesia, hyperbaric bupivacaine, hypotension, hypertension
Background
The most common and severe complication of spinal anesthesia is hypotension. The incidence of hypotension in non-obstetric patients ranges between 5% and 66%, depending on the definition of hypotension limit, timing of measurements, and differences between data acquisition methods and patient characteristics. Previous cohort studies reported that hypotension due to spinal anesthesia was approximately twice as common in hypertensive patients [1–3].
It has been shown that intraoperative hypotension is associated with myocardial ischemia and increased stroke risk. In addition, because hypertension is an independent risk factor for coronary heart disease, congestive heart failure, and cerebrovascular diseases, it is considered that the negative effects of hypotension will occur more easily in this patient group [4,5].
The number of hypertensive patients in the adult population in Turkey is estimated to be approximately 15 million (31.8%), and almost one-third is unaware of their condition. Although hypertension is a problem frequently encountered by anesthesiologists, and despite advances in the definition and management of hypertension, there are only a few randomized controlled studies that have investigated the hemodynamic effects of spinal anesthesia in these patients [6,7].
In this study we aimed to compare normotensive patients and hypertensive patients under control by medical treatment, with respect to the hemodynamic effects of spinal anesthesia performed with hyperbaric bupivacaine.
Material and Methods
The study was approved by the ethics committee of Haseki Training and Research Hospital, and all patients gave written and verbal consent prior to the study. Sixty patients ages 18–65 with ASA scores I–II and who were scheduled for elective surgeries of lower abdomen, lower limbs, and perineum under spinal anesthesia were included into the study. The patients were evaluated at the bedside 1 day before the operation. Blood pressures and heart rates were measured under standard conditions and the basal values were recorded. The patients were separated into 2 groups. Patients with a history of hypertension and who regularly used antihypertensive medications constituted Group H (n=30), and patients with basal blood pressure values below 140/90 mm Hg and no history of use of drugs effective on the cardiovascular system constituted Group N (n=30). Hypertension was inadequately controlled but blood pressure was below 180/100 mm Hg in all patients of Group H. Antihypertensive drugs were given on the morning of surgery. Patients in whom lumbar puncture failed in the first attempt, who had blood loss necessitating transfusion, or had any contraindications against spinal anesthesia were excluded from the study.
An intravenous catheter was placed in the premedication room, then the ECG, non-invasive blood pressure (NIBP) and peripheral oxygen saturation (SpO2) were monitored (Dräger Infinity Delta, Dräger Medical Systems, Inc. Danvers, MA, USA). The patients were premedicated with 1–1.5 mg midazolam and received 10 ml/kg isotonic NaCl according to the ideal body mass calculated with formula ([height−100− (height−150)/4] for male and [height−100− (height−150)/4]×0.9 for female) in 20 minutes, then they were taken into the operating room. Spinal anesthesia injected with a 22-gauge Quincke spinal needle from the L3–4 intervertebral space with the patient in sitting position. As the local anesthetic, 3.5 ml of 0.5% hyperbaric bupivacaine (Marcaine 0.5%, Spinal Heavy 0.5% (5 mg/ml) AstraZeneca) was applied to the subarachnoid space for 15 seconds. The patients were then immediately brought into supine position with the head elevated to 30 degrees.
Sensory block was evaluated with the pinprick test, and motor block was evaluated with the modified Bromage scale (0=no motor block, 1=inability to raise extended leg, but able to move knees and feet, 2=inability to raise extended leg and move knee, but able to move feet, 3=inability to flex ankle joint). Surgery was allowed to start when the sensory block reached the T10 level.
The systolic (SBP), diastolic (DBP), and mean (MBP) blood pressures, and heart rate (HR) were measured and recorded by an assistant at the following time points: basal (the average of 3 consecutive measurements in the premedication room), after fluid loading, and 1, 3,5, 10, 20, 30, 40, 50, and 60 minutes after spinal anesthesia. A decrease of MBP of more than 30% from the baseline was accepted as hypotension and was treated with intravenous ephedrine 5 mg. A decrease in HR to below 50 beats/minute was accepted as bradycardia and was treated with intravenous atropine 0.5 mg. Patients who received ephedrine and atropine, and the time of treatment were recorded. During surgery, all patients inspired 100% O2 at a flow rate of 2 L/minute using a face mask, and isotonic NaCl was infused at 8 ml/kg/hour.
Data were evaluated with the SPSS 17.0 statistics software for Windows (SPSS Inc., Chicago, IL, USA). Categoric variables between the groups (sex, ASA, the frequency of hypotension and bradycardia) were compared with the chi-square test. Numeric variables (sex, body weight, height, and hemodynamic parameters) were evaluated with the Kolmogorov-Smirnov test with respect to normal distribution. Comparison of parametric data between the groups was made with the t test. Repeated measurements analysis of variance (RMANOVA) was used for intragroup comparison of the hemodynamic data, and Bonferroni test was used for post hoc multiple comparison. The data are shown as mean ±S.D. and p<0.05 was accepted as statistically significant.
Results
There were 60 patients included into the study and 10 of them were female. Mean age was 62±14 years. There was no significant difference between the groups with respect to age, sex, weight, height, maximal height of sensory block, incidences of hypotension and bradycardia, or the amount of fluids infused (p>0.05). However, in Group H the number of patients with ASA II physiologic scores was statistically higher compared to Group N (p<0.001) (Table 1). Antihypertensive medications used by the patients are also shown in Table 1.
Table 1.
Characteristics of the groups.
| Group H (n=30) | Group N (n=30) | |
|---|---|---|
| Age (years) | 65±11 | 58±14 |
|
| ||
| Sex M/F (n) | 27/3 | 23/7 |
|
| ||
| Weight (kg) | 73±9 | 73±10 |
|
| ||
| Height (cm) | 169±7 | 171±8 |
|
| ||
| ASA I/II (n) | 0/30 | 29/1# |
|
| ||
| Duration of hypertension (years) | (2–9) | – |
|
| ||
| Antihypertensive medications (n) | ||
| Calcium channel blockers | 11 | – |
| Diuretics | 13 | – |
| ACE inhibitors | 12 | – |
| β blockers | 3 | – |
|
| ||
| Hypotension (n) | 6 (10%) | 1(3.3%) |
| Observation time (min.)* | 20 (5–40) | 15 |
|
| ||
| Bradycardia (n) | 6 (%20) | 7 (%23) |
| Observation time (min.)* | 20 (5–40) | 15 (5–40) |
|
| ||
| Maximal sensory block level (dermatome)* | T8 (T6–T11) | T9 (T6–T12) |
|
| ||
| Prehidration (ml) | 640±40 | 650±50 |
|
| ||
| Total fluid infusion (ml)** | 510±25 | 520±30 |
Data are given as mean ±SD or number of cases.
Median (maximum-minimum);
amount of fluid given within the first hour after injection (added to prehidration);
P<0.05.
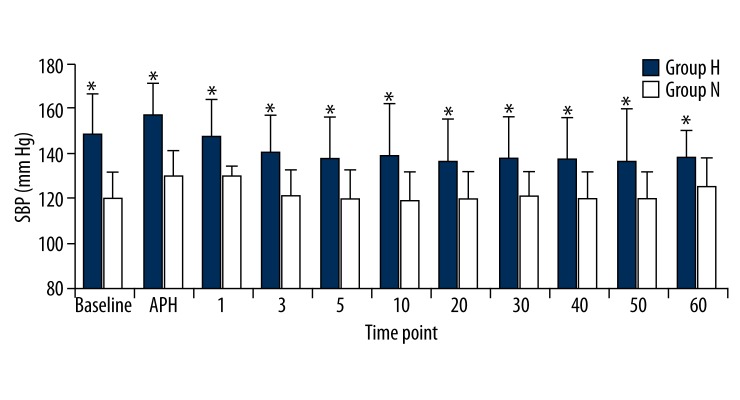
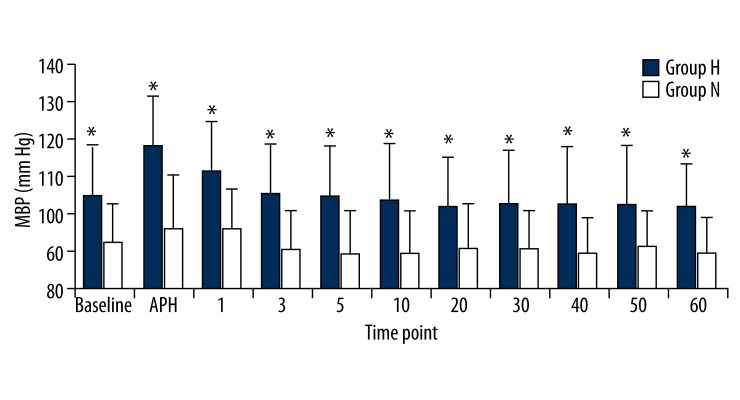
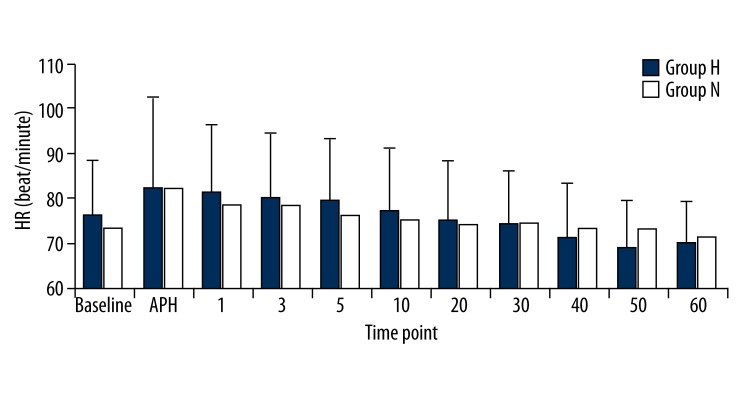
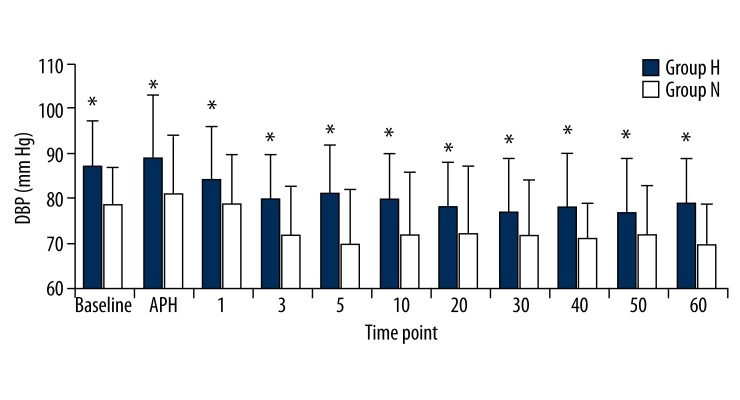
In the hypertensive patient group, the SBP, DBP, and MBP values were significantly higher than the normotensive patient group at all measurement times (p<0.05). Comparison within the groups did not reveal any significant differences in either group compared to the basal values (p>0.05; Figures 1–3). There were no significant differences between the HR values of the groups. We also found that HR values did not show a significant change compared to the basal measurements (p>0.05; Figure 4).
Figure 1.
Sistolic blood pressures (SBP) of groups. Numbers represents minutes after spinal anesthesia. * p<0.05.
Figure 3.
Mean blood pressures (SBP) of groups. Numbers represents minutes after spinal anesthesia. * p<0.05.
Figure 4.
Heart rates (HR) of groups. Numbers represents minutes after spinal anesthesia.
Six patients in Group H and 7 patients in Group N received atropine due to bradycardia. We observed that bradycardia resolved with a single dose of 0.5 mg intravenous atropine. In patients who developed hypotension, a single dose of 5 mg ephedrine treatment was sufficient (Table 1).
Discussion
In this study we showed that there was no difference between the incidence of hypotension in hypertensive versus normotensive patients who underwent spinal anesthesia with hyperbaric bupivacaine. However, the number of patients who developed hypotension was greater in the hypertensive group (6/30) compared to the normotensive group (1/30).
In contrast to our results, Racle et al. [8] reported that hypotension due to spinal anesthesia with 0.5% isobaric bupivacaine was more frequent in hypertensive patients than in normotensives. In their study, the incidence rate of hypotension was 33% (10 out of 30 patients) in the hypertensive group and 10% (3 out of 30 patients) in the normotensive group, which were higher than our results. On the other hand, 2 other studies showed, as in our study, that the incidence rates of hypotension in hypertensive and normotensive patient groups were not different. However, the rates of hypotension were 55.5% and 67% in hypertensive patients, and 43.8% and 73% in normotensive patients, which were higher than our findings [9,10].
The patients in the studies of Racle [8] and Nishikawa [10] were 1 or 2 decades older than ours (mean age was above 75). Hypotension due to spinal anesthesia is caused by decreased systemic vascular resistance and venous return to the heart, caused by sympathetic system blockage [11]. There is an increase in the basal sympathetic system activity and plasma norepinephrine levels associated with aging [12]. Therefore, sympathetic blockage after spinal anesthesia may cause a greater decrease in the systemic vascular resistance in elderly patients. The age factor was one of the reasons why we encountered less hypotension.
Hypertensive patients also have increased sympathetic activity and norepinephrine levels, as well as decreased parasympathetic activity. Persistent sympathetic stimulation, independent of hypertension itself, causes loss of elasticity in the arterial wall and induces structural changes that in turn result in increases in peripheral vascular resistance [13]. It has been shown that there is a close relationship between basal sympathetic activity and decrements in blood pressure that occur after sympathetic blockage [14]. The reduction in blood pressure after spinal anesthesia is correlated with the degree of preoperative blood pressure [8]. Fukuda [9] and Nishikawa [10] found that when preoperative blood pressure was normalized with antihypertensive treatment, there was no difference between the incidence of hypotension following spinal anesthesia in hypertensive versus normotensive patients. Our results support these findings – the reason for this is the improvement in vascular structural changes and the achievement of a decrease in basal sympathetic activity with the use of effective antihypertensive treatment [15].
Intravascular fluid volume is regulated by the sympathetic nervous system. Dehydration due to inadequate fluid intake causes increased sympathetic activity [16]. Prehydration under the guidance of heart rate variability, an indicator of sympathovagal balance, was seen to decrease the risk of hypotension after spinal anesthesia [17]. Venous capacitance is decreased in hypertensive patients [18]; therefore it is thought that a sympathetic block after spinal anesthesia will cause a further decrease in the central volume and venous return to the heart. The fact that antihypertensive treatment provides an improvement in venous capacitance as much as peripheral vascular resistance explains why we did not find any significant decrease in the incidence of hypotension between normotensive and hypotensive patients [15].
In this study, although we showed that there was no difference between the 2 groups with respect to the frequency of hypotension, the SBP, DBP, and MBP were higher in hypertensive patients compared to normotensive patients at all measurement times – this is related to the higher basal blood pressure values in the hypertensive group compared to the normotensive group.
Our study did not measure the basal sympathetic activities of the patients, which is this study’s most significant limiting factor. The most important reason for increased hypotension risk is the increase in sympathetic activity [13,16]. Although an improvement in this increase and blood pressure regulation is achieved with appropriate antihypertensive treatment, lack of measurement of the basal sympathetic activities of the patients was the most significant limiting factor.
Conclusions
In conclusion, we found that there was no significant difference between normotensive and hypertensive patients in the incidence of hypotension caused by spinal anesthesia with 0.5% hyperbaric bupivacaine.
Figure 2.
Diastolic blood pressures (DBP) of groups. Numbers represents minutes after spinal anesthesia. * p<0.05.
Footnotes
Source of support: Departmental sources
References
- 1.Singla D, Kathuria S, Singh A, et al. Risk factors for development of early hypotension during spinal anesthesia. J Anaesth Pharmacol. 2006;22(4):387–93. [Google Scholar]
- 2.Carpenter RL, Caplan RA, Brown DL, et al. Incidence and risk factors fors ide effects of spinal anethesia. Anesthesiology. 1992;76(6):906–16. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann B, Junger A, Klasen J, et al. The incidence and risk factors for hypotension after spinal anesthesia induction: an analysis with automated data collection. Anesth Analg. 2002;94(6):1521–29. doi: 10.1097/00000539-200206000-00027. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Juelsgaard P, Sand NP, Felsby S, et al. Perioperative myocardial ischaemia in patients undergoing surgery for fractured hip randomized to incremental spinal, single-dose spinal or general anaesthesia. Eur J Anaesthesiol. 1998;15(6):656–63. doi: 10.1097/00003643-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Sabate S, Mases A, Guilera N, et al. Incidence and predictors of major perioperative adverse cardiac and cerebrovascular events in non-cardiac surgery. Br J Anaesth. 2011;107(6):879–90. doi: 10.1093/bja/aer268. [DOI] [PubMed] [Google Scholar]
- 6.Altun B, Arici M, Nergizoğlu G, et al. Turkish Society of Hypertension and Renal Diseases: Prevalence, awareness, treatment and control of hypertension in Turkey (the PatenT study) in 2003. J Hypertens. 2005;23(10):1817–23. doi: 10.1097/01.hjh.0000176789.89505.59. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Fagard R, Norkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task force for the management of arterial hypertension of the European Society of hypertension (ESC) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281, 357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 8.Racle JP, Poy JY, Haberer JP, Benkhadra A. A comparison of cardiovascular responses of normotensive and hypertensive elderly patients following bupivacine spinal anesthesia. Regional Anesthesia. 1989;14(2):66–71. [PubMed] [Google Scholar]
- 9.Fukuda T, Dohi S, Naito H. Comparisons of tetracaine spinal anesthesia with clonidine or phenylephrine in normotansive and hypertensive humans. Anesth Analg. 1994;78:106–11. doi: 10.1213/00000539-199401000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa K, Yamakage M, Omote K, Namiki A. Prophylactic IM small-dose phenylephrine blunts spinal anesthesia-induced hypotensive response during surgical repair of hip fracture in the elderly. Anesth Analg. 2002;95(3):751–56. doi: 10.1097/00000539-200209000-00040. [DOI] [PubMed] [Google Scholar]
- 11.Brown DL. Spinal, Epidural and Caudal Anesthesia. In: Miller RD, editor. Miller’s Anesthesia. 7th ed. Philadelphia, PA, USA: Churchill Livingstone Elsevier; 2010. pp. 1611–38. [Google Scholar]
- 12.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528(3):407–17. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139(9):761–76. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 14.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93(6):715–24. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Colle S, Morello F, Rabbia F, et al. Antihypertensive drugs and the sympathetic nervous system. J Cardiovasc Pharmacol. 2007;50(5):487–96. doi: 10.1097/FJC.0b013e318135446c. [DOI] [PubMed] [Google Scholar]
- 16.Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009;84(9):822–30. doi: 10.4065/84.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanss R, Bein B, Francksen H, et al. Heart rate variability-guided prophylactic treatment of severe hypotension after subarachnoid block for elective cesarean delivery. Anesthesiology. 2006;104(4):635–43. doi: 10.1097/00000542-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Delaney EP, Young CN, Disabatino A, et al. Limb venous tone and responsiveness in hypertensive humans. J Appl Physiol. 2008;105(3):894–901. doi: 10.1152/japplphysiol.90574.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]