Abstract
We previously reported that retrovirally transduced platelet-derived growth factor-B (PDGFB) in glial progenitors of the rat cerebral white matter, subventricular zone, or brain stem induced malignant brain tumors closely resembling human glioblastoma (GBM). While human GBMs may progress over the period of several months to a few years, prospective, long-term in-vivo observation of histological changes of the tumor tissues is not feasible in these models, because the animals undergo rapid tumor progression and mortality within approximately 1 month. We thus performed successive, long-term in-vivo transplantation of the PDGFB-induced tumor cells into the rat cerebrum. Primary retroviral transduction of PDGFB in the glial progenitors of the rat basal ganglia induced malignant glioma resembling human GBM or anaplastic oligodendroglioma (AOL) consisting of relatively monomorphous tumor cells expressing markers for the oligodendrocyte lineage. In the course of long-term successive transplantation, tumor cells presented pleomorphism as well as focal GFAP expression. This suggests that secondary chromosomal aberration and dysregulation of gene expression following accelerated cell cycle by PDGFB stimulation would induce morphological and immunophenotypic changes in tumor cells. Furthermore, while the primary tumors contained only a minor fraction of proviral GFP-expressing or hemagglutinin-expressing cells, most tumor cells came to express these proviral genes in the course of serial transplantation suggesting a persistent role of PDGFB-expressing cells in maintenance and growth of the tumors. This model would be useful for investigation of the long-term effects of PDGFB stimulation in glioma tissues on anaplastic evolution.
Keywords: Oligodendrocyte precursors, PDGF, Glioma, Animal model, Successive transplantation
Introduction
Glioblastoma (GBM) is the most malignant form of gliomas and is usually considered to be the highest grade astrocytoma (grade IV) [1, 2], although it has been recently recognized that some GBMs contain foci resembling oligodendroglioma [3].
Alteration of platelet-derived growth factor-B (PDGFB) signaling is commonly observed in human gliomas of different histopathological types and grades including both oligodendroglial and astrocytic tumors [4–6]. Several lines of evidence have shown that platelet-derived growth factor (PDGF) plays an important role in the migration, proliferation, and differentiation of glial progenitors, particularly in a subset of progenitors that express PDGF receptor α (PDGFRα). In-vitro studies have shown that PDGF directly stimulates the migration and proliferation of glial progenitors [7–9]. PDGF supplementation in cultures will keep glial precursors cycling and inhibit their differentiation for a limited number of passages, after which they differentiate along the oligodendrocyte lineage [9]. If PDGF is given in combination with basic fibroblast growth factor the cells will remain proliferative and immature indefinitely [10, 11].
Animal models have demonstrated a causal connection between aberrant PDGF signaling and the formation of gliomas [12–18]. We and others previously generated rat cerebral and brainstem malignant glioma models by retroviral transduction of PDGFB in glial progenitors [17, 19, 20]. Histopathological examination of these tumors revealed cellular, diffuse proliferation of glioma cells with relatively monomorphous, small sized nuclei actively infiltrating the surrounding brain tissue. Brisk mitotic activity, pseudopalisading necrosis, and glomeruloid blood vessels were also noted. Immunohistochemistry revealed the tumor cells expressed markers for immature oligodendrocytes, but not those for the astrocytic lineage [17, 19,20]. Interestingly, tumor cells immunopositive for proviral genes constituted only a minor fraction of these cerebral tumor tissues [17, 19]. This suggested either recruitment of non-transformed glial progenitors in tumor progression, silencing of the proviral genes in once-transformed cells [16], or both. Either way, it is interesting that the tumor tissues are maintained and keep growing with only a small number of transgene-expressing cells. While human GBMs may progress over a period of several months to a few years, long-term in-vivo observation of histological changes of the tumor tissues is not feasible with these models because the animals undergo rapid tumor formation and mortality within approximately 1 month. Recently, Calzolari et al. [16] has suggested malignant progression of PDGF-induced murine gliomas with time; however, prospective histopathological changes in their model were not fully documented. We thus conducted successive in-vivo transplantation of PDGFB-induced tumor cells into rat brains to prospectively investigate the long-term changes in the cellular pathology, tissue structure, and the role of proviral gene-expressing cells in the maintenance of the tumor tissue.
Materials and methods
Retrovirus production and injection
A retrovirus vector expressing PDGFB together with hemagglutinin (HA)-tag and enhanced green fluorescent protein (EGFP), pQ-PDGFB-HA-IRES-EGFP, was generated as described elsewhere [17, 19, 20]. Five microliters of the PDGF-green fluorescent protein (GFP) retrovirus (at a titer of 5.0 × 104 CFU/ml) was stereotactically injected into the basal ganglia of postnatal day 5–10 (P5–P10) Sprague–Dawley (SD) rats using a glass capillary (n = 17, stereotactic coordinates relative to bregma: 1.8 mm lateral, 2.0 mm rostral, and 2.5–2.8 mm deep). All animal work was performed according to Institutional Animal Care and Use Committee (IACUC) guidelines of Kyushu University.
Magnetic resonance imaging study
Of the seventeen rats injected with PDGF-GFP retrovirus, 13 randomly selected rats were subjected to magnetic resonance imaging (MRI) studies at 30 days (n = 4), 31 days (n = 1), 34 days (n = 6), 42 days (n = 1), and 84 days (n = 1) after the retroviral injection (dpi). Of the six rats imaged at 34 dpi, 5 were subjected to second imaging at 48 dpi to follow up the tumor growth. The animals were anesthetized by intraperitoneal administration of 2,2,2-tribromoethanol (2.5 mg/10 g body weight; Sigma–Aldrich, St Louis, MO, USA) and immobilized in a Plexiglas frame. T1 and FLAIR images were collected with a 0.3 T open MRI unit (AIRIS-II, Hitachi Medical Corporation, Tokyo, Japan). For contrast enhancement of the tumor tissue, 0.05 ml gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) (OmniScan®; Daiichi Sankyo Company, Tokyo, Japan) was given intraperitoneally before imaging.
Immunohistochemistry
Rat brains were fixed with 4% paraformaldehyde, by transcardial perfusion or immersion, and embedded in paraffin. Five micrometer-thick tissue sections were routinely stained with hematoxylin and eosin. For immunohistochemistry, sections were incubated with the following primary antibodies overnight at 4°C: rabbit anti-Olig2 (1:10000; kind gift from Dr Hideaki Yokoo, Gunma University, Gunma, Japan), mouse anti-nestin (1:500; Developmental Studies Hybridoma Bank, Iowa, USA), rabbit anti-NG2 (1:200; Chemicon, Temecula, CA, USA), rabbit anti-PDGFRα (1:100; Cell Signaling, Danvers, MA, USA), rabbit anti-GFP (1:500; Invitrogen, Eugene, OR, USA), mouse anti-HA (1:500; Covance, Berkeley, CA, USA), mouse anti-proliferating cell nuclear antigen (PCNA) (1:200; NovoCastra, Newcastle, UK), and rabbit anti-glial fibrillary acetic protein (GFAP) (1:1000; Dako, Glostrup, Denmark) each diluted in 5% normal goat serum–phosphate-buffered saline. Immunoreactivity was detected by the streptavidin–biotin complex method. Images were acquired with a microscope equipped with a CCD camera (Olympus AX80 and DP20; Olympus, Tokyo, Japan). PCNA-positive, GFP-positive, and HA-positive ratios were measured in representative microscopic fields (200× power field).
Cell culture
From some of the animals that formed PDGF-induced tumors (n = 8), tumor tissues were freshly dissected out and dissociated with Cell Strainers (BD Falcon™; Franklin Lakes, NJ, USA), and then processed for cell culture as described elsewhere [21, 22]. The cells were cultured in serum-free medium (Neurobasal medium, L-glutamine, B-27, and penicillin/streptomycin/amphotericin, all purchased from Invitrogen, Carlsbad, CA, USA) supplemented with 20 ng/ml epidermal growth factor (EGF, mouse; Sigma, St Louis, MO, USA) and 10 ng/ml basic-fibroblast growth factor (bFGF, human recombinant; Sigma). The culture medium was changed twice a week, and the cells were replated every week. For detection of GFP signals, the cells were grown on poly-L-lysine-coated cover slips, fixed with 4% paraformaldehyde, counterstained with DAPI, and then images were captured with a fluorescent microscope (Olympus BX50).
Long-term serial transplantation in vivo
After establishing long-term cultures of the PDGF-induced tumor cells, we generated a serial transplantation line (line 1) starting from tumor cells prepared from one of the anaplastic oligodendroglioma (AOL)-like tumors (Table 1). The culture was maintained in vitro for 178 days, as described, without losing high proliferative activity. The tumor cells were then injected into the basal ganglia of the second-generation rats (n = 4, 3 × 104 cells in 5 μl suspension for each rat) using the same stereotactic procedures as already described. When the animals showed signs of morbidity for example weight loss, seizures, abnormal posture, and/or periorbital hemorrhage, they were sacrificed and the tumor tissues were freshly dissected out. Thereafter, a serial transplantation was performed through the fourth generation in a similar manner using at least 6.5 × 103 tumor cells per animal prepared from one of the animals from the previous generation (n = 2–4 for each generation), except that the tumor cells were directly transplanted to the next generations immediately after dissociation without intermittent cell culture. The dissociated tumor cells from each generation were partly saved and maintained in culture for enzyme-linked immunosorbent assay (ELISA, see below). After sampling vital tumor cells for transplantation and ELISA, the remaining brain tissues, and the brain tissues from the other animals of the same generations, were fixed and subjected to histopathological study.
Table 1.
Summary of PDGF-GFP retrovirus injection
| Days of age at injection |
Sacrifice (dpi) |
MRI findings |
Histology |
|---|---|---|---|
| P5 | 37 | 34○ | Not confirmeda |
| P5 | 48 | 34○ | AOL |
| 48○ | |||
| P5 | 49 | 34○ | Mixed |
| 48○ | |||
| P5 | 49 | 34× | Mixed |
| 48○ | |||
| P5 | 49 | 34× | GBM |
| 48○ | |||
| P5 | 49 | 34× | No tumor |
| 48× | |||
| P6 | 41 | Not done | Mixed |
| P6 | 41 | Not done | AOLb |
| P6 | 53 | Not done | AOL |
| P6 | 89 | Not done | Not confirmed |
| P10 | 42 | 30○ | GBM |
| P10 | 42 | 42○ | GBM |
| P10 | 48 | 31○ | AOL |
| P10 | 59 | 30○ | Mixed |
| P10 | 59 | 30○ | GBM |
| P10 | 87 | 84○ | Not confirmed |
| P10 | 120 | 30× | No tumor |
○ and × in MRI findings indicate tumor formation-positive and negative at the indicated days post injection, respectively
P, postnatal days; dpi, days post injection; AOL, anaplastic oligodendroglioma; GBM, glioblastoma; Mixed, mixed AOL + GBM
In-vivo serial transplantation line 2
In-vivo serial transplantation line 1
In addition, we prepared another serial transplantation line through the second generation using tumor cells from a different PDGF-induced tumor (of which histotype was not examined, Table 1) that had been maintained for 114 days in vitro (div) before starting the transplantation (line 2).
PDGFB ELISA
Confluent cultures of primary PDGF-induced tumor cells from the second to the fourth generations of the successive transplantation line 1 were rinsed in PBS and incubated with the above described serum-free medium. Conditioned medium was collected after 24 h and spun at 10,000 rpm, and 100 μl of the medium was used for ELISA. Quantikine kits (R&D Systems, Minneapolis, MN, USA) were used for quantification of PDGFB according to the manufacturer’s instructions. As controls, we used the C6 glioma cell line and glial progenitors isolated from the cerebral subventricular zone (SVZ) of P3 SD rats as described previously [21, 22]. Control cells were cultured in the same serum-free medium for 7 days, by which time they had reached confluence. Experiments were performed in triplicate, and results are presented as mean ± SEM. Optical densities were obtained by measurement of indicator color shift at 450 nm (with a reference filter of 570 nm) on a microplate reader (Multiscan MS, Labsystems, Finland), and concentrations were determined in combination with a software assisted calculation program (Genesis, Lite 3.03, Life Sciences, UK). Statistical analysis was performed by Tukey’s test using JMP® Statistical Discovery Software (SAS Institute Japan, Japan).
Results
PDGF-expressing retrovirus induces tumors that closely resemble human glioblastoma or anaplastic oligodendroglioma
After stereotactic injection of PDGF-expressing retrovirus into the basal ganglia of neonatal rats, brain tumors developed in 88.2% of the animals (15 of 17) (Table 1). The histopathology was examined in 12 of the 15 tumors. The histopathological features of the tumors corresponded to either those of human GBM (4 of 12), diffuse cellular proliferation of small sized, highly anaplastic glioma cells with hyperchromatic nuclei, brisk mitotic activity, pseudopalisading necrosis, and endothelial proliferation; AOL (4 of 12), diffuse cellular proliferation, monomorphous tumor cells, round nuclei, perinuclear halo, brisk mitotic figures, and pseudopalisading necrosis, or a mixture of these (AOL + GBM) (4 of 12) (Fig. 2).
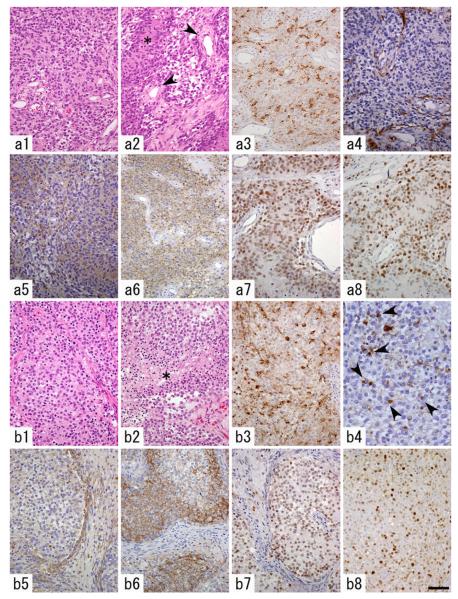
Fig. 2.
Histopathological features of a PDGF-induced glioblastoma (GBM)-like tumor (a) and an anaplastic oligodendroglioma (AOL)- like tumor (b). a1 Cellular, diffuse proliferation of tumor cells with hyperchromatic nuclei. a2 Pseudopalisading necrosis (asterisk) and endothelial proliferation (arrowheads) were observed. a3 A subset of the tumor cells are immunopositive for proviral HA-tag. a4 GFAP expression is restricted to entrapped, non-neoplastic astrocytes. a5–a7 Tumor cells are diffusely immunopositive for nestin (a5), NG2 (a6) and Olig2 (a7). a8 PCNA-immunostaining reveals high proliferative activity of the tumor cells. b1 Diffuse proliferation of tumor cells with round, monomorphous nuclei and perinuclear halo. b2 Necrosis of the tumor tissue is frequently seen (asterisk). b3 A subset of the tumor cells are immunopositive for proviral HA-tag. b4 GFAP expression is noted in minigemistocyte-like or gliofibrillary oligodendrocyte-like tumor cells (arrowheads) and a small number of entrapped, nonneoplastic astrocytes. b5 Nestin expression is not as prominent as that in GBM-like tumors. b6, b7 Tumor cells are diffusely immunopositive for NG2 (b6) and Olig2 (b7). b8 PCNA-immunostaining reveals the high proliferative activity of the tumor cells. Scale bar 20 μm (b4), 50 μm (a1–a8, b1–b3, b5–b8)
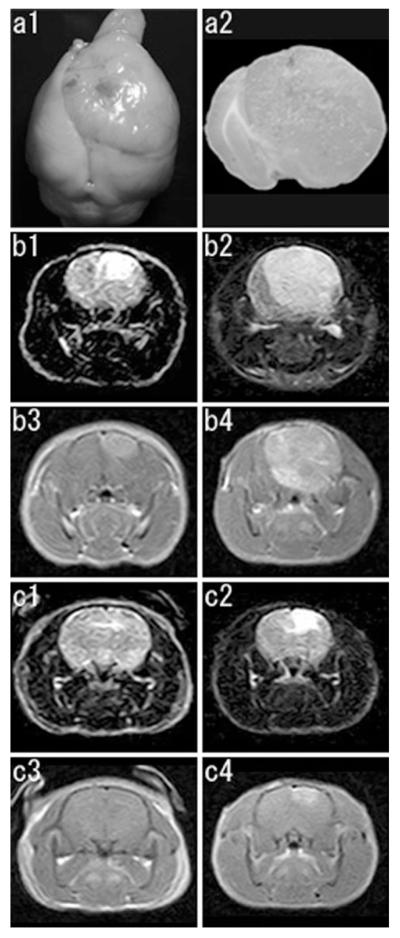
We could follow development and progression of the tumors by MRI scanning in 11 of the 13 rats. PDGF retrovirus-induced tumor was visible by 30 dpi at the earliest with edema around a small tumor. The tumors were uniformly hypointense to isointense in T1-weighted imaging and hyperintense in FLAIR imaging, and showed strong, diffuse enhancement on Gd-DTPA administration. Of the five rats subjected to the serial imaging at 34 and 48 dpi, 2 showed tumor formation at 34 dpi and rapid progression during the following 2 weeks. Two of the remaining 3 animals were tumor-negative at 34 dpi, but developed tumors at 48 dpi (Fig. 1; Table 1). We could compare MRI findings and histopathology in 7 animals. Of these, two showed GBM-like features, 2 showed AOL-like features, 2 showed mixed (GBM + AOL) features, and the remaining 1 showed no tumor formation even on microscopic inspection. There was no correlation among the days of age at retroviral injection, MRI findings, and histopathology of the tumors (Table 1).
Fig. 1.
a Macroscopic features of a PDGF-induced tumor (GBM, viral injection at P10, 42 days post injection, the same animal as presented in b). Dorsal view of the whole brain (a1),and a coronal brain slice at the level of the viral injection (a2). A large tumor is noted with remarkable midline shift. b, c Serial MRI studies of PDGF-induced tumors. Representative coronal images are presented (b1, b2, c1, c2, FLAIR images; b3, b4, c3, c4, T1 weighted images with gadolinium enhancement). b This animal shows development of a tumor at 34 dpi (b1, b3) and its rapid progression at 48 dpi (b2, b4). c This animal shows no tumor formation at 34 dpi (c1, c3), but development of a tumor at 48 dpi (c2, c4)
To characterize the cellular composition of the tumors, we performed immunohistochemical analysis for retrovirally encoded genes, GFP and HA, and a variety of glial markers. Irrespective of the histotypes, the vast majority of the tumor cells expressed markers for oligodendrocyte lineage, including NG2 (Fig. 2a6, b6) and Olig2 (Fig. 2a7, b7). The tumor cells were generally negative for GFAP (Fig. 2a4), however, AOL-like tumors showed a fraction of GFAP-immunopositive tumor cells morphologically resembling minigemistocytes or gliofibrillary oligodendrocytes (Fig. 2b4). GBM-like tumors and AOL-like tumors showed similar PCNA-labeling indices (59 and 60.5%, respectively, in a representative 200× microscopic field in each histotype; Fig. 2a8, b8). On the other hand, GBM-like tumors showed more prominent immunopositivity for nestin than AOL-like ones (Fig. 2a5, b5), suggesting that the former had a more undifferentiated phenotype. Only a subset of the tumor cells expressed a detectable level of proviral GFP or PDGF-HA in both GBM-like and AOL-like tumors. HA-positive cells constituted 24.9 and 17.1% of the all tumor cells in a representative 200× microscopic field in each histotype, as previously reported by Assanah et al. [17]. The vast majority of tumor cells expressed PDGFRα (data not shown), as previously reported [17, 19, 20].
PDGF-induced tumor cells can be maintained in vitro and passaged by serial transplantation in vivo with histological changes
Primary PDGF-induced tumor cells from all the 8 tumors tested could be kept in long-term culture. In one of the primary cultures, we maintained cells for up to 365 div. The cells well endured weekly passage during this period of time and their proliferative activity did not show apparent decrease (data not shown). In the course of longterm culture we noticed that GFP-negative cells initially having constituted a large population in the primary culture eventually died off and only GFP-positive cells survived (Fig. 3a1–3).
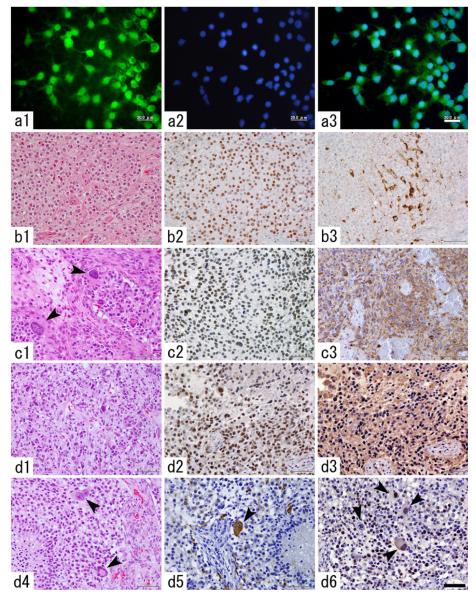
Fig. 3.
Long-term in-vitro culture and in vivo serial transplantation of PDGF-induced AOL-like tumor cells. a1–3 GFP-positive tumor cells are enriched in the course of long term culture (a1 GFP-immunocytochemistry, a2 nuclear staining with DAPI, a3 merged image). Scale bar 20 μm. b–d Histological changes in the tumor tissues over a series in-vivo transplantations (b1–3 the first-generation tumor, c1–3 the second-generation tumor, d1–6 the fourth-generation tumor). While the first-generation tumor showed diffuse proliferation of the tumor cells with round, monomorphous nuclei and perinuclear halo (b1), the second-generation tumor shows the presence of giant tumor cells (c1, arrowheads). Most of the tumor cells are immunopositive for Olig-2 throughout the generations (b2, c2, d2). The first generation tumor contains a subset of proviral HA-expressing tumor cells (b3), however, in the second generation and thereafter, most of the tumor cells show diffuse HA expression (c3, d3). The fourth generation tumor focally shows increased nuclear polymorphism (d1) and the presence of bizarre, multinucleated giant cells (d4). A fraction of giant tumor cells strongly express GFAP (d5, arrowhead). There are giant tumor cells with proliferative activity as revealed by PCNA-immunostaining (d6, arrowheads). Scale bar 50 μm
In the in vivo serial transplantation line 1, the histology of the original, primary tumor showed diffuse, cellular proliferation of glioma cells with round nuclei and perinuclear halo. Brisk mitotic activity and pseudopalisading necrosis were observed, resembling human AOL (Fig. 3b1).The second-generation rats (n = 4) presented symptoms and were sacrificed at 66, 101, 115, and 115 dpi. Histologically, the tumor tissues of the second generation basically showed AOL-like features as in the original tumor. However, there were many large tumor cells with markedly increased nuclear size that had never been detected in the original tumor tissue (Fig. 3c1). The third-generation rats (n = 2) presented signs of morbidity and were sacrificed at 35 and 41 dpi. The tumor tissues of the third-generation rats showed similar histological features to those of the tumors of the second-generation rats (data not shown). The fourth-generation rats (n = 4) showed signs of morbidity at 40, 40, 40, and 47 dpi. The tumor tissues of the fourth-generation rats focally showed further increase of cellular polymorphism, nuclear atypia, and the presence of multinucleated giant cells (Fig. 3d1, d4).
In line 2, three of the eleven animals of the second generation developed symptoms by 49 dpi, and were sacrificed at that point. All the tumors histologically showed not only pseudopalisading necrosis, microvascular endothelial proliferation, and brisk mitotic activity, but also cellular polymorphism, and nuclear atypia of the tumor cells, including multinucleated giant cells (data not shown).
Immunohistological differences in tumor tissues among the generations
To characterize and compare the cellular composition of the tumors among generations of the serial transplantation, we performed immunohistochemical analysis for proviral genes, GFP and HA, and a variety of glial markers. In all generations, the vast majority of the tumor cells were diffusely immunopositive for Olig2 (Fig. 3b2, c2, d2). In the first generation, a small number of tumor cells were immunopositive for GFP and HA (Fig. 3b3). However, in the second generation and thereafter, most tumor cells showed cytoplasmic staining for GFP and HA (Fig. 3c3, d3). The first and the fourth generations showed similar PCNA-labeling indices (60.5 and 67.2% in a representative 200× microscopic field, respectively, compare Figs. 2b8 and 3d6).
The tumor cells were generally negative for GFAP from the first through the fourth generations except for minigemistocyte-like or gliofibrillary oligodendrocyte-like cells, however, from the second generation onward, some of the giant tumor cells also exhibited immunoreactivity for GFAP (compare Figs. 2b4 and 3d5).
We defined giant cells as cells with more than twice the nuclear size of other monomorphic tumor cells. We evaluated 100 giant tumor cells for proliferative activity and transgene expression. Positive ratios of PCNA and GFP expression in giant tumor cells were 43 and 47%, respectively.
PDGF-induced tumor cells keep secreting PDGF over serial transplantation
Persistent expression of proviral genes in PDGF-induced tumor tissues over serial transplantation indicated that the tumor cells kept secreting PDGF to their local environment. To directly address this issue, we measured, by ELISA, the levels of PDGFB in conditioned medium of tumor cells cultured from the second to fourth generations. This revealed that tumor cells from each generation secreted high levels of PDGFB with significantly increasing levels over generations (Fig. 4), whereas C6 cells and oligodendrocyte precursors showed no detectable level of PDGFB (data not shown).
Fig. 4.
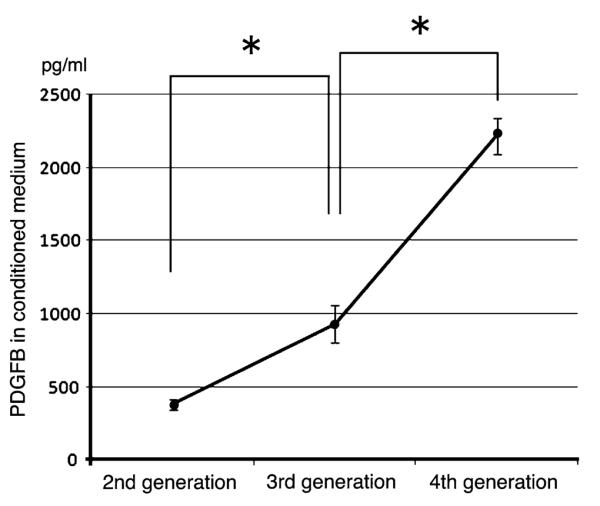
Quantification of PDGF-B expression levels in PDGF-induced tumors over in-vivo serial transplantation. The concentration of PDGF-B was measured by ELISA in conditioned medium of the tumor cells isolated from the second to fourth generations of serial transplantation. The values represent the mean ± SEM from three separate experiments. Asterisks indicate p < 0.05
Discussion
In this study, overexpression of PDGFB in the glial progenitors of the neonatal rat basal ganglia efficiently induced either high-grade oligodendroglial (AOL-like) tumors or GBM-like tumors, both of which consisted of tumor cells with immunophenotype of the oligodendrocyte lineage. Primary PDGF-induced tumor cells could be cultured and indefinitely propagated in vitro, and proviral gene-expressing tumor cells were enriched in the course of long-term culture. These cultured cells formed secondary tumors on transplantation into rat brain, and long-term histological follow-up of the tumor tissues by serial transplantation revealed changes of cell morphology, immunophenotypes, and ratio of proviral gene-positive cells. Furthermore, we confirmed by ELISA that tumor cells kept expressing PDGFB with increasing expression levels over serial transplantation.
We previously reported that injecting the same PDGF-expressing retrovirus into the subcortical white matter of adult rats consistently induced the formation of brain tumors with GBM-like histology [17]. However, in this study, in which we infected glial progenitors in the neonatal basal ganglia, the tumors exhibited histological features of either AOL, GBM, or mixed AOL + GBM. We currently do not know what difference determined the histotypes of the tumor in this model. One possibility is that there are intrinsic differences in the tumorigenic potential of neonatal versus adult progenitor populations. In line with these findings, Appolloni et al. [15] demonstrated that infecting embryonic neural progenitors with PDGFB-expressing retrovirus induced tumors that express markers of the oligodendroglial lineage, even when displaying histopathological traits typical of GBM, such as necrosis, pseudopalisades, and widespread angiogenesis. Furthermore, the PDGF expression level has been shown to correlate with tumor grade; higher levels of PDGF were expressed in anaplastic astrocytomas and glioblastomas than in low-grade gliomas [6, 23]. Both in a mouse glioma model and primary human glioma tissues, it has been shown that increased levels of PDGFB correlated with high-grade features of tumors including shortened latency and histological anaplasia [24, 25]. It is thus possible that the current retrovirus vector could induce oligodendroglial tumors of different grades depending on subtle differences in microenvironment that affect the local level of PDGF concentration.
In vivo successive transplantation induced increased nuclear atypia and cellular polymorphism of the tumor cells, and appearance of giant tumor cells, as in human GBMs, whereas the primary tumor cells showed small sized, relatively monomorphous nuclei [17, 19, 20], even in those presenting otherwise GBM-like histological features. Basically, tumor cells were diffusely immunopositive for oligodendroglial markers from the first through fourth generations. Although most tumor cells were negative for GFAP in the primary tumors, a small fraction of the tumor cells morphologically resembling minigemistocytes or gliofibrillary oligodendrocytes expressed GFAP in AOL-like tumors. In the second-generation tumors onward, however, a small number of PCNA-positive and/or GFP-positive giant tumor cells were immunopositive for GFAP. There is a possibility that long-term in-vitro culture followed by in-vivo culture led to dysregulation of gene expression in some tumor cells originating from the oligodendrocyte lineage. It is also possible that minigemistocyte-like or gliofibrillary oligodendrocyte-like cells developed into the GFAP-positive giant tumor cells.
Currently, only astrocytic tumors are recognized to reach grade IV in the World Health Organization (WHO) classification [3] although the existence of “glioblastoma with oligodendroglioma components”, which contains foci that resemble oligodendroglioma in the background of GBM, has been increasingly acknowledged [2, 3]. Recent gene expression-based molecular classification of GBMs revealed the “proneural” subtype of GBMs exhibited a phenotype of the oligodendroglial lineage [26]. This suggests the possibility that some GBMs could have oligodendrocyte origin. Indeed, we clearly demonstrated that gliomas initially showing features of oligodendroglioma developed into more pleomorphic tumors with proliferative GFAP-positive tumor cells that no longer retained the morphological characteristics of either minigemistocytes or gliofibrillary oligodendrocytes, their features resembling glioblastoma with oligodendroglioma components. The tumor cells in the current serial transplantation model survived many accelerated cell cycles, and this could have induced mitotic errors and accumulation of additional genetic alterations, which might have led to the above-mentioned histological changes. These findings thus indirectly support the notion that at least a part of “glioblastomas with oligodendroglial component” [3] could indeed be regarded as “grade IV oligodendroglioma”, as stated by Calzolari et al. [16].
ELISA revealed that PDGF-induced tumor cells kept secreting PDGFB at high levels throughout the course of serial transplantation. This suggests a persistent role of PDGF in maintenance and growth of the tumor tissue. The increase of proviral gene-immunopositive cells in the tumor tissue in the serial transplantation may correlate with elevation of the secreted PDGF level in the later generations. It has been reported that the development of PDGFB-induced tumors is dependent on continuous PDGFB signaling [24, 27]. Our histopathological findings suggest that in oligodendroglial tumors, not only tumor cell proliferation but also anaplastic transformation may be heralded by PDGF signaling. Our data provide further evidence that fully progressed tumors remain addicted to their triggering oncogenic stimulation.
Previous studies have demonstrated that resident progenitors in the adult white matter and the neonatal SVZ of the cerebrum could be recruited into the tumor via paracrine signaling, implying a novel mechanism of tumor growth [17, 19]. We also observed that only a minor fraction of the primary PDGF-induced tumor cells were immunopositive for proviral gene products, suggesting the recruitment of non-transformed glial progenitors. To test this possibility, we infected glial progenitor cells in culture with either PDGF-IRES-DSRED or control GFP expressing retroviruses and then co-injected the red and green cells into the subcortical white matter of naïve adult rats. We found that both populations proliferated massively, giving rise to GBM-like tumors that were composed of PDGF-expressing red cells and greens cells that had been driven to proliferate by paracrine PDGF stimulation [17]. On the other hand, it is also possible that proviral gene expression is silenced in some of the retrovirus infected cells, as Calzolari et al. [16] reported. We observed that the ratio of proviral gene-expressing cells increased during long-term culture and that the high level was maintained over serial transplantation. This suggests that the retrovirally transduced PDGF gene could remain activated for a long period of time in at least a subset of the primary tumor cells. These cells and their progenies would play a persistent, key role in tumor progression. It is necessary to further confirm whether the GFP/HA-negative tumor cells have proviral genes by real-time PCR on sorted GFP-positive and negative cell fractions.
Little is known about the in-vivo mechanisms of PDGF signaling during the initiation and progression of primary human gliomas. Data obtained with our serial transplantation model have potential implications for human pathology including the role of PDGF stimulation in neoplastic transformation and anaplastic progression of the oligodendrocyte lineage.
Acknowledgments
The authors would like to thank Dr Kensuke Sasaki for instruction in statistical analysis, Dr Yoshihiro Seki for helpful comments and supports, and Ms Sachiko Nagae and Kimiko Sato for their excellent technical instruction and assistance (all from Department of Neuropathology, Kyushu University). They also thank Dr Hideki Horikawa (Department of Neuropsychiatry, Kyushu University) for instruction of ELISA, and Dr Sumako Nishimura and Dr Takeshi Yasunaga (Hitachi Medical Corporation) for their excellent technical assistance with open-MRI.
Contributor Information
Rina Torisu, Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Satoshi O. Suzuki, Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan
Kenta Masui, Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
Koji Yoshimoto, Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Masahiro Mizoguchi, Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Makoto Hashizume, Department of Advanced Medical Initiatives, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Peter Canoll, Department of Pathology, Columbia University College of Physicians and Surgeons, New York, USA.
James E. Goldman, Department of Pathology, Columbia University College of Physicians and Surgeons, New York, USA
Tomio Sasaki, Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Toru Iwaki, Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.
References
- 1.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Holland EC, Cairncross JG. Glioma classification: a molecular reappraisal. Am J Pathol. 2001;159:779–786. doi: 10.1016/S0002-9440(10)61750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Rocco F, Carroll RS, Zhang J, et al. Platelet-derived growth factor and its receptor expression in human oligoden-drogliomas. Neurosurgery. 1998;42:341–346. doi: 10.1097/00006123-199802000-00080. [DOI] [PubMed] [Google Scholar]
- 5.Nister M, Libermann TA, Betsholtz C, et al. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988;48:3910–3918. [PubMed] [Google Scholar]
- 6.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–3219. [PubMed] [Google Scholar]
- 7.Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. doi: 10.1002/jnr.490270319. [DOI] [PubMed] [Google Scholar]
- 8.Frost EE, Zhou Z, Krasnesky K, et al. Initiation of oligodendrocyte progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochem Res. 2008;34:169–181. doi: 10.1007/s11064-008-9748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble M, Murray K, Stroobant P, et al. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- 10.Bogler O, Wren D, Barnett SC, et al. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci USA. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinnon RD, Matsui T, Dubois-Dalcq M, et al. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- 12.Dai C, Celestino JC, Okada Y, et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesselager G, Uhrbom L, Westermark B, et al. Complementary effects of platelet-derived growth factor autocrine stimulation and p53 or Ink4a-Arf deletion in a mouse glioma model. Cancer Res. 2003;63:4305–4309. [PubMed] [Google Scholar]
- 14.Uhrbom L, Hesselager G, Nister M, et al. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58:5275–5279. [PubMed] [Google Scholar]
- 15.Appolloni I, Calzolari F, Tutucci E, et al. PDGF-B induces a homogeneous class of oligodendrogliomas from embryonic neural progenitors. Int J Cancer. 2009;124(10):2251–2259. doi: 10.1002/ijc.24206. [DOI] [PubMed] [Google Scholar]
- 16.Calzolari F, Appolloni I, Tutucci E, et al. Tumor progression and oncogene addiction in a PDGF-B-induced model of gliomagenesis. Neoplasia. 2008;10:1373–1382. doi: 10.1593/neo.08814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assanah M, Lochhead R, Ogden A, et al. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Assanah MC, Bruce JN, Suzuki SO, et al. PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia. 2009;57(16):1835–1847. doi: 10.1002/glia.20895. [DOI] [PubMed] [Google Scholar]
- 20.Masui K, Suzuki SO, Torisu R, et al. Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. Glia. 2010;58(9):1050–1065. doi: 10.1002/glia.20986. [DOI] [PubMed] [Google Scholar]
- 21.Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. [PubMed] [Google Scholar]
- 22.Mason JL, Goldman JE. A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- 23.Fleming TP, Saxena A, Clark WC, et al. Amplification and/or overexpression of platelet derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–4553. [PubMed] [Google Scholar]
- 24.Shih AH, Dai C, Hu X, et al. Dose-dependent effects of platelet derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar K, Radotra BD, Vasishta RK, et al. Platelet-derived growth factor expression correlates with tumor grade and proliferative activity in human oligodendrogliomas. Surg Neurol. 2009;72:54–60. doi: 10.1016/j.surneu.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Verhaak RGW, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uhrbom L, Nerio E, Holland EC. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nat Med. 2004;10:1257–1260. doi: 10.1038/nm1120. [DOI] [PubMed] [Google Scholar]