Abstract
Introduction
Most studies on quality of life of breast cancer survivors have not had adequate representation of ethnic minorities. The purpose of this study was to determine whether racial/ethnic differences in quality of life exist between white, African American, and Latina women in the early stages of survivorship.
Methods
2268 women were identified by two Surveillance, Epidemiology and End Results (SEER) registries (6/05-2/07) and asked to complete a survey (mean 9 months post-diagnosis, 72.1% response rate). Latina and African American women were over-sampled. Regression models compared quality of life across race/ethnicity (white, African American, Latina [low vs. high acculturation]), sequentially controlling for sociodemographics, clinical, and treatment factors.
Results
There were significant racial/ethnic differences in quality of life controlling for sociodemographics, clinical factors and treatment factors. Lower acculturated Latinas compared to whites had significantly lower functional well-being, emotional well-being, and breast cancer concerns (p values <0.05). African Americans had significantly higher emotional well-being than whites. Age, co-morbidities, cancer stage, and chemotherapy also influenced quality of life. A significant interaction was found between race/ethnicity and age for physical well-being (p=0.041) and for emotional well-being (p=0.042). Specifically, racial/ethnic differences were only observed among older women (≥50 years), with less acculturated Latinas reporting the lowest quality of life.
Conclusions
Racial/ethnic differences in quality of life exist during the cancer survivorship period. Latinas with low acculturation are a particularly vulnerable subgroup.
Implications
Greater attention should be devoted to identifying women disproportionately affected by breast cancer and developing interventions targeting their unique survivorship concerns.
Keywords: Breast Cancer, Quality of Life, Minority Health, Racial/Ethnic Differences
INTRODUCTION
Improving the quality of life (QOL) of cancer survivors is a priority of the Institute of Medicine and was emphasized by cancer patients themselves at the 2004 President’s Cancer Panel [1, 2]. A recent Institute of Medicine report also highlighted the need to evaluate QOL during the key period following the end of initial diagnosis and treatment [3]. While a number of studies have focused on the QOL of breast cancer survivors [4–12], the extent to which racial/ethnic disparities in QOL exist in the survivorship period is not well known. The proportion of breast cancer survivors who are racial/ethnic minorities is increasing, and this group may be especially vulnerable to poor QOL outcomes relative to white women [13–16]. Few studies, however, have had sufficient minority patients to effectively address this issue [11, 17–19].
Published findings regarding racial/ethnic differences in QOL have been somewhat mixed in studies on cancer survivors. Two recent studies that included white, African American and Latina women found lowest overall QOL among Latina breast cancer survivors [20, 21]. Results from a number of qualitative studies have suggested that Latina women may face some unique challenges during the survivorship period [13, 18]. Results from studies that focused on differences between African American and white women [15, 18, 21–24] have suggested that African American women report better emotional well-being and mental health but lower levels of physical functioning [21, 24, 25].
Whether racial/ethnic differences in QOL vary by levels of sociodemographic or clinical/treatment factors has not been well explored. While some studies have shown that QOL is associated with age at diagnosis [4, 5, 7, 10, 20, 26, 27, 28–30], education [5, 27], the presence of other comorbidities at the time of the cancer diagnosis [20, 31], cancer stage [32], and the receipt of chemotherapy [5, 8, 30, 33], they have not evaluated the association between these factors, race/ethnicity, and QOL.
The association between race/ethnicity and QOL needs to be evaluated in population-based studies with sufficient numbers of racial/ethnic minority breast cancer survivors. In particular, studies are needed that have sufficient numbers of Latinas to examine differences depending on level of acculturation. Lower acculturated Latinas may be at particular risk for poor QOL due to limited access to culturally and linguistically appropriate services and cancer care support [10, 18, 34, 35].
To address these gaps in the literature we conducted a study to address the following questions:
Are there racial/ethnic differences in QOL as women with breast cancer transition into survivorship?
Do these racial/ethnic differences persist after adjustment for other sociodemographic characteristics, clinical factors, and treatment factors?
Do racial/ethnic differences in QOL vary by levels of sociodemographic characteristics, clinical factors, or treatment factors?
METHODS
Study Population
Between June 2005 and February 2007, 3252 women aged 20–79 years diagnosed with primary ductal carcinoma in-situ (DCIS) or invasive breast cancer stages I, II, or III [32] in Los Angeles (LA) and Detroit were selected for the study. Of these women, 119 were excluded because: 1) a physician did not want the patient contacted (n=20), 2) the woman did not speak English or Spanish (n=17), 3) the woman was too ill or incompetent to participate (n=59), or 4) the woman denied having cancer (n=23). Of the 3133 eligible women included in the final sample, 432 (13.8%) could not be contacted, 411 (13.1%) were contacted but did not participate, and 30 (0.9%) women completed the survey but their information could not be merged to SEER data. Thus, 2268 (72.1% of eligible patients) were included in the final analytic sample (96.5% completed written survey, 3.5% completed telephone survey). An analysis of non-respondents versus respondents showed there were no significant differences by age at diagnosis or Hispanic ethnicity. However, non-respondents were more likely to be African American (34.9% vs. 26.2%, p<0.001), to have never married (23.0% vs. 19.3%, p=0.01), to have cancer stage II or III (43.4% vs. 40.5%, p=0.005), and to be less likely to receive lumpectomy (54.5% vs. 63.2%, p=0.02).
Population Sampling and Data Collection
Female breast cancer cases meeting the selection criteria were accrued via rapid case ascertainment as they were reported to the LA Cancer Surveillance Program (LACSP) and the Metropolitan Detroit Cancer Surveillance System (MDCSS) – the Surveillance, Epidemiology and End Results (SEER) program registries for the metropolitan areas of LA, California and Detroit, Michigan. All African American women were selected based on demographic information from the treating hospitals. Because Latina status may not be accurately collected by hospitals, the following sampling strategy was used to increase representation of Latina women in Los Angeles. All women who were designated as Hispanic by the hospital were selected, as well as all women whose surname indicated a high probability of being Latina, based on a list generated from the 1980 US Census. A random sample of the remaining white (non-Spanish surnamed) patients in LA and Detroit were selected to reach the targeted accrual number. Asian women in LA were excluded because these women were enrolled in studies being conducted by other investigators.
Physicians were notified of our intent to contact their selected patients, and if no objection was received, the patients were mailed an introductory letter, survey materials and a $10 cash gift. The survey instrument was translated into Spanish using a standard approach [36]. Los Angeles women likely to be Latina were sent both English and Spanish study materials. The Spanish version of the survey was not sent to Detroit participants because there are very few monolingual Spanish speaking women in metropolitan Detroit. The Dillman survey method was employed to encourage survey response [37]. Information from the survey was merged to SEER data for all patients in the final sample. The study protocol was approved by the Institutional Review Boards of the University of Michigan, University of Southern California, and Wayne State University.
Measures
Sociodemographic characteristics
A variable was created combining survey information on race, ethnicity, and language. Women were asked to indicate their race (White, Black/African American, American Indian or Alaska Native, Asian or Pacific Islander, or some other race) and if they were Hispanic/Latina (yes/no). The Short Acculturation Scale for Hispanics (SASH) was used to determine language preference for Latina women. This measure has been shown to be an efficient, reliable, and valid measure to identify Latinas with low or higher acculturation [22]. The four items in SASH indicate the preference for English or Spanish in different contexts (usually read/speak, think, use at home, use with friends) on a 5-point scale (from “English only” to “Spanish only”). We aggregated across the four items to calculate a mean language preference score. Fifty-five percent of the Latina patients scored ≥ 4 on the 5-point scale (strongly preferring Spanish across contexts). Race/ethnicity was thus divided into four categories (White, African American, Latinas-high acculturation [Latinas-high], and Latinas-low acculturation [Latinas-low]). Compared to Latinas-high, Latinas with less acculturation were much more likely to be foreign born (99.4% vs. 35.2%).
Additional sociodemographic variables obtained from the survey were age at the time of diagnosis (<50, 50–70, >70), level of education (< high school [H.S.], H.S. diploma, > H.S. diploma), employment status at diagnosis (yes/no), and marital status (currently married/partner, divorced/widowed/separated, never married).
Clinical factors
Clinical factors included in the survey were family history of breast cancer (first degree, second degree, no history) and number of comorbidities (0, 1, 2, or more). Information on breast cancer stage was obtained via SEER data using criteria set forth by the American Joint Committee on Cancer (0, I, II, III) [32].
Treatment factors
Treatment factors were obtained from the patient survey and included surgical procedure for breast cancer (lumpectomy or mastectomy), radiation therapy (yes/no), and chemotherapy (yes/no).
Quality of Life
Quality of life was measured by the Functional Assessment of Cancer Therapy-Breast (FACT-B) questionnaire, a 44-item self-report instrument designed to measure multidimensional QOL in breast cancer. The FACT-B consists of the FACT-General (FACT-G) [38] and includes four subscales: Physical Well-Being (7 items), Emotional Well-Being (7 items), Functional Well-Being (6 items), and Social/Family Well-Being (7 items). There is also a Breast Concerns Subscale (breast-specific concerns) with 9 items [39]. Possible responses ranged from 0 (not at all) to 4 (very much). We aggregated across all items to create a dimension score that ranged from 0–28 for physical, functional, and social well-being, 0–24 for emotional well-being, and 0–36 for breast concerns subscale. Higher scores indicate better QOL. The reliability and validity of the FACT-B is well established [39] and we found comparable reliability (Cronbach’s alphas ranged from 0.73–0.89).
Analysis Plan
Of the 2268 available for analysis, we omitted 23 women with stage IV disease and an additional 753 women who had not finished their primary treatment course at the time of the survey (surgical procedure, followed by radiation therapy, and/or chemotherapy if needed). Thus the final analytic sample included 1492 women. All analyses were performed using SAS V9 programming language. Sample weights were applied in analyses, to adjust for design effects resulting from differential selection by race/ethnicity and non-response. SAS is designed for the inclusion of sample weights in all statistical procedures. Descriptive statistics were used to characterize the distribution of study covariates overall and by race/ethnicity. Bivariate associations were investigated between QOL measures and each of the sociodemographic, clinical, and treatment factors, and between race/ethnicity and all study measures. Multivariable linear regression models investigated associations between QOL and race/ethnicity before and after adjustment for sociodemographic, clinical, and treatment factors using a series of sequential models. We estimated the adjusted mean difference in QOL between Latinas (low and high acculturated) and whites and between African Americans and whites adjusting for age (Model 1). We then added other sociodemographic (Model 2), clinical (Model 3), and treatment variables (Model 4). In the final model (Model 4) we tested all two-way interactions between race/ethnicity and the other sociodemographic, clinical, and treatment factors. Two-way interactions were retained in final models if p<0.05.
RESULTS
Table 1 displays the sample characteristics overall and by race/ethnicity. The mean age of the sample was 57.5 (SD=11.3) years and had the following racial/ethnic distribution: white (49.9%), African American (26.5%), Latinas-high (11.0%), and Latinas-low (12.6%). There were significant differences by race/ethnicity across all demographic characteristics, family history, and number of comorbidities (all p values <0.05), but not for treatment factors (all p values >0.08). African American and Latina women were more likely than white women to be <50 years at diagnosis. Latinas-low were far more likely to have less than a high school education than whites, and African American women were less likely than white women to be married.
Table 1.
Sample Characteristics Overall and by Race/Ethnicity (N=1492)
| Overall |
White N=726 | African American N=386 | Latina-high N=160 | Latina-low N=184 | p-value | ||
|---|---|---|---|---|---|---|---|
| N* | % | %** | %** | %** | %** | ||
| Sociodemographic | |||||||
| Age | |||||||
| <50 | 402 | 24 | 21 | 29 | 34 | 30 | 0.037 |
| 50–70 | 842 | 59 | 61 | 54 | 50 | 58 | |
| >70 | 248 | 17 | 18 | 17 | 16 | 13 | |
| Education | |||||||
| < H.S. diploma | 252 | 12 | 5 | 12 | 22 | 70 | <0.001 |
| H.S. diploma | 309 | 20 | 21 | 17 | 17 | 20 | |
| > H.S. diploma | 905 | 68 | 74 | 71 | 61 | 10 | |
| Employment status | |||||||
| Yes | 658 | 56 | 56 | 57 | 61 | 44 | 0.006 |
| No | 820 | 44 | 44 | 43 | 39 | 56 | |
| Marital status | |||||||
| Married/partner | 866 | 62 | 66 | 41 | 68 | 59 | <0.001 |
| Divorced/wid/sep | 460 | 30 | 28 | 42 | 24 | 31 | |
| Never married | 148 | 8 | 6 | 17 | 8 | 9 | |
| Clinical | |||||||
| Family history | |||||||
| 1st degree relative | 283 | 20 | 22 | 19 | 14 | 16 | <0.001 |
| 2nd degree relative | 205 | 14 | 16 | 12 | 11 | 7 | |
| No history | 1004 | 66 | 62 | 70 | 74 | 77 | |
| Breast cancer stage | |||||||
| 0 | 272 | 18 | 17 | 20 | 16 | 21 | 0.445 |
| I | 593 | 43 | 44 | 38 | 41 | 35 | |
| II | 459 | 29 | 28 | 30 | 29 | 33 | |
| III | 168 | 11 | 10 | 12 | 14 | 12 | |
| # co-morbidities | |||||||
| 0 | 579 | 42 | 44 | 31 | 46 | 48 | <0.001 |
| 1 | 425 | 31 | 32 | 30 | 29 | 25 | |
| 2+ | 414 | 27 | 24 | 39 | 25 | 27 | |
| Treatment | |||||||
| Surgical Procedure | |||||||
| Lumpectomy | 1165 | 80 | 81 | 82 | 76 | 76 | 0.296 |
| Mastectomy | 312 | 20 | 19 | 18 | 24 | 24 | |
| Radiation therapy | |||||||
| Yes | 1315 | 89 | 89 | 92 | 89 | 84 | 0.082 |
| No | 161 | 11 | 11 | 8 | 11 | 16 | |
| Chemotherapy | |||||||
| Yes | 797 | 50 | 47 | 54 | 56 | 61 | 0.256 |
| No | 655 | 50 | 53 | 46 | 44 | 39 | |
< 2% missing data on all study variables
Percents are weighted
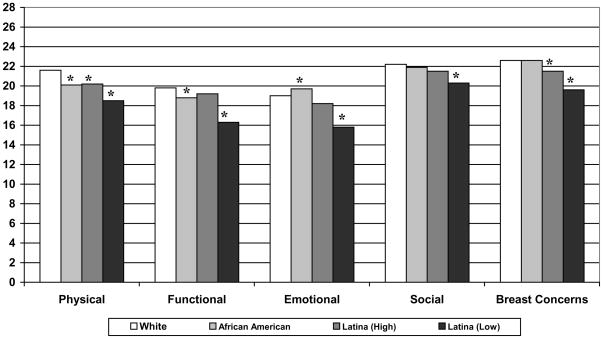
Figure 1 shows the unadjusted mean FACT-B scores by race/ethnicity. All racial/ethnic minority groups reported lower physical well-being relative to white women. African American women reported significantly lower functional well-being but higher emotional well-being than whites. Latinas-high also reported more breast concerns than whites. Latinas-low had significantly (p<0.001) worse scores than white women for physical well-being, functional well-being, emotional well-being, social well-being and breast concerns.
Fig. 1.
Mean QOL (FACT-B) scores by race/ethnicity
Social: (p=0.004); all else (p<0.001);
Range of FACT-B scales: Physical, Functional, Emotional, Social (0–28); Breast Concerns (0–40)
Table 2 presents findings for models on racial/ethnic differences in QOL that control for age, other sociodemographics, clinical factors, and, finally, treatment factors in a sequential pattern. When age is controlled for (Model 1) all unadjusted race/ethnicity findings remain significant except Latinas-high no longer have greater breast concerns compared to white women. When other sociodemographics are added to the model (Model 2) no significant racial/ethnic differences in social well-being remain, Latinas-high are no longer significantly different than white women on physical well being and African Americans are no longer significantly different from white women on functional well-being. When clinical factors are added (Model 3), African Americans no longer report significantly lower physical well-being than white women. In the final model (Model 4) that includes treatment factors as well as sociodemographics and clinical factors, Latinas-low continue to have significantly lower QOL scores than white women for functional well-being, emotional well-being, and breast concerns (all p values <0.05), with physical well-being becoming marginally significant (p=0.053). African American women still have significantly better emotional well-being than white women. Although our primary comparison for racial/ethnic differences uses white women as the reference, in additional analyses we found Latinas-low also were more likely to report lower levels of functional and emotional well-being and more breast concerns as compared to Latinas-high and African American women, adjusting for all factors in Model 4 (all p values < 0.05).
Table 2.
Adjusted Mean Difference in Quality of Life by Race/ethnicity
| Physical | Functional | Emotional | Social | Breast Concerns | |
|---|---|---|---|---|---|
| Model 1: Age-Adjusted | |||||
| Latina (low) | −2.92*** | −3.26*** | −2.98*** | −1.66*** | −2.78*** |
| Latina (high) | −1.28* | −0.34 | −0.57 | −0.53 | −0.74 |
| African American | −1.20** | −0.80* | 0.80* | −0.19 | 0.28 |
| White ( | -- | -- | -- | -- | -- |
| F-test (p-value) | <0.001 | <0.001 | <0.001 | 0.020 | <0.001 |
| Model 2: +Sociodemographics | |||||
| Latina (low) | −2.31** | −2.69*** | −2.80*** | −1.05 | −2.40*** |
| Latina (high) | −1.19 | −0.24 | −0.55 | −0.41 | −0.66 |
| African American | −1.03* | −0.51 | 0.87** | 0.09 | 0.36 |
| White | -- | -- | -- | -- | -- |
| F-test (p-value) | 0.003 | 0.003 | <0.001 | 0.388 | <0.001 |
| Model 3: + Clinical | |||||
| Latina (low) | −2.11** | −2.64*** | −2.70*** | −1.07 | −2.19*** |
| Latina (high) | −0.93 | 0.06 | −0.34 | −0.20 | −0.57 |
| African American | −0.52 | −0.10 | 1.01** | 0.42 | 0.64 |
| White | -- | -- | -- | -- | -- |
| F-Test (p-value) | 0.024 | 0.004 | <0.001 | 0.305 | <0.001 |
| Model 3: +Treatment | |||||
| Latina (low) | −1.90* | −2.41** | −2.58*** | −1.02 | −1.94** |
| Latina (high) | −0.89 | −0.01 | −0.40 | −0.26 | −0.35 |
| African American | −0.53 | −0.18 | 0.90* | 0.32 | 0.71 |
| White | -- | -- | -- | -- | -- |
| F-Test (p-value) | 0.053 | 0.014 | <0.001 | 0.385 | 0.002 |
Model 1: race/ethnicity, age
Model 2: Model 1 + education, income, marital status
Model 3: Model 2 + family history, number of co- morbidities, cancer stage
Model 4: Model 3 + surgical procedure, receipt of chemotherapy, receipt of radiation therapy
p < 0.001;
p<0.01,
p<0.05
Table 3 presents our final model showing all associations between sociodemographic, clinical, and treatment factors in relation to QOL. We found consistent patterns between QOL and age, number of comorbidities, cancer stage at diagnosis, and receipt of chemotherapy. Generally, younger women (compared to those 50–70 and those >70 years) reported significantly worse QOL. Women with earlier cancer stage at diagnosis reported higher physical and functional well-being and less breast concerns than those women with later stage cancer. The presence of other comorbidities (≥1) resulted in lower levels of QOL across all domains except emotional well-being. The receipt of chemotherapy remained significantly associated with lower scores on physical, functional, emotional, and on the breast concerns subscale. Factors that did not significantly impact QOL scores in multivariable models included education, employment, marital status, and family history.
Table 3.
Adjusted Mean Difference in Quality of Life by Sociodemographic, Clinical, and Treatment Factors
| Physical | Functional | Emotional | Social | Breast Concerns | |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age | |||||
| <50 | −3.33*** | −2.89*** | −3.15*** | −2.72*** | −3.72*** |
| 50–70 | −2.12*** | −1.44** | −1.27*** | −1.49 | −1.75*** |
| >70 | -- | -- | -- | -- | -- |
| F-test p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Race/ethnicity | |||||
| Latina (low) | −1.90 | −2.41** | −2.58*** | −1.02 | −1.94** |
| Latina (high) | −0.89 | −0.01 | −0.40 | −0.26 | -0.35 |
| African American | −0.53 | −0.18 | 0.90* | 0.32 | 0.71 |
| White | -- | -- | -- | -- | -- |
| F-test p-value | 0.053 | 0.014 | <0.001 | 0.385 | 0.002 |
| Education | |||||
| < H.S. diploma | −0.95 | −0.62 | −0.41 | −0.67 | −0.71 |
| H.S. diploma | −0.18 | −0.01 | 0.16 | 0.37 | −0.09 |
| > H.S. diploma | -- | -- | -- | -- | -- |
| F-test p-value | 0.339 | 0.613 | 0.498 | 0.251 | 0.429 |
| Employed (yes) | −0.07 | 0.96** | 0.04 | 0.27 | −0.11 |
| Marital status | |||||
| Married/partner | −0.09 | 0.21 | −0.25 | 0.56 | −0.04 |
| Divorced/wid/sep | −0.57 | −0.27 | −0.50 | −0.01 | −0.22 |
| Never married | -- | -- | -- | -- | -- |
| F-test p-value | 0.406 | 0.417 | 0.49 | 0.220 | 0.825 |
| Clinical | |||||
| Family history | |||||
| 1st degree relative | 0.47 | 1.00* | 0.57 | 0.79 | −0.41 |
| 2nd degree relative | 0.14 | 0.57 | 0.54 | 0.56 | 0.34 |
| No family history | -- | -- | -- | -- | -- |
| F-test p-value | 0.533 | 0.044 | 0.073 | 0.092 | 0.276 |
| Breast cancer stage | |||||
| 0 | 2.68** | 2.21** | 0.67 | −0.96 | 0.86 |
| I | 1.31 | 1.68* | 0.04 | −0.19 | 0.27 |
| II | 1.04 | 0.62 | 0.24 | 0.02 | −0.55 |
| III | -- | -- | -- | -- | -- |
| F-test p-value | 0.006 | 0.020 | 0.308 | 0.323 | 0.047 |
| # co-morbidities | |||||
| 0 | 2.58*** | 2.45*** | 0.55 | 0.64 | 1.54*** |
| 1 | 2.29*** | 2.38*** | 0.52 | 1.08** | 1.24** |
| 2+ | -- | -- | -- | -- | -- |
| F-test p-value | <0.001 | <0.001 | 0.189 | 0.036 | <0.001 |
| Treatment | |||||
| Lumpectomy | −0.05 | −0.68 | −0.06 | −0.53 | 0.12 |
| Radiation therapy | −0.30 | −0.51 | −0.09 | −0.65 | −0.03 |
| Chemotherapy | −2.42*** | −2.39*** | −0.87* | −0.10 | −3.08*** |
p-value ≤0.05;
p-value ≤0.01;
p-value ≤0.001
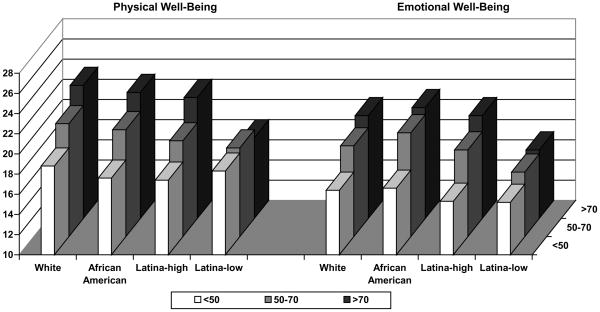
Finally, we examined all interactions with race/ethnicity and each of the sociodemographic, clinical and treatment factors. There was a significant interaction between race/ethnicity and age for physical well-being (p=0.041) and emotional well-being (p=0.042) (Figure 2). Specifically, there was no statistically significant racial/ethnic variation in physical and emotional well-being for younger women (< 50 years of age) indicating that younger women with breast cancer, irrespective of race/ethnicity, had lower levels of physical and emotional well-being than older women. However, there was significant racial/ethnic variation for older women. In the older age groups (50–70, >70) Latinas-low had worse physical well-being and emotional well-being as compared to white women (p values <0.001), and worse emotional well-being compared to African American women (p values <0.01). Latinas-low also had worse physical well-being compared to Latinas high among women 50–70 years of age (p value = 0.01.). A similar interaction was found between race/ethnicity and the number of comorbidities for the breast concerns subscale (p=0.030). Among women with zero or one comorbidity, Latinas-low had more breast concerns as compared to white women; however, there was no significant racial/ethnic variation among women with multiple comorbidities. There was a significant interaction between race/ethnicity and family history for the breast concerns subscale (p=0.037). For this interaction, there were significant racial/ethnic differences with Latinas-low experiencing the worst QOL across all levels of the interacting covariates and thus we did not focus on these results.
Fig. 2.
Adjusted Mean Physical and Emotional Well-Being Scores by Race/Ethnicity and Age
Note: p-value for interaction between race/ethnicity and age is 0.041 for Physical well-being and 0.042 for Emotional well-being
DISCUSSION
In this diverse, population-based study, race, ethnicity, and extent of acculturation were associated with quality of life after controlling for other sociodemographic, clinical, and treatment factors. This is one of the first studies to evaluate these factors with a large sample including both low and more highly acculturated Latinas, allowing us to compare differences between these groups. Latinas with low acculturation reported significantly worse QOL compared to white women. In contrast, highly acculturated Latinas generally reported QOL scores similar to non-Latina white women. African American women reported higher emotional well-being compared with white women. While younger women reported worse QOL than older women, age played a moderating effect on racial/ethnic and acculturation differences in QOL. Latinas with low acculturation experienced worse physical and emotional well-being compared to white women only among the older age groups, and there were no racial/ethnic or acculturation differences in QOL among younger women. Similarly, racial/ethnic differences in the breast concerns subscale were modified by the number of comorbidities, and significant racial/ethnic differences were no longer present among women with two or more comorbid illnesses.
The relative importance of other sociodemographic factors must be considered when examining the influence of race/ethnicity in QOL in cancer survivors [5, 7, 8, 30]. The disproportionate negative impact of breast cancer and its treatment for younger women has been reported in many previous studies [4, 5, 7, 20, 26, 27, 28–30]. Possible explanations include that younger women may experience more discordance between their health expectations and a cancer diagnosis [4], experience greater disruption in their daily lives, work schedules, and financial stability [5, 27], or possess fewer coping strategies to manage life-threatening situations [28]. Our findings suggest that older, less acculturated Latinas may also be vulnerable to poor QOL in cancer survivorship. Thus interventions designed to address QOL disparities need to focus not just on race/ethnicity but also on age-specific issues.
Some researchers have suggested that poorer QOL among minority women in the survivorship period may be the result of more advanced disease at diagnosis, requiring more extensive treatment and increasing the risk for side effects and lower physical and emotional functioning [40–42]. While we found that breast cancer stage was a significant factor in physical and functional well-being, there were no racial/ethnic differences in QOL by cancer stage. In a similar fashion, while we found that chemotherapy was associated with lower QOL, consistent with the findings of others [5, 8, 33], racial, ethnic, and acculturation differences in QOL were not explained by receipt of chemotherapy. In fact, very little was gained in explaining observed racial/ethnic differences in QOL from clinical or treatment factors once sociodemographic factors were controlled for in our sequential modeling procedure.
Our results suggest the need to consider mechanisms beyond demographics, clinical, and treatment factors to understand racial/ethnic differences in QOL. A number of previous studies have documented the relationship between exposure to cancer information and care support and better QOL outcomes [43, 44]. If minority women receive less information and/or support, such differences may contribute to differences in QOL outcomes during survivorship. In a previous study we reported that Latinas with low acculturation had the most unmet information needs [10]. We also found that this group had the lowest amount of satisfaction with the treatment decision making process [45]. Previous studies have suggested that Latinas (especially those with low acculturation) face special challenges understanding information provided in the medical care setting [10, 13, 34, 35]. These informational barriers highlight the need for professional translational services and culturally appropriate written information for less acculturated Latinas.
The presence of supportive relationships during the cancer experience also has been associated with better QOL [46]. Previous research indicates that African American breast cancer survivors report higher levels of social support from all sources than whites [29] and that Latinas report having the lowest level of social support [10, 20]. Older Latinas may be especially socially isolated with limited access to culturally appropriate cancer support groups, especially web-based support groups. Racial/ethnic minority cancer survivors in general have less access to, and are less likely to become members of, organized cancer support groups [10, 35]. Some evidence for the importance of social support was found in a recent study where more acculturated Korean immigrant cancer survivors reported stronger social networks that in turn contributed to better QOL. [47] Thus improving access to culturally appropriate social and peer support during the transition from patient to survivorship could have a positive impact on QOL.
Another mechanism that may contribute to racial/ethnic differences in QOL is the role of religion during stressful health events. While we did not measure the role of religion, others have shown African American survivors use religion/spirituality as a strategy for coping with breast cancer [18, 21, 48]. There may be more variation in the role of religion among Latina survivors. One study reported that higher levels of religiosity in Latinas was positively correlated with better QOL [49] while another reported that, when confronted with health concerns, some older Mexican Americans choose to “suffer in silence” and not to avail themselves of support from others [50]. Finally, a recent study found that low vs. high acculturated Latinas reported greater levels of spirituality, and perceived more social support, which enhanced their life satisfaction in cancer survivorship [51]. Further research on the intersection between religion, social support, and behavior in health crises may add to the understanding of the observed racial/ethnic differences in cancer survivorship.
Limitations
Study findings are limited by the cross-sectional study design that did not allow us to examine QOL over time. The mean time from diagnosis to survey completion was 9.2 months, therefore, longer term consequences of the cancer experience on QOL were not assessed. There is a need to conduct multi-ethnic longitudinal evaluations of QOL in order to design effective interventions to reduce any observed disparities. In addition, the generalizability of our findings regarding racial/ethnic differences is limited to those groups included in the sample. A major strength of this study was the large population based sample with sufficient numbers of Latina women allowing us to examine the relevance of acculturation. However, the origin of the Latina population in Los Angeles County is predominantly from Mexico and Central America. The U.S. Hispanic population is a diverse population, and it may not be appropriate to generalize our findings to Latinas from other cultural backgrounds.
Implications
The findings from this population-based sample have implications for patients, providers, and policy-makers. We need to identify and target women disproportionately affected by breast cancer and its treatment such as younger women and less acculturated Latinas. Behavioral and counseling interventions must be targeted to their unique issues and concerns which may have a positive impact on their quality of life. High quality care is dependent on patients understanding information about their care at both the time of initial cancer treatment and in the long-term survivorship period. Possible interventions to improve QOL include access to: a) professional translational services, b) language and reading-level appropriate written information, c) support groups and peer counseling, and d) web-based social networks of cancer survivors. However, these interventions must be sensitive to factors influenced by culture and ethnicity. For example, a cognitive reframing intervention focused on helping women to reinterpret problems as manageable and see them as sources of opportunity was more effective with African American than whites [52]. More attention must also be paid to the competing demands of managing comorbid conditions among cancer survivors, particularly for African American women [52].
Continuing research to untangle important factors contributing to racial/ethnic differences in QOL in cancer survivorship is warranted. The relative influence of racial/ethnic variation, acculturation, information and care support, and treatment patterns resulting in differences in women’s QOL deserves further research.
Acknowledgments
This work was funded by grants R01 CA109696 and R01 CA088370 from the National Cancer Institute (NCI) to the University of Michigan. Dr. Katz was supported by an Established Investigator Award in Cancer Prevention, Control, Behavioral, and Population Sciences Research from the NCI (K05CA111340).
The collection of Los Angeles County cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI’s Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35139 awarded to the University of Southern California, contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute. The collection of metropolitan Detroit cancer incidence data was supported by the NCI SEER Program contract N01-PC-35145. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Special thanks to Ain Boone, Cathey Boyer, and Deborah Wilson for their data collection efforts at the Metropolitan Detroit Cancer Surveillance System (MDCSS) and to Alma Acosta, Marlene Caldera, Norma Caldera, Maria Isabel Gaeta, Urduja Trinidad, and Mary Lo at the Cancer Surveillance Program in Los Angeles; and to Barbara Salem, Ashley Gay and Paul Abrahamse at the University of Michigan.
References
- 1.Smedley BD, Stith A, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: Institute of Medicine: The National Academy Press; 2003. [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services, Nation Institutes of Health, National Cancer Institute. Living beyond cancer: Finding a new balance. President’s Cancer Panel: 2003–2004 Annual Report; Washington, D.C. 2004. [Google Scholar]
- 3.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academy Press; 2006. [Google Scholar]
- 4.Bloom JR, Steward SL, Chang S, Banks P. Then and now: quality of life of young breast cancer survivors. Psycho-Oncology. 2004;13:147–160. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- 5.Costanzo ES, Lutgendorf SK, Mattes ML, Trehan S, Robinson CB, Tewfik F, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97(12):1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton SP, Levine EG, Fitzpatrick CM, Dold KH, Targ E. Exploring the relationships among spiritual well-being, quality of life, and psychological adjustment in women with breast cancer. Psycho-oncology. 1999;8(5):429–438. doi: 10.1002/(sici)1099-1611(199909/10)8:5<429::aid-pon420>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Friedman LC, Kalidas M, Elledge R, Chang J, Romero C, Husain I, et al. Optimism, social support and psychosocial functioning among women with breast cancer. Psycho-oncology. 2006;15(7):595–603. doi: 10.1002/pon.992. [DOI] [PubMed] [Google Scholar]
- 8.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin T. Quality of life in long term disease free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 9.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women; quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21:4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 10.Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113:1058–1067. doi: 10.1002/cncr.23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack L, Greer GE, Rowland JH, Miller A, Doneski D, Coughlin SS, et al. Cancer survivorship: a new challenge in comprehensive cancer control. Cancer Causes Control. 2005;16:51–59. doi: 10.1007/s10552-005-0452-x. [DOI] [PubMed] [Google Scholar]
- 12.Trentham-Dietz A, Sprague BL, Klein R, Klein BE, Cruickshanks KJ, Fryback DG, et al. Health-related quality of life before and after breast cancer diagnosis. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9653-1. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashing-Giwa KT, Padilla GV, Bohorquez DE, Tejero JS, Garcia M. Understanding the breast cancer experience of Latina women. J Psychosoc-Oncol. 2006;24:19–52. doi: 10.1300/J077v24n03_02. [DOI] [PubMed] [Google Scholar]
- 14.Garofalo JP, Hammann HA, Ashworth K, Baum A. Stress and quality of life in African American breast cancer survivors. Ethn Dis. 2006;16:732–738. [PubMed] [Google Scholar]
- 15.Powe B, Hamilton J, Hancock N, Johnson N, Finnie R, Ko J, et al. Quality of life of African American cancer survivors. Cancer. 2007;109:435–445. doi: 10.1002/cncr.22358. [DOI] [PubMed] [Google Scholar]
- 16.Katz SJ, Lantz PM, Paredes Y, Janz NK, Fagerlin A, Liu L, et al. Breast cancer treatment experiences of Latinas in Los Angeles County. Am J Public Health. 2005;95:2225–2230. doi: 10.2105/AJPH.2004.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayanian JZ, Jacobsen PB. Enhancing research on cancer survivors. J Clin Oncol. 2006;24:5149–5153. doi: 10.1200/JCO.2006.06.7207. [DOI] [PubMed] [Google Scholar]
- 18.Fatone AM, Moadel AB, Foley FW, Fleming M, Jandorf L. Urban voices: the quality-of-life experience among women of color with breast cancer. Palliat Support Care. 2007;5:115–125. doi: 10.1017/s1478951507070186. [DOI] [PubMed] [Google Scholar]
- 19.Gil KM, Mishel MH, Belyea M, Germino B, Porter LS, Carlton Laney I, et al. Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2004;31:633–639. doi: 10.1188/04.onf.633-639. [DOI] [PubMed] [Google Scholar]
- 20.Ashing-Giwa K, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16:413–428. doi: 10.1007/s11136-006-9138-4. [DOI] [PubMed] [Google Scholar]
- 21.Bowen DJ, Alfano CM, McGregor BA, Kuniyuki A, Bernstein L, Meeske K, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106:85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashing-Giwa K. Quality of life of African-American and white long term breast carcinoma. Cancer. 1999;85:418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Northouse LL, Caffey M, Deichelbohrer L, Schmidt L, Guziatek-Trojniak L, West S, et al. The quality of life of African American women with breast cancer. Res Nurs Health. 1999;22:449–60. doi: 10.1002/1098-240x(199912)22:6<449::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Rao D, Debb S, Blitz D, Choi SW, Cella D. Racial/ethnic differences in the health-related quality of life of cancer patients. J Pain Symptom Manage. 2008;36:488–496. doi: 10.1016/j.jpainsymman.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: Comparing African Americans, Hispanics, and non-Hispanic Whites. Psycho-Oncology. 2002;11:495–504. doi: 10.1002/pon.615. [DOI] [PubMed] [Google Scholar]
- 26.Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychol. 1999;18:159–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- 27.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: how are they faring? Cancer. 2005;104:2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 28.Kornblith AB, Powell M, Regan MM, Bennett S, Krasner C, Moy B, et al. Long-term psychosocial adjustment of older vs. younger survivors of breast and endometrial cancer. Psycho-oncology. 2007;16:895–903. doi: 10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- 29.Giedzinska AS, Meyerowitz BE, Ganz PA, Rowland JH. Health-related quality of life in a multiethnic sample of breast cancer survivors. Ann Behav Med. 2004;28:39–51. doi: 10.1207/s15324796abm2801_6. [DOI] [PubMed] [Google Scholar]
- 30.Janz NK, Mujahid MS, Lantz PM, Fagerlin A, Salem B, Morrow M, et al. Population-based study of the relationship of treatment and sociodemographics on quality of life for early stage breast cancer. Qual Life Res. 2005;14:1467–1479. doi: 10.1007/s11136-005-0288-6. [DOI] [PubMed] [Google Scholar]
- 31.Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Holzel D. Predictors of quality of life of breast cancer patients. Acta Oncol. 2003;42:710–718. doi: 10.1080/02841860310017658. [DOI] [PubMed] [Google Scholar]
- 32.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM. AJCC Cancer Staging Manual. 6. Philadelphia, PA: Lippincott Raven Publishers; 2002. [Google Scholar]
- 33.Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Titus-Ernstoff L, et al. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol. 2005;23:4399–4405. doi: 10.1200/JCO.2005.03.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore RJ, Butow P. Culture and oncology: impact of context effects. New York, NY: Kluwer; 2004. [Google Scholar]
- 35.Aziz NM, Rowland JH. Cancer survivorship research among ethnic minority and medically Underserved groups. Oncol Nurs Forum. 2002;29:789–801. doi: 10.1188/02.ONF.789-801. [DOI] [PubMed] [Google Scholar]
- 36.Marin G, Van Oss Marin B. Applied Social Research Methods Series. 23. Sage Publications; Newbury Park, CA: 1991. Research with Hispanic populations. [Google Scholar]
- 37.Dillman DA. Mail and Telephone Surveys: The Total Design Method. New York, NY: John Wiley and Sons; 1997. [Google Scholar]
- 38.Cella D, Tulsky D, Gray G. The Functional Assessment of Cancer Therapy Scale: Development and Validation of the General Measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 39.Brady M, Cella D, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and Validity of the Functional Assessment of Cancer Therapy-Breast Quality-of-Life Instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 40.Bradley CJ, Neumark D, Luo Z, Schenk M. Employment and cancer: findings from a longitudinal study of breast and prostate cancer survivors. Cancer Invest. 2007;25:47–54. doi: 10.1080/07357900601130664. [DOI] [PubMed] [Google Scholar]
- 41.Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Lantz PM, Mujahid M, Schwartz K, Janz NK, Fagerlin A, Salem B, et al. The influence of race, ethnicity and individual socioeconomic factors of breast cancer stage at diagnosis. Am J Public Health. 2006;96:2173–2178. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arora NK, Johnson P, Gustafson DH, McTavish F, Hawkins RP, Pingree S. Barriers to information access, perceived health competence, and psychosocial health outcomes: test of a mediation model in a breast cancer sample. Patient Educ Couns. 2002;47:37–46. doi: 10.1016/s0738-3991(01)00170-7. [DOI] [PubMed] [Google Scholar]
- 44.Griggs JJ, Sorbero ME, Mallinger JB, Quinn M, Waterman M, Brooks B, et al. Vitality, mental health, and satisfaction with information after breast cancer. Patient Educ Couns. 2007;66:58–66. doi: 10.1016/j.pec.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Hawley ST, Janz NK, Hamilton A, Griggs JJ, Alderman AK, Mujahid M, Katz SJ. Latina patient perspectives about informed treatment decision making for breast cancer. Patient Educ Couns. 2008;73:363–70. doi: 10.1016/j.pec.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sammarco A, Konecny LM. Quality of life, social support, and uncertainty among Latina breast cancer survivors. Oncol Nurs Forum. 2008;35(5):844–849. doi: 10.1188/08.ONF.844-849. [DOI] [PubMed] [Google Scholar]
- 47.Lim J, Yi J, Zebrack B. Acculturation, social support, and quality of life for Korean immigrant breast and gynecological cancer survivors. Ethn Health. 2008;13(3):243–260. doi: 10.1080/13557850802009488. [DOI] [PubMed] [Google Scholar]
- 48.Ashing-Giwa K, Padilla GV, Tejero JS, Kim J. Breast cancer survivorship in a multiethnic sample. Cancer. 2004;101:450–465. doi: 10.1002/cncr.20370. [DOI] [PubMed] [Google Scholar]
- 49.Wildes KA, Miller AR, San Miguel de Majors S, Ramirez AG. The religiosity/spirituality of Latina breast cancer survivors and influence on health-related quality of life. Psycho-Oncology. 2008 doi: 10.1002/pon.1475. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krause N, Batida E. Religion, suffering, and health among older Mexican Americans. J Aging Stud. 2009;23:114–123. doi: 10.1016/j.jaging.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephens C, Stein K, Landrine H. The role of acculturation in life satisfaction among Hispanic cancer survivors: results of the American Cancer Society’s study of cancer survivors. Psycho-Oncology. 2009 doi: 10.1002/pon.1566. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 52.Porter LS, Clayton MF, Belyea M, Mishel MH, Gil KM, Germino B. Predicting negative mood state and personal growth in African American and White long term breast cancer survivors. Ann Behav Med. 2006;31(3):195–204. doi: 10.1207/s15324796abm3103_1. [DOI] [PubMed] [Google Scholar]