Abstract
Background
A high occurrence of knee injuries have been observed in women during the menstrual cycle (MC). As a result, numerous studies have been conducted regarding knee ligament elasticity during the MC. Some researchers believe that since estrogen receptor b exists in ligaments and tendons in the knee, estrogen may modulate towards a state of laxity. However, increased tissue temperature also observed during the MC can predispose ligament and tendon laxness. Therefore, the purpose of this study was to assess in women the relationship between Estradiol (E2) serum concentrations and tissue temperature during the MC and their combined effect on knee laxity.
Material/Methods
Ten non-athletic young healthy females, 18 to 30 years of age participated in the study. E2 serum concentrations, anterior cruciate ligament (ACL) elasticity, and force to flex the knee (FFK), knee flexion-extension hysteresis (KFEH) were assessed both at ambient temperature (22°C) and after 38°C warming. Testing was performed multiple times during the participant’s MC, for one full MC.
Results
ACL elasticity was significantly higher (P<0.01) and FFK and KFEH were significantly lower (p<0.05) during ovulation when E2 levels were highest. ACL elasticity was still higher during ovulation after warming to 38°C. But, the effects of MC on FFK and KFEH were reduced by tissue warming.
Conclusions
ACL elasticity, FFK, and KFEH was affected not only by E2 but also tissue temperature. However, E2 had more impact on ACL elasticity while tissue temperature had more impact on FFK and KFEH at 38°C warming.
Keywords: estrogen, menstrual cycle, knee elasticity, temperature
Background
In the adult population, numbers of knee injuries arise every year during sports activities and exercise [1]. The most common injuries are ligament sprains, muscle strains, and contusions [2]. In the annually recorded 150,000 injuries affecting the knee, the Anterior Cruciate Ligament (ACL) injuries are the most frequently occurring injury.
According to the Sports Medicine Media Guide for 2011, the cost of ACL injuries has been estimated at half a billion dollar annually. Additionally, people who have a history of ACL injury are at a higher risk of potential arthritis later in their life and will cost an additional millions of dollars (Sports medicine media guide, 2011). Previous studies have indicated that women have a 2 to 8 times higher risk factor of knee injuries than men, specifically, involving the ACL [3–7]. There has been ongoing debate on why women are more susceptible.
The differences are commonly assumed to be caused by anatomical, neuromuscular, behavior patterns, and hormonal influences that make women more susceptible to ACL injuries [7,8]. Recently investigators have examined the association between female reproductive hormones and structure material and mechanical properties of the ACL. There has been a common theory of the presence of 17-beta estradiol receptor in ACL for decades. The constant changes in the levels of estrogen during the menstrual cycle have been investigated to see if sex hormones are related to ligament laxity [9–12]. Human tendon, muscle, and ligament are composed of collagen fibers closely packed together. Due to the decrease of collagen formation and fibroblast proliferation with an increased estrogen serum concentration, the decreased collagen synthesis causes ligament looseness, weak muscle strength and tendon elasticity all of which makes the ACL more susceptible to injuries [9,10,13]. Estradiol is the estrogens in women and is the most commonly measured estrogen in young non-pregnant females [14]. Since most ACL injuries occur in young healthy women, most investigators have used estradiol as the major estrogen biomarker.
However, there has been controversy on the result of ACL laxity changes across the menstrual cycle. A systemic review on the effects of the menstrual cycle on anterior knee laxity by Zazulak et al. showed that only three of the nine studies reported a significant difference on ACL laxity during the menstrual cycle [5]. Although this study found limited evidence of the correlation between ACL laxity and sex hormones, Zazulak et al. claimed that cycle phases might have an effect on anterior knee laxity. These researchers suggested that future investigators should consider the limitation in present methodologies that they had discovered [5]. They also point out confounding variables that might affect anterior knee elasticity during the menstrual cycle.
Temperature is one of the factors that affects elasticity in muscles [15], such that, an increase in tissue temperature by doing a warm-up exercise will increase muscle and tendon extensibility [16]. Normal core temperature is maintained between 36–37.5° C [17,18]. The core temperature shows a fluctuation across the menstrual cycle; it is at a peak during the middle luteal phases while in the early follicular and the ovulation it is relatively low [19–21]. Skin temperature of the arms and legs is commonly measured around 6°C below the core, 30–31.5°C since human legs and arms tissues are used as a radiator to remove excess heat from the core [22,23]. Due to the core temperature changes throughout the menstrual cycle, skin temperature can also be altered because of the core temperature changes. To increase core temperature, arm and leg temperature is reduced at first to conserve heat. To lower core temperature, arm and leg temperature increases to dissipate heat [24]. Since, ligament, muscle, and tendon extensibility will decrease and increase with decreased or increased tissue temperature, it is of no surprise that the menstrual cycle has dramatic effects on tissue extensibility. Although tissue extensibility decreases with decreased tissue temperature, many studies have reported that anterior knee laxity is greater in the ovulation phases where the core temperature is comparatively lower than other phases [25,26]. However, core temperature increases after ovulation. To keep core temperature lower at ovulation, the shell must be dissipating more heat to keep the core cooler [20]. Therefore, shell must be warmer after ovulation. These findings may explain that anterior knee laxity changes are a consequence of estrogen fluctuations alone during the menstrual cycle. The reported change may be temperature related to and not a direct effect of estradiol. Thus, the aim of the present study is to examine the relationship between estradiol serum concentration and ACL elasticity, knee flexibility and how this relates to tissue temperature in young healthy women with a regular menstrual cycle.
Material and Methods
Ten non-athletic young healthy females with a regular menstrual cycle participated in this study. They were recruited by word of mouth and selected by their self-reported menstrual cycle. Participants were 18 to 30 years of age with a body mass index (BMI) between 15 and 30 who had a regular menstrual cycle for at least one year (Table 1). Participants had no history of pregnancy, neuropathy, myopathy or knee injury, and were not taking any medication that could alter sex hormones. The study was approved by Institutional Review Board of Loma Linda University and all participants signed an informed consent.
Table 1.
Mean (SD) of general demographics (N=10).
| Age (years) | Height (cm) | Weight (kg) | BMI | Cycle length (d) | |
|---|---|---|---|---|---|
| Mean (SD) | 24.7 (2.0) | 164.6 (3.4) | 57.1 (5.0) | 21.0 (1.3) | 29.5 (3.2) |
SD – standard deviation.
Trained researchers measured all outcome variables throughout the experiments. The outcome variables assessed were ACL elasticity, force to flex the knee (FFK), knee flexion-extension hysteresis (KFEH), leg skin temperature, and estradiol serum concentration.
Estradiol serum concentration
Two certified phlebotomists drew 4cc of venous blood samples from each participant’s antecubital area with 23-guage needles to determine estradiol serum concentration. The serum was separated from plasma by centrifuging the blood. The centrifuge was set at 3000 rpm for 10 minutes (Beckman Coulter Inc., Brea, CA). The serum was kept at a temperature of −80°C. After all samples of serum were collected, Estradiol serum concentration was assessed using the TOHSO Estradiol ST AIA assay (Fisher Healthcare Inc. Houston, TX).
Leg skin temperature
Leg skin temperature was measured using the Forward Looking Infrared 660 IR Camera (FLIR systems, Danderyd, Sweden). This device was validated to accurately detect the temperature of the skin [27]. To acquire best images, the readings were at a perpendicular angle and at a distance of one meter away from the skin. Dark colored rooms were preferred to minimize infrared interference so that test was performed in the room with uniform Light Emitting Diode (LED) [28]. Baseline leg skin temperature was taken before ACL laxity and quadriceps elasticity measurements after stabilizing leg temperature in a neutral environment for 20 minutes and leg skin temperature was measured immediately when the heat pads at 38°C were removed.
Anterior ligament elasticity
Knee elasticity of ACL was measured by a KT-2000 knee arthrometer (MEDmetric Corp, San Diego, California). This device had been validated for over twenty years in both clinical and research areas [29,30]. First, the knee was placed in a predetermined position while the participant lays supine. With the knee at 90 degrees, a strain gauge was used to measure the force necessary to generate an anterior glide of the proximal end of the tibia on the femoral condyles. This generated a force curve of elasticity in the anterior cruciate ligaments. The process was accomplished by supporting both limbs with a firm, comfortable platform placed proximal to the popliteal space. This helped keep the patient’s knee flexion angle constant for the duration of the test. Along with this device, a foot support accessory supplied with the Arthrometer®, positioned the feet symmetrically allowing the leg position to be optimal for the test while reducing external rotation of the tibia. For the most comfortable position during the flexion angle test, knee flexion angle was held at 25 degrees (Figure 1). A thigh strap, provided, controlled hip external rotation while offering support that allows patients to be most relaxed. Force used for the experiment was applied at 30 lbs (133N) of force. The output of ACL elasticity was displaced on an X–Y plotter. A vernier caliper was used to interpret anterior tibal displacement (ATD) graph. A single trained investigator measured ACL laxity measures throughout the study to minimize potential inter investigation variation.
Figure 1.
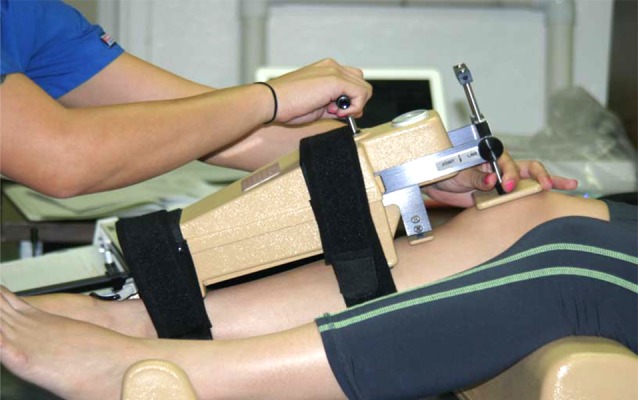
Experimental setup for ADT measured the ACL elasticity. The subject was positioned supine with their knee flexed to 25 degrees.
Muscle and tendon flexibility
Muscle and tendon flexibility was measured by a continuous passive motion device (CPM). Participants were asked to sit on the edge of the table while the leg hung freely. The knee was moved through 35 degrees of motion from 90 to 125 of knee flexion by CPM. The FFK and KFEH measured the flexibility of muscle and tendon. An electronic goniometer was placed on lateral side of the thigh leg to measure the degrees of knee flexion while passively moving the knee. The goniometer used a ruby bearing 360 degree 5000 ohm potentiometer (Figure 2). The output was digitized on BioPac MP 150 data collection system (BioPac System, Goleta, CA). The force needed to move the leg at 115 degrees of knee flexion was used to assess difference in FFK and KFEH.
Figure 2.

Experimental setup for the FFK and KFEH measured the flexibility of muscle and tendon. The subject was positioned sit with their hip and knee flexed to 90 degrees.
The elastic hysteresis curve, which is the difference between the FFK and force to extend the knee (FEK), was also measured by CPM (Figure 3). Potential elastic energy stored during the loading (flexion) is greater than that of unloading (extension); thus, flexing the knee requires greater force than extending the knee [31]. A lower elastic hysteresis value indicates more elasticity of the muscle and tendon.
Figure 3.
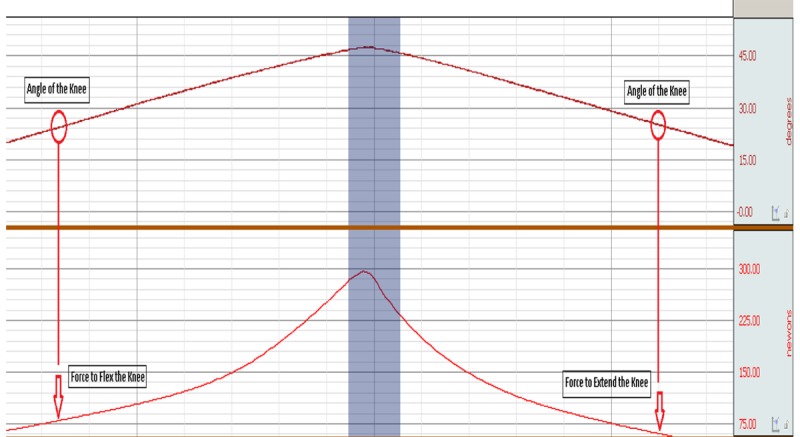
The elastic hysteresis curve, which is the difference between the FFK and for to extend the knee (FEK), was measured by CPM.
Control of quadriceps and knee temperature
Quadriceps and knee temperature were controlled with Berg’s polar care therapy heat exchange unit (Berg Inc. East Carlsbad, CA). Water temperature of the bath was maintained 40°C by a water bath. A heating water bath circulator (Fisher scientific Inc. Pittsburgh, PA) was attached to the water bath to control the water bath temperature. Since water temperature dropped about 2°C from the water bath to the therapy pad. Water temperature was kept at 40°C. This way each participant was given 38°C heat on their knee and quadriceps.
Procedures
Participants signed an informed consent form prior to their involvement. All tests were performed seven times depending on each participant’s menstrual phases throughout one full menstrual cycle. Since the estradiol serum concentration in blood and body temperature fluctuates during the day, the tests were performed at the same time each experiment day.
On the first day, physical characteristics were measured including height, weight, BMI, and self-reported menstrual cycle. Upon arriving to the examination area, participants rested comfortably in a regulated ambient temperature of 25°C for 20 minutes to stabilize their body temperature in a neutral environment. First, 4cc of blood samples were taken to analyze estradiol serum concentration before measuring leg skin temperature. Next, ACL elasticity, FFK, and KFEH were measured. After this, two 38°C heat pads were placed on each participant’s quadriceps and knee for 20 minutes to control their leg temperature, skin temperatures, ACL elasticity, FFK, and KFEH measurements were repeated.
Data analysis
Kolmogrov-Smirnov test was conducted to examine the distribution of estradiol serum concentration, leg skin temperature, ACL elasticity, FFK, and KFEH. One-way repeated measures analysis of variance (ANOVA) was used to examine mean estradiol serum concentration, leg skin temperature, ACL elasticity, and the FFK at seven different phases in the menstrual cycle at ambient temperature. LSD pairwise comparisons test for multiple comparisons was used to compare means of variables between any two different testing phases. A mixed factorial ANOVA was conducted to compare cycle phases with respect to the effect of estradiol on ACL elasticity, FFK, and KFEH at ambient temperature and 38°C warming. The level of significance was set at p<.05.
Results
The distribution of estradiol serum concentration, leg skin temperature, ACL elasticity, FFK, and KFEH was approximately normal (p>0.05). Mean and Standard Deviation (SD) of number of days, estradiol serum concentration, and leg skin temperature in seven different phases of the menstrual cycle is listed in Table 2. The Participant’s phases were determined by their self-report and estradiol serum concentration. Mean (SD) of the Anterior Tibial Displacement (ATD), FFK and KFEH at ambient temperature and 38°C warming in seven different testing phases during the menstrual cycle are shown in Table 3.
Table 2.
Summary of outcome variables in 7 different test phases during the menstrual cycle (N=10).
| Outcome measure | M | EF | LF | O | EL | ML | LL | p-value |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Number of days | 2.2 (0.8) | 6.3 (1.38) | 10.2 (1.28) | 14.2 (2.2) | 17.8 (2.7) | 23.4 (2.4) | 28.1 (3.2) | <0.001 |
| Estradiol, pg/ml | 51.4 (9.0) | 68.3 (14.3) | 82.5 (17.6) | 175.8 (45.9) | 103.9 (26.3) | 130.3 (38.8) | 68.7 (17.0) | <0.001 |
| Knee skin temperature, °C | 31.8 (0.7) | 31.4 (0.4) | 31.7 (1.0) | 31.5 (0.8) | 31.9#* (0.8) | 31.8# (0.5) | 31.8# (0.6) | 0.39 |
| Quadriceps skin temperature, °C | 31.8 (0.9) | 31.5 (0.5) | 31.8 (1.1) | 31.6 (0.7) | 32.1#* (1.0) | 31.9# (0.7) | 31.9 (0.98) | 0.29 |
SD – standard deviation; M – menstruation; EF – early follicular; LF – late follicular; O – ovulation; EL – early luteal; ML – middle luteal; LL – late luteal.
Significant changes from early follicular phase;
significant changes from ovulation phase.
Table 3.
Summary of biomechanical variables in 7 different test phases during the menstrual cycle at ambient temperature and 38°C warming (N=10).
| Phase | ATD, mm | FFK, N | FKEH, N | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre heat Mean (SD) | Post heat Mean (SD) | p-value a | Pre heat Mean (SD) | Post heat Mean (SD) | p-value a | Pre heat Mean (SD) | Post heat Mean (SD) | p-value a | |
| M | 5.1 (1.5)*^ | 5.5 (1.6)*^ | <0.01 | 126.0 (35.4)*^ | 113.2 (23.8) | 0.04 | 34.4 (9.1)*^ | 28.4 (8.0) | <0.01 |
| EF | 5.4 (1.5)*^ | 5.5 (1.8)* | 0.43 | 123.9 (34.2) | 112.0 (31.3) | 0.05 | 30.0 (8.7)* | 26.9 (10.4) | 0.07 |
| LF | 5.4 (5.9)* | 5.6 (1.7) | 0.24 | 116.6 (38.0) | 110.9 (34.6) | 0.01 | 28.9 (7.4)^ | 25.0 (9.2) | 0.03 |
| O | 5.9 (1.7)* | 6.0 (1.8) | 0.41 | 111.2 (24.6) | 109.0 (25.9) | 0.36 | 27.0 (6.0) | 24.2 (8.7) | 0.44 |
| EL | 5.4 (1.6)* | 5.8 (1.7) | 0.02 | 122.4 (31.9) | 111.5 (31.2) | <0.01 | 30.6 (7.2)* | 25.5 (7.8) | 0.05 |
| ML | 5.7 (1.7)^ | 5.8 (1.6) | 0.66 | 115.2 (18.4) | 108.9 (30.2) | 0.2 | 30.0 (7.0)* | 25.6 (5.3) | 0.02 |
| LL | 5.2 (1.2)* | 5.4 (1.0) | 0.26 | 122.1 (27.7) | 112.0 (27.4) | 0.02 | 30.9 (9.1) | 24.9 (6.8) | <0.01 |
| p-value b | 0.03 | 0.35 | 0.2 | 0.82 | 0.06 | 0.71 | |||
SD – standard deviation; M – menstruation; EF – early follicular; LF – late follicular; O – ovulation; EL – early luteal; ML – middle luteal; LL – late luteal.
Significant changes from menstruation;
significant changes from early follicular phase;
significant changes from ovulation phase;
p-values for the null hypothesis that there is a no difference between pre and post heat;
p-values for the null hypothesis that there is a no difference across 7 phases of the menstrual cycle.
Estradiol serum concentration
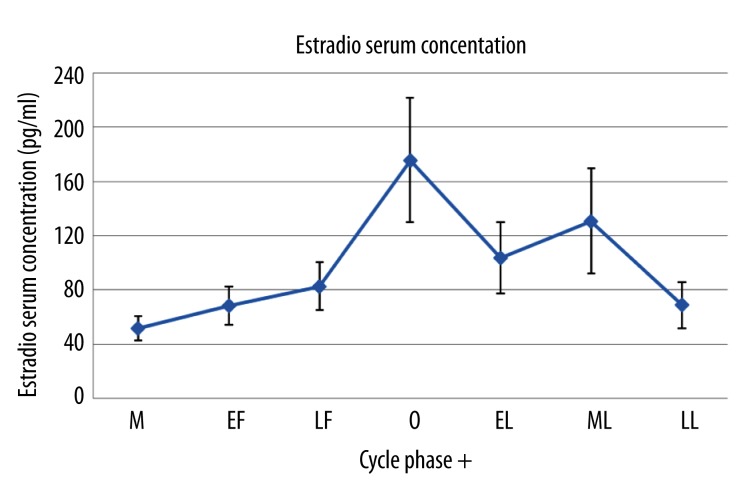
As shown in Figure 4, estradiol concentrations significantly changed throughout the menstrual cycle (p<0.001). The lowest estradiol concentration was found during menstruation (51.4±9.0 pg/ml) and the peak of estradiol concentration was found during ovulation (175.8±45.9 pg/ml, Table 2).
Figure 4.
Mean ±SD of estradiol serum concentration measured in 7 different phases during the menstrual cycle. M – menstruation; EF – early follicular; LF – late follicular; O – ovulation; EL – early luteal; ML – middle luteal; LL – late luteal.
Leg skin temperature
Results indicated that there were no significant differences in knee and quadriceps skin temperatures during the menstrual cycle at ambient temperature (p>0.05, Table 2). However, there was a significant difference in knee skin temperature between the early follicular and the early luteal phase (31.4±0.4°C vs. 31.9±0.8°C, p=0.01). The quadriceps skin temperature was also significantly higher in the early luteal phase than early follicular phases (32.3±1.0°C vs. 31.5±0.5°C, p=0. 04). Leg skin temperature significantly increased and held at 38°C warming when two heat pads were applied on the knee and quadriceps.
Anterior knee ligament elasticity
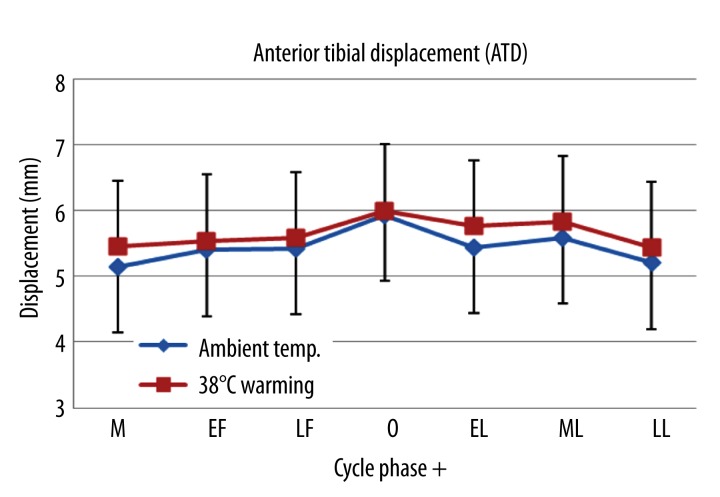
Anterior knee ligament elasticity was a measure of ATD. The greatest ATD during ovulation occurred both at ambient temperature and after 38°C warming (Figure 5). ATD during ovulation was significantly higher than any other phase at ambient temperature (p=.03). There was also a significant difference in ATD between menstruation and ovulation as well as between early follicular and menstruation at 38°C warming (p<.05, Table 3). The greatest ATD was observed when estradiol peaks while the opposite occurred with lower estradiol concentration across the seven phases during the menstrual cycle. There were significant differences in ADT between ambient temperature and 38°C warming during menstruation and early luteal phases (Table 3).
Figure 5.
Mean ±SD of ATD measured in 7 different phases during the menstrual cycle at ambient temperature and 38°C warming. M – menstruation; EF – early follicular; LF – late follicular; O – ovulation; EL – early luteal; ML – middle luteal; LL – late luteal.
Measurement of quadriceps muscle and tendon flexibility
Force to flex the knee (FFK)
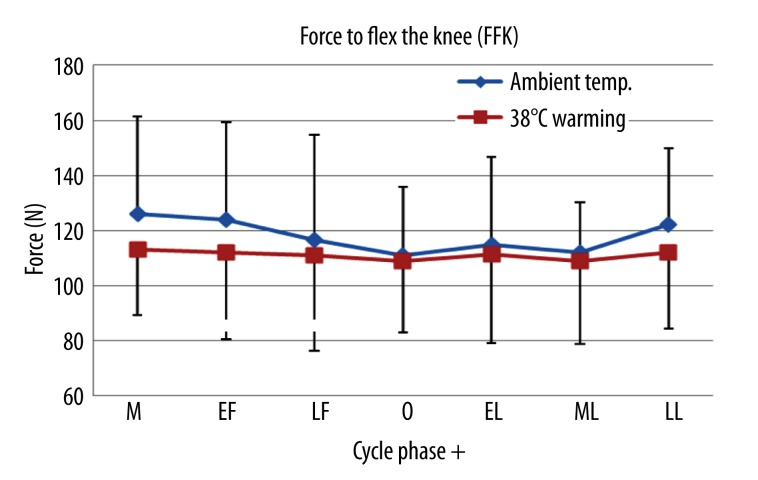
The FFK decreased 15% from menstruation to ovulation at ambient temperature (p=0.4, Table 3). However, no significant changes in FFK across the menstrual phases at 38°C warming were detected (p>0.05, Table 3). Also, a significant difference was found in the FFK in between the ambient temperature and 38°C warming all the phases except the ovulation and the middle luteal phase in which estradiol concentrations were relatively higher than other phases (Table 3, Figure 6).
Figure 6.
Mean ±SD of the FFK measured in 7 different phases during the menstrual cycle at ambient temperature and 38°C warming. M – menstruation; EF – early follicular; LF – late follicular; O – ovulation; EL – early luteal; ML – middle luteal; LL – late luteal.
Knee flexion-extension hysteresis (KFEH)
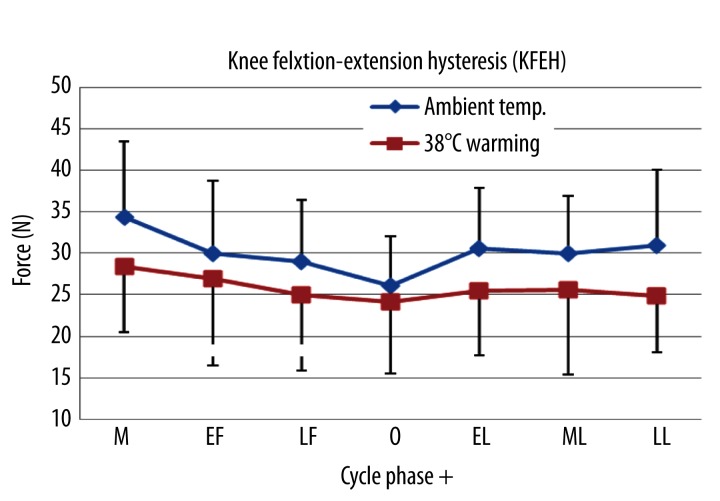
Figure 4 shows the KFEH curve across the seven phases. As shown in Figure 7, there were significant differences of KFEH over the phases at ambient temperature. The KFEH increased 25% from ovulation to menstruation (26.0±6.0 N vs. 34.5±9.1 N, p<0. 01), 15% from ovulation to the early luteal (26.0±6.0 N vs. 30.63±7.23 N, p=0. 04), and 13% from ovulation to the middle luteal phases (26.0±6.0 N vs. 29.97±6.98 N, p=0. 03). However, there were no significant changes in the KFEH over the phases at 38°C warming (p>0.05). The KFEH was significantly higher at 38°C warming as compared to that at ambient temperature in all phases except the ovulation (P<0. 05, Figure 6).
Figure 7.
Mean ±SD of KFEH measured across the 7 different phases during the menstrual cycle at ambient temperature and 38°C warming. M – menstruation; EF – early follicular; LF – late follicular; O – ovulation; EL – early luteal; ML – middle luteal; LL – late luteal.
Discussion
ACL injury is more common in women compared to men who participate in the same activities. Numerous studies have been conducted on changes of knee ligament elasticity during the menstrual cycle to examine the correlation between sex hormones, which fluctuate over the menstrual cycle, and ACL laxity [32,33]. In spite of that, researchers are still debating on the results of these studies because the study methods and procedures were vastly different. Increased tissue temperature could be one factor that causes ligament and tendon laxness (Jarosch, 2011). In this investigation, ACL elasticity, FFK, and KFEH were measured during seven different phases of the menstrual cycle at ambient temperature and after warming the leg to 38°C in young healthy women with a regular menstrual cycle.
The most important finding of the present study was a significantly greater ACL elasticity, less FFK, and KFEH during ovulation where estradiol concentration peaks compared to the mensturation where estradiol level is lowest during the menstrual cycle. The relationship between sex hormones, especailly, estrogen and knee ligament elasticity as well as between estrogen and musculotendinous stifnesss in young women with a regular mentrual cycle, has been demonstrated [12,34–36]. However, this study is the first to examine the relationship between estradiol serum concentration and knee elasticity and its relationship to tissue temperature in young healthy women with a regular menstrual cycle. Greater ACL elasticity was found to occur during ovulation. There are two factors that may cause this: 1) estrogen and 2) tissue temperature. Heat is generally used to increase the elasicity and flexibility of soft tissues (LaBella et al., 2011). Ligaments must have more elasticity with relatively higher temperature since these are elastic stuctures. In this investigation, skin temperature was comparably lower during ovulation than mesntruation and the early follicular phase. But the only improtant temperature is the temperature of deep tissue. There are very few papers on deep shell tissue temperature in deep tissues during the menstrual cycle [21]. These investigations found that muscle temperature was significantly elevated during ovulation and the well documented increase in core temperature. This is because heat has to be conserved by skin to get increased body temperature. Heat goes through the muscle and leaves the skin by radiation, convection, conduction, and evaporation. Skin needs to be cooler than the core temperature to aid in heat loss or conserve heat [24]. Therefore, to keep the body cool at ovulation, the shell needs to remove more heat, hence muscle temperature increases at ovulation.
When the knee was heated to 38°C, there was still difference in ACL elasticity at ovulation when compared to other phases of the menstrual cycle. Therefore, while the temperature change in deep tissues causes increased laxness, so must estradiol. Both are synergistic at ovulation in increasing ACL elasticity. However, previous studies examining ACL elasticity did not control tissue temperature. Therefore, if it was a cool day, the cool temperature of the shell would increase ACL stiffness and its effect would be cancelled. Clothing and ambient temperature become important to consider in studies and may be the reason the results were so varied in different studies. No previous study has even had subjects acclimatized to room temperature before measuring measurements.
This study also measured the FFK and KFEH in knee felxion and extension. These measures examined muscle and tendon flexibility. Human muscles and tendons are soft tissues composed of collagen fibers which may be also affected by estradiol concentration and temperature as explained above. Results of this sutdy showed a significantly lower FFK and KFEH at ovulation compared to menstruation. This finding indicates that less muscle and tendon stiffness are shown in the ovulation than menstruation statge. In addition, this result may infer that less muscle flexibility occurs with increased estradiol concentration and lower skin temperature which may lead to higher muscle tissue temperautre in young healthy women. However, for the knee, the probable effect of estradiol is different. There was more energy storage at ovulation showing increased laxity. When the leg was warmed to the core temperature, this effect disappeared. This result implies that the flexibility is more affected by temperature than estrogen; although estrogen is responsible for the temperature fluctuation. Thus, using heat would be beneficial on increasing knee flexibility; the optimal time would be in the menstruation and early follicular phase, where the less flexibility occurs.
Knee joint stiffness is a major risk factor for non-contact ACL injuries [37]. It is so common for athletes to do warm-up stretching or use heat packs to decrease stiffness before exercise for injury prevention [38]. Women have greater knee stiffness due to estradiol and temperature effects during menstruation. However, this investigation did not examine how knee stiffness is related to injuries. Laxity may cause injuries or may not. Myklebust et al. and Slatuterbeck et al. reported that there are more ACL injuries during menstruation where less ACL elasticity was found [39,40]. On the other hand, Wojtys et al. claimed that more ACL injuries occur during ovulation where greater ACL elasticity was found [41]. However, these studies did not control environment or tissue temperature. Temperature may be another factor that affects ACL injuries but previous studies did not examine ACL elasticity under the same condition such as controlling the effects of clothing or environment temperature. If women play sports during ovulation in a hot environment, both temperature and estrogen causes excessive knee laxity which can leads to more knee injuries. However, if women play sports during menstruation in cold environments, both temperature and estrogen cause excessive knee stiffness which can lead to more knee injuries. Thus, in further investigation, both estrogen and temperature should be considered as key risk factors that can cause ACL injuries during menstrual cycle.
Conclusions
ACL elasticity, FFK, and KFEH were affected not only by estradiol serum concentration but tissue temperature during the menstrual cycle. However, estradiol serum concentration had more impact on ACL laxity while tissue temperature had more impact on FFK and KFEH at 38°C warming.
Footnotes
Source of support: Departmental sources
References
- 1.Pappas E, Zampeli F, Xergia SA, Georgoulis AD. Lessons learned from the last 20 years of ACL-related in vivo-biomechanics research of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):755–66. doi: 10.1007/s00167-012-1955-0. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DF, Connolly DA, Beynnon BD. Risk factors for lower extremity injury: a review of the literature. Br J Sports Med. 2003;37(1):13–29. doi: 10.1136/bjsm.37.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SK, Stefanyshyn DJ, Ramage B, et al. Relationship between knee joint laxity and knee joint mechanics during the menstrual cycle. Br J Sports Med. 2009;43(3):174–79. doi: 10.1136/bjsm.2008.049270. [DOI] [PubMed] [Google Scholar]
- 4.Vauhnik R, Morrissey MC, Rutherford OM, et al. Knee anterior laxity: a risk factor for traumatic knee injury among sportswomen? Knee Surg Sports Traumatol Arthrosc. 2008;16(9):823–33. doi: 10.1007/s00167-008-0559-1. [DOI] [PubMed] [Google Scholar]
- 5.Zazulak BT, Paterno M, Myer GD, et al. The effects of the menstrual cycle on anterior knee laxity: a systematic review. Sports Med. 2006;36(10):847–62. doi: 10.2165/00007256-200636100-00004. [DOI] [PubMed] [Google Scholar]
- 6.Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34(2):86–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8(3):141–50. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Barber-Westin SD, Noyes FR, Smith ST, Campbell TM. Reducing the risk of noncontact anterior cruciate ligament injuries in the female athlete. Phys Sportsmed. 2009;37(3):49–61. doi: 10.3810/psm.2009.10.1729. [DOI] [PubMed] [Google Scholar]
- 9.Yu WD, Liu SH, Hatch JD, et al. Effect of estrogen on cellular metabolism of the human anterior cruciate ligament. Clin Orthop Relat Res. 1999;(366):229–38. doi: 10.1097/00003086-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Liu SH, Al-Shaikh RA, Panossian V, et al. Estrogen affects the cellular metabolism of the anterior cruciate ligament. A potential explanation for female athletic injury. Am J Sports Med. 1997;25(5):704–9. doi: 10.1177/036354659702500521. [DOI] [PubMed] [Google Scholar]
- 11.Hansen M, Kongsgaard M, Holm L, et al. Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol. 2009;106(4):1385–93. doi: 10.1152/japplphysiol.90935.2008. [DOI] [PubMed] [Google Scholar]
- 12.Shultz SJ, Kirk SE, Johnson ML, et al. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36(7):1165–74. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SK, Stefanyshyn DJ, Ramage B, et al. Alterations in knee joint laxity during the menstrual cycle in healthy women leads to increases in joint loads during selected athletic movements. Am J Sports Med. 2009;37(6):1169–77. doi: 10.1177/0363546508330146. [DOI] [PubMed] [Google Scholar]
- 14.Nintasen R, Riches K, Mughal RS, et al. Divergent effects of 17-beta-estradiol on human vascular smooth muscle and endothelial cell function diminishes TNF-alpha-induced neointima formation. Biochem Biophys Res Commun. 2012;420(4):828–33. doi: 10.1016/j.bbrc.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 15.Jarosch R. The Different Muscle-Energetics during Shortening and Stretch. Int J Mol Sci. 2011;12(5):2891–900. doi: 10.3390/ijms12052891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasanen K, Parkkari J, Pasanen M, Kannus P. Effect of a neuromuscular warm-up programme on muscle power, balance, speed and agility: a randomised controlled study. Br J Sports Med. 2009;43(13):1073–78. doi: 10.1136/bjsm.2009.061747. [DOI] [PubMed] [Google Scholar]
- 17.Petrofsky JS, Laymon M. Heat transfer to deep tissue: the effect of body fat and heating modality. J Med Eng Technol. 2009;33(5):337–48. doi: 10.1080/03091900802069547. [DOI] [PubMed] [Google Scholar]
- 18.Petrofsky J, Bains G, Prowse M, et al. Dry heat, moist heat and body fat: are heating modalities really effective in people who are overweight? J Med Eng Technol. 2009;33(5):361–69. doi: 10.1080/03091900802355508. [DOI] [PubMed] [Google Scholar]
- 19.Janse DEJXA, Thompson MW, Chuter VH, et al. Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med Sci Sports Exerc. 2012;44(11):2190–98. doi: 10.1249/MSS.0b013e3182656f13. [DOI] [PubMed] [Google Scholar]
- 20.Petrofsky J, Al Malty A, Suh HJ. Isometric endurance, body and skin temperature and limb and skin blood flow during the menstrual cycle. Med Sci Monit. 2007;13(3):CR111–17. [PubMed] [Google Scholar]
- 21.Petrofsky JS, LeDonne DM, Rinehart JS, Lind AR. Isometric strength and endurance during the menstrual cycle. Eur J Appl Physiol Occup Physiol. 1976;35(1):1–10. doi: 10.1007/BF00444652. [DOI] [PubMed] [Google Scholar]
- 22.Yim J, Petrofsky J, Berk L, et al. Differences in endothelial function between Korean-Asians and Caucasians. Med Sci Monit. 2012;18(6):CR337–43. doi: 10.12659/MSM.882902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrofsky J, Goraksh N, Alshammari F, et al. The ability of the skin to absorb heat; the effect of repeated exposure and age. Med Sci Monit. 2011;17(1):CR1–8. doi: 10.12659/MSM.881315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrofsky J, Paluso D, Anderson D, et al. The contribution of skin blood flow in warming the skin after the application of local heat; the duality of the Pennes heat equation. Med Eng Phys. 2011;33(3):325–29. doi: 10.1016/j.medengphy.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Park SK, Stefanyshyn DJ, Loitz-Ramage B, et al. Changing hormone levels during the menstrual cycle affect knee laxity and stiffness in healthy female subjects. Am J Sports Med. 2009;37(3):588–98. doi: 10.1177/0363546508326713. [DOI] [PubMed] [Google Scholar]
- 26.Heitz NA, Eisenman PA, Beck CL, Walker JA. Hormonal changes throughout the menstrual cycle and increased anterior cruciate ligament laxity in females. J Athl Train. 1999;34(2):144–49. [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Nakhli HH, Petrofsky JS, Laymon MS, et al. The use of thermal infrared imaging to assess the efficacy of a therapeutic exercise program in individuals with diabetes. Diabetes Technol Ther. 2012;14(2):159–67. doi: 10.1089/dia.2011.0187. [DOI] [PubMed] [Google Scholar]
- 28.Al-Nakhli HH, Petrofsky JS, Laymon MS, Berk LS. The use of thermal infra-red imaging to detect delayed onset muscle soreness. J Vis Exp. 2012;22(59) doi: 10.3791/3551. pii: 3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawhorn KW, Howell SM, Traina SM, et al. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079–86. doi: 10.1016/j.arthro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Araki D, Kuroda R, Kubo S, et al. The use of an electromagnetic measurement system for anterior tibial displacement during the Lachman test. Arthroscopy. 2011;27(6):792–802. doi: 10.1016/j.arthro.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Nordez A, McNair PJ, Casari P, Cornu C. The effect of angular velocity and cycle on the dissipative properties of the knee during passive cyclic stretching: a matter of viscosity or solid friction. Clin Biomech (Bristol, Avon) 2009;24(1):77–81. doi: 10.1016/j.clinbiomech.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Karageanes SJ, Blackburn K, Vangelos ZA. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin J Sport Med. 2000;10(3):162–68. doi: 10.1097/00042752-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Van Lunen BL, Roberts J, Branch JD, Dowling EA. Association of Menstrual-Cycle Hormone Changes with Anterior Cruciate Ligament Laxity Measurements. J Athl Train. 2003;38(4):298–303. [PMC free article] [PubMed] [Google Scholar]
- 34.Shultz SJ, Levine BJ, Nguyen AD, et al. A comparison of cyclic variations in anterior knee laxity, genu recurvatum, and general joint laxity across the menstrual cycle. J Orthop Res. 2010;28(11):1411–17. doi: 10.1002/jor.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eiling E, Bryant AL, Petersen W, et al. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):126–32. doi: 10.1007/s00167-006-0143-5. [DOI] [PubMed] [Google Scholar]
- 36.Bell DR, Blackburn JT, Norcorss MF, et al. Estrogen and muscle stiffness have a negative relationship in females. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):361–67. doi: 10.1007/s00167-011-1577-y. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Suggs J, Gill T. The effect of anterior cruciate ligament injury on knee joint function under a simulated muscle load: a three-dimensional computational simulation. Ann Biomed Eng. 2002;30(5):713–20. doi: 10.1114/1.1484219. [DOI] [PubMed] [Google Scholar]
- 38.Herman K, Barton C, Malliaras P, Morrissey D. The effectiveness of neuromuscular warm-up strategies, that require no additional equipment, for preventing lower limb injuries during sports participation: a systematic review. BMC Med. 2012;10:75. doi: 10.1186/1741-7015-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myklebust G, Engebretsen L, Braekken IH, et al. Prevention of noncontact anterior cruciate ligament injuries in elite and adolescent female team handball athletes. Instr Course Lect. 2007;56:407–18. [PubMed] [Google Scholar]
- 40.Slauterbeck JR, Fuzie SF, Smith MP, et al. The Menstrual Cycle, Sex Hormones, and Anterior Cruciate Ligament Injury. J Athl Train. 2002;37(3):275–78. [PMC free article] [PubMed] [Google Scholar]
- 41.Wojtys EM, Huston LJ, Boynton MD, et al. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am J Sports Med. 2002;30(2):182–88. doi: 10.1177/03635465020300020601. [DOI] [PubMed] [Google Scholar]