Improvement in clinical imaging technologies has made it possible to resolve small, previously invisible lesions in the brains of elderly humans. Initially, these lesions were called “silent strokes” because they do not present with dramatic acute symptoms like major stroke. It was later shown that these small lesions have cognitive consequences, and are a contributing factor to age-related mental decline and dementia 1-3. In analogy to large strokes, these microscopic lesions are thought to be caused by disruptions of small blood vessels. The lesions are small simply because the territory covered by a small vessel is limited in size. There are two categories of vascular events that could lead to these lesions. First, occlusions of small vessels can prevent blood flow from reaching a region of brain tissue leading to ischemic damage. In addition, microscopic deposits of red blood cell lysis products found in postmortem human studies suggest that microvessels might also hemorrhage 4. Understanding the causes and effects of these small lesions is complicated by the small size of the vessels thought to be involved, resulting in a limited number of animal models that reflect the human pathology. Recent advances in optical tools, both in chronic, high-resolution imaging with multiphoton microscopy and in the innovative use of laser ablation and photothrombosis to generate targeted vascular lesions, have enabled controlled studies of how clotting or hemorrhage of small vessels affect the health and function of brain cells 5, 6. Femtosecond laser ablation, in which a high power laser pulse can be used to selectively ionize a portion of a blood vessel even when it is below the surface of brain tissue, can be used to generate both occlusions and hemorrhages in microvessels (Figure 1) 5. Combined with two-photon imaging in transgenic animals that express fluorescent proteins in specific cells, the response of brain cells to these lesions can be carefully tracked. These advances enable investigations in experimental animal models that can recapitulate the variety found in human pathology. Recent studies suggest that the story may not be quite as simple as once thought and that the pathological processes underlying these lesions and their relation to cognitive function can have several possible mechanisms.
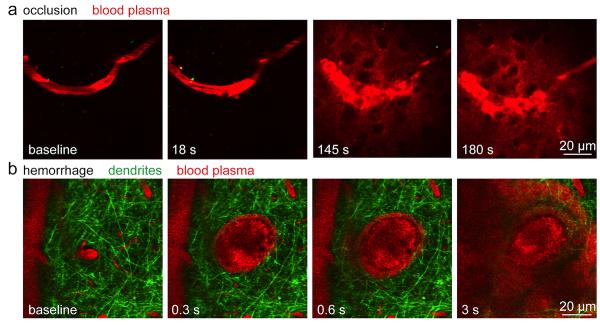
Figure 1. Femtosecond laser ablation can be used to generate experimental models of ischemic and hemorrhagic microstroke.
a) Time-lapse, two-photon imaging of fluorescently labeled blood plasma (red) during the formation of an occlusion in a targeted sub-surface brain arteriole. Irradiation by a series of tightly-focused femtosecond laser pulses is used to injure the endothelium of the targeted vessel and trigger clotting. b) Hemorrhage from a penetrating arteriole generated by irradiation with a single high-energy laser pulse. Neuronal processes (green) are displaced by the expanding hematoma while blood plasma (red) pushes into the brain parenchyma. Red blood cells are visualized as dark volumes within the fluorescent blood plasma. Time stamps indicate the time after the first laser irradiation.
Occlusions of penetrating arterioles lead to microinfarction
Strokes can range in size from affecting nearly the entire brain to lesions only observable with a microscope. In models of occluded vessels, the extent of tissue affected by a blockage depends critically on the kind of vessel blocked and the degree of collateralization 6-9. Penetrating arterioles represent a critical bottleneck in flow to the cortex. These terminal vessels branch off from the highly-collateralized surface arteriole network and feed the capillary beds in the cortex. They dive almost straight down into the brain do not make connections with other arterioles. One determinant of the severity of the impact of a vessel occlusion is the amount of blood flow change in downstream vessels. Femtosecond laser ablation can be used to injure the endothelium of a targeted vessel and trigger clotting (Figure 1a). Blood flow changes can then be determined by tracking red blood cell motion in two-photon images. Occlusions in the penetrating arterioles cause a severe drop in flow in the downstream capillaries and lead to a region of neuronal death 6, 10, 11. Dendrite degeneration, which can been observed in real time using transgenic mice that express fluorescent proteins in neurons, is observed within minutes of vascular occlusion (Figure 2a) 12. This degeneration seems to occur in stages and may be correlated with a wave of depolarization similar to spreading depression 12. Over time, this progresses to cellular death in the ischemic region (Figure 2a) 13. The clear damage to neurons suggests that the cognitive impact of small vessel occlusions is due the to neural death in critical brain regions. It is likely that even in less critical regions, loss of neurons could contribute to functional impairments. These lesions are also accompanied by activation of inflammatory cells such as microglia (Figure 2b) and may, like their larger counterparts, involve leukocyte invasion. This inflammatory response may also play a role in the long-term impact of the occlusion on the brain.
Figure 2. Differing modes of pathology and dysfunction around occlusions and hemorrhages of a penetrating arteriole.
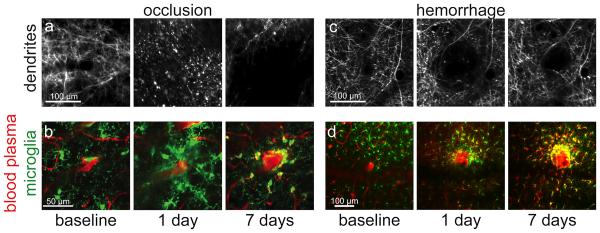
a) Nearby neuronal processes degenerate rapidly and fail to recover over time after a penetrating arteriole occlusion. b) Microglia activate and increase in density near an occlusion. c) Dendrites around a microhemorrhage are displaced by the bleed, but remain structurally sound and relax back toward their original location over time. d) Microglia rapidly activate after a microhemorrhage and leave a long-lived dense shell of ameboid cells immediately adjacent to the lesion. Panels a, c and d are adapted from Rosidi, et al 16.
Experimental hemorrhages of microvessels resemble human microhemorhhages
In addition to ischemic lesions, postmortem histological studies and improved MRI imaging have suggested that dementia patients often have microscopic bleeds in their brain. Although the number of lesions detected by MRI is correlated with cognitive decline 14, 15, post-mortem pathology studies often do not show an infarct near microhemorrhages. Using femtosecond laser ablation, hemorrhages as small as 50 μm in diameter that match the size observed in human studies can be generated in rodent models (Figure 1b). One surprising observation was that a hematoma made of densely packed red blood cells can form around a ruptured arteriole and compress the surrounding tissue, but still leave the bleeding vessel patent and free of significant blood flow changes 5. The bleeding is likely limited by thrombosis along the ruptured vessel wall so that after a brief time, the hematoma size stabilizes 16. The fluid component of the blood penetrates much further into the tissue than red blood cells, essentially replacing all the interstitial fluid with blood plasma within several hundred micrometers of the hemorrhaged vessel.
Microhemorrhages cause sub-lethal inflammation that may lead to neural dysfunction
Recent experiments with this new model of microhemorrhage have suggested a very different mechanism for neuron dysfunction after microhemorrhages compared to ischemic lesions. Unlike occlusions of the same class of vessels, a small hemorrhage does not seem to cause neuronal death or even obvious structural alterations in dendrites (Figure 2c) 16. Over several days, the hematoma appears to degrade somewhat, allowing some structures displaced by the hematoma, such as neuronal processes, to relax back into their original position. This apparent lack of direct damage to neurons or their processes is consistent with the observation in human samples that although some microbleeds show some apoptosis in nearby cells, many bleeds show no evidence of cell death and there is almost never signs of true infraction 17. Inflammatory changes, however, happen within minutes of the hemorrhage, as evidenced by a redirection of microglia processes toward the hemorrhage site. Over the next several days, microglia density near the hemorrhage increases and these cells display an activated, amoeboid morphology (Figure 2d). Astrocytes within this area also show increased reactivity as reported by increased expression of glial fibrillary acid protein. Studies of sterile, laser-based non-vascular injuries to the brain suggest that the initial activation of astrocytes provide directional cues that polarize the microglia in the direction of the injury 18, 19. Human studies also find inflammatory cells around microbleeds, including macrophages, activated microglia and lymphocytes 17.
Over about a week, most of the activated microglia within a few hundred micrometers of the hemorrhage return to normal shapes, but a dense cluster of microglia or perhaps cells derived from blood-borne monocytes remains packed around the lesion (Figure 2d) 16. Such chronic inflammation can remain at the lesion site for many months. Recent data indicating that microglia and astrocytes play an active role in regulating synaptic strength and perhaps even connectivity 20, 21 suggest that this rapidly-initiated but chronically sustained inflammatory response could have functional consequences for neuronal wiring and activity. Microglia are now recognized as having an active role in modulating synapse number in both development and throughout life and might be involved in pruning unwanted connections between neurons 22, 23. Could changes in microglia activity and number after a microhemorrhage lead to an altered ability of microglia of properly regulate synapses? Increases in the turnover rate of dendritic spines can be caused by mild inflammatory injuries such as those caused by cranial window surgeries and infection 24. Could the microglial activation observed near a microhemorrahge lead to similar changes in spine turnover rates near the hemorrhage? Could such inappropriate rewiring of neural connectivity be a novel mechanism through which microhemorrhages, or other conditions that drive neuroinflammation, exert a cognitive effect?
Novel optical tools have enabled the creation of robust animal models of microvascular clots and hemorrhages as well as enabled the detailed study of the response of different classes of brain cells to these lesions. These studies suggest that for neurons, ischemia caused by occlusions is more damaging than a bleed from the same vessel class. However, microhemorrhages cannot be ignored because they can lead to long-lasting alterations in the composition and function of cells around the neurons, which might lead to unknown cognitive effects.
Acknowledgments
Sources of Funding. This work was supported by the American Heart Association (0735644T to C.B.S) and the NIH (AG031620 to N.N. and NS065357 to C.B.S.).
Footnotes
Disclosures. The authors have no financial disclosures or other conflicts of interest.
References
- 1.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 2.Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, et al. Cerebral microbleeds are associated with worse cognitive function: The rotterdam scan study. Neurology. 2012;78:326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 3.Gregoire SM, Smith K, Jager HR, Benjamin M, Kallis C, Brown MM, et al. Cerebral microbleeds and long-term cognitive outcome: Longitudinal cohort study of stroke clinic patients. Cerebrovasc Dis. 2012;33:430–435. doi: 10.1159/000336237. [DOI] [PubMed] [Google Scholar]
- 4.Cullen KM, Kocsi Z, Stone J. Pericapillary haem-rich deposits: Evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab. 2005;25:1656–1667. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura N, Schaffer CB, Friedman B, Tsai PS, Lyden PD, Kleinfeld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: Three models of stroke. Nat Methods. 2006;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaffer CB, Friedman B, Nishimura N, Schroeder LF, Tsai PS, Ebner FF, et al. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol. 2006;4:e22. doi: 10.1371/journal.pbio.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen J, Nishimura N, Fetcho RN, Iadecola C, Schaffer CB. Occlusion of cortical ascending venules causes blood flow decreases, reversals in flow direction, and vessel dilation in upstream capillaries. J Cereb Blood Flow Metab. 2011;31:2243–2254. doi: 10.1038/jcbfm.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: An anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 10.Drew PJ, Shih AY, Driscoll JD, Knutsen PM, Blinder P, Davalos D, et al. Chronic optical access through a polished and reinforced thinned skull. Nat Methods. 2010;7:981–984. doi: 10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura N, Rosidi NL, Iadecola C, Schaffer CB. Limitations of collateral flow after occlusion of a single cortical penetrating arteriole. J Cereb Blood Flow Metab. 2010;30:1914–1927. doi: 10.1038/jcbfm.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih AY, Driscoll JD, Drew PJ, Nishimura N, Schaffer CB, Kleinfeld D. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J Cereb Blood Flow Metab. 2012;32:1277–1309. doi: 10.1038/jcbfm.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Won Seo S, Hwa Lee B, Kim EJ, Chin J, Sun Cho Y, Yoon U, et al. Clinical significance of microbleeds in subcortical vascular dementia. Stroke. 2007;39:1949–1951. doi: 10.1161/STROKEAHA.106.477315. [DOI] [PubMed] [Google Scholar]
- 15.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Rosidi NL, Zhou J, Pattanaik S, Wang P, Jin W, Brophy M, et al. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PloS One. 2011;6:e26612. doi: 10.1371/journal.pone.0026612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrag M, McAuley G, Pomakian J, Jiffry A, Tung S, Mueller C, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: A postmortem mri study. Acta Neuropathol. 2010;119:291–302. doi: 10.1007/s00401-009-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hines DJ, Hines RM, Mulligan SJ, Macvicar BA. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia. 2009;57:1610–1618. doi: 10.1002/glia.20874. [DOI] [PubMed] [Google Scholar]
- 19.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. Atp mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 24.Kondo S, Kohsaka S, Okabe S. Long-term changes of spine dynamics and microglia after transient peripheral immune response triggered by lps in vivo. Mol Brain. 2011;4:27. doi: 10.1186/1756-6606-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]